Introduction
Estimates from the USA indicate that 1.2 million residents were living with human immunodeficiency virus (HIV) infection at the end of 2013; >800 000 were infected with hepatitis B virus (HBV); and approximately 4.6 million have ever been infected with hepatitis C virus (HCV) [1–Reference Edlin3]. Although effective therapies are available for managing HIV, HBV and HCV infections, these infections sometimes remain undiagnosed because of their often asymptomatic nature [Reference Spradling4–Reference Hall6]. Public health efforts to test and link persons with HIV and viral hepatitis infections to care are of crucial importance for mitigating associated morbidity and mortality [Reference Asselah and Marcellin7–Reference Montaner9].
Because social factors that place persons at risk for acquiring HIV, HBV and HCV are similar and these conditions share some transmission routes, patients can often be coinfected with viral hepatitis and HIV. Although the proportion and prevalence of coinfection vary on the basis of disease epidemiology, worldwide estimates report that approximately 10% of persons living with HIV infection are coinfected with HBV and 25% are coinfected with HCV [Reference Thio10–13]. HIV infection can increase susceptibility to subsequent infection with HBV or HCV, and concomitant HIV infection can result in an increase in HBV or HCV viraemia, thus accelerating liver damage [Reference Colin14–Reference Operskalski and Kovacs17]. Coinfected persons are at greater risk for liver and all-cause morbidity and mortality, compared with those who are monoinfected [Reference Konopnicki18–Reference Teira20]. Identifying coinfected persons and linking them to care and management of both their HIV and viral hepatitis infections is essential. Highly active antiretroviral therapy for HIV, antiviral therapy for HBV and direct-acting antivirals that can cure HCV infection can improve outcomes for coinfected patients [Reference Hua11, Reference Taylor, Swan and Mayer16, Reference Operskalski and Kovacs17].
Communicable disease surveillance data help identify trends and risks associated with infectious agent transmission and guide development and evaluation of public health initiatives [Reference Adekoya21]. Individual states and cities collect communicable disease data and transmit de-identified records to the Centers for Disease Control and Prevention (CDC) [22]. HIV and viral hepatitis infections are nationally notifiable in the USA but are maintained in disparate surveillance systems within jurisdictions and at CDC. Health departments’ surveillance activities for HIV, acute and chronic HBV, and acute and chronic HCV vary by jurisdiction. Although some health departments have used their surveillance data to quantify the number and characteristics of HIV and viral hepatitis coinfections, approaches used for identifying coinfections and analysing results vary greatly [Reference Sanchez23–Reference Prussing27]. Routine linkages of HIV and viral hepatitis surveillance data are necessary to monitor health status, including assessments of the risk for a geographically focused outbreak [Reference Van Handel28]. This study examined characteristics of HIV and viral hepatitis coinfections by using surveillance data from 15 US states and two cities with a standardised method for matching and analysis.
Methods
Jurisdiction selection
All 65 health departments funded as part of CDC's National HIV Surveillance System were contacted to identify jurisdictions interested in developing a standardised approach for using HIV and viral hepatitis surveillance data for assessing HIV and hepatitis coinfection. Fifteen states (Arizona, Connecticut, Florida, Iowa, Louisiana, Maryland, Massachusetts, Michigan, Minnesota, North Dakota, South Carolina, Texas, Virginia, Washington and Wisconsin) and two independently funded cities (New York City, New York and San Francisco, California) conducted linkages in accordance with their local data security and confidentiality policies and provided de-identified data to CDC. The independently funded city of Houston, Texas, participated in the project, but we limited our analysis to results reported by Texas to avoid duplication of reported cases. We used information collected as part of routine public health surveillance activities classified as non-research; therefore, institutional review board review was not required.
Hepatitis case selection
Jurisdictions varied by viral hepatitis conditions that were reportable and by when each condition became reportable (Table 1). Data were extracted from surveillance systems used to maintain viral hepatitis data in each jurisdiction and input into SAS® (SAS Institute, Inc., Cary, North Carolina, USA) datasets. Datasets included acute HBV, acute HCV, chronic HBV and chronic HCV conditions, with case classifications consistent with applicable CDC/Council of State and Territorial Epidemiologists case definitions [29]. Each jurisdiction was responsible for assigning case classifications to viral hepatitis cases by using the applicable case definition. Chronic HBV and chronic HCV are not reportable in Texas; therefore, standard definitions in alignment with the chronic HCV case definition were applied to HCV laboratory data reported electronically to identify cases in Texas. Hepatitis event date was determined for each hepatitis case by a CDC-developed hierarchy of dates associated with the condition [30]. Each jurisdiction determined the earliest event date and conditions to be included on the basis of the jurisdiction's hepatitis surveillance practices (Table 1). Health departments de-duplicated their viral hepatitis data to create a unique identifier for each person across all reported conditions or to create an identifier for each person separately by HBV and HCV conditions.
Table 1. Comparison of the earliest yeara included in the analysis and the year registry started, HIV and hepatitis surveillance registries, 15 US states and two cities
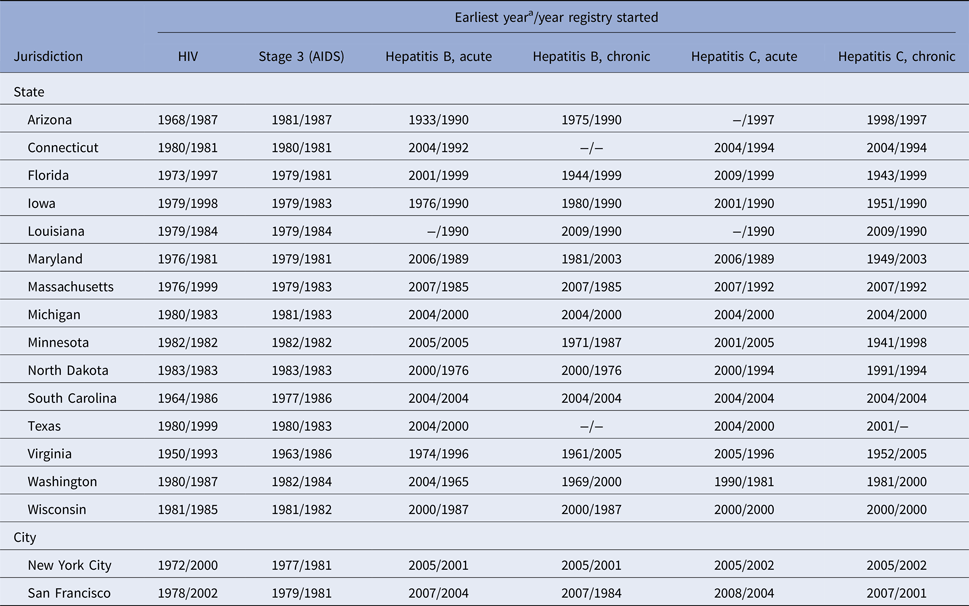
AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; MMWR, Morbidity and Mortality Weekly Report.
a For HIV and stage 3 (AIDS), based on the earliest diagnosis date included in the analysis; the HIV surveillance system automatically calculated the diagnosis date by using recorded laboratory and clinical (non-laboratory) evidence. For hepatitis, based on the earliest event date included in the analysis as selected by each jurisdiction. The event date was determined for each hepatitis case by a hierarchy of dates associated with the condition (i.e., onset date, diagnosis date, laboratory report date or first report to the public health system, state or MMWR date).
HIV case selection
All jurisdictions have reported HIV infection stage 3 (AIDS) since the beginning of the epidemic in the early 1980s. However, HIV infection reporting was implemented at different times across US jurisdictions (Table 1). Data were extracted from the HIV surveillance system within each jurisdiction by using a standardised SAS program and input into a SAS dataset. All jurisdictions had routine quality-assurance procedures in place, including a requirement to de-duplicate HIV cases on a monthly basis. Datasets included all persons with HIV infection reported to health departments and meeting data completeness eligibility criteria for transfer to CDC (unpublished data CDC, 2017).
Data matching
All jurisdictions used an automated hierarchical deterministic matching method to link HIV and hepatitis datasets to reduce the matching time and to minimise variation in manual adjudication. A SAS program was developed for matching data on 14 keys (i.e., character string of values from a variable or combination of variables) (Table 2) and was similar to the method previously described by New York City [Reference Drobnik26]. Six jurisdictions validated the deterministic matching method against their existing matching methods that included a probabilistic matching component. Manual review was required only when multiple records in one dataset matched to a single record in the other dataset on the same lowest key number.
Table 2. Matching keys used by 15 US states and two cities for the deterministic matching methoda
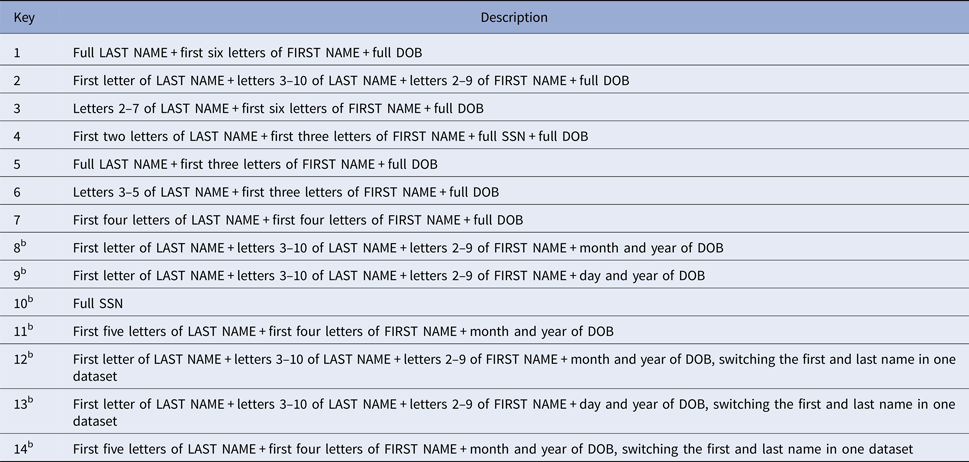
DOB, date of birth; HIV, human immunodeficiency virus; SSN, social security number.
a Automated SAS® (SAS Institute, Inc., Cary, North Carolina, USA) program used to match records on 14 keys. Manual review was required only when multiple records from one dataset matched to a single record in the other dataset on the same lowest key value.
b If matched on this key, the following three additional criteria had to be met to be considered a match:
(1) Value of sex had to be same in both datasets or the full date of birth and digits one through four and six through nine of the social security number had to be the same in both datasets.
(2) First name in the HIV dataset was not among the 20 most common names in the HIV dataset for the jurisdiction.
(3) Last name in the HIV dataset was not among the 20 most common names in the HIV dataset for the jurisdiction.
Analysis
All jurisdictions used a standardised SAS program to summarise results from the matched datasets. Aggregate data from each jurisdiction were combined. Coinfections were defined as both HIV and viral hepatitis (HBV or HCV) infections in the same person. We examined characteristics of coinfections within three cohorts: (1) persons living with diagnosed HIV as of 31 December 2014; (2) persons ever reported with HBV; and (3) persons ever reported with HCV. When assessing coinfections among persons living with diagnosed HIV infection, HIV cases were restricted to those among persons meeting the following criteria: (1) HIV infection diagnosis date on or before 31 December 2014; (2) alive as of 31 December 2014; and (3) most recent known address on or before 31 December 2014 was in the jurisdiction. When assessing coinfections among persons with a viral hepatitis condition, HIV cases were restricted to persons with HIV infection diagnosed on or before 31 December 2014 who were reported to the jurisdiction regardless of vital status and residence. When assessing coinfections among all three cohorts described previously, viral hepatitis cases were restricted to those with a condition event date on or before 31 December 2014 reported to the jurisdiction regardless of residence or vital status. Among persons with multiple reported HBV conditions (e.g., reported with both an acute and a chronic condition), the HBV condition with the earliest event date was used when summarising the coinfection; the same method was used among persons with multiple reported HCV conditions. When assessing coinfections among persons living with diagnosed HIV as of 31 December 2014, we included persons ever diagnosed with a viral hepatitis condition and reported with a condition event date on or before 31 December 2014; due to limitations of viral hepatitis surveillance data we could not determine whether individuals had cleared their viral hepatitis infections before 31 December 2014. Because the number of persons coinfected with HIV, HBV and HCV was expected to be low, our analysis was not designed to identify these coinfections. If a person was coinfected with all three conditions, both the HIV and HBV coinfection information and the HIV and HCV coinfection information would be summarised.
Age group was based on age at diagnosis of HIV or viral hepatitis infection; age for coinfections was based on age at diagnosis of the second reported virus. Transmission category was selected from the most likely route of transmission of HIV on the basis of a hierarchy of reported risk information [1]. Among coinfected persons, sex and race/ethnicity were first derived from the HIV dataset, and supplemented with information from the hepatitis dataset if missing from the HIV dataset. For HIV infection, sex indicated sex at birth. For viral hepatitis cases, sex was not uniformly defined across all jurisdictions and indicated sex at birth, sex at the time of viral hepatitis event or current sex at the time the data were extracted depending on the jurisdiction. Among coinfected persons, the timing of when coinfection became known was determined by comparing the HIV diagnosis date and hepatitis event date. This represented the earliest known date associated with each virus but might not reflect the true order of infection.
Results
The earliest year included in the analysis and the year the registry started for viral hepatitis and HIV data varied across the 15 states and two cities (Table 1). Of 504 398 persons living with diagnosed HIV infection as of 31 December 2014 in 17 total jurisdictions, 10 216 (2.0%; range: 0.1–4.5%) were coinfected with HBV, and 33 993 (6.7%; range: 0–11.3%) were coinfected with HCV (Table 3). Of 269 884 persons ever reported with HBV, 14 117 (5.2%; range: 2.6–12.2%) were coinfected with HIV. Of 1 093 050 persons ever reported with HCV, 47 240 (4.3%; range: 0.2–13.3%) were coinfected with HIV.
Table 3. Number and percentage of HIV and hepatitis coinfections among persons living with diagnosed HIV infection and among persons with hepatitis infection, 15 US states and two cities
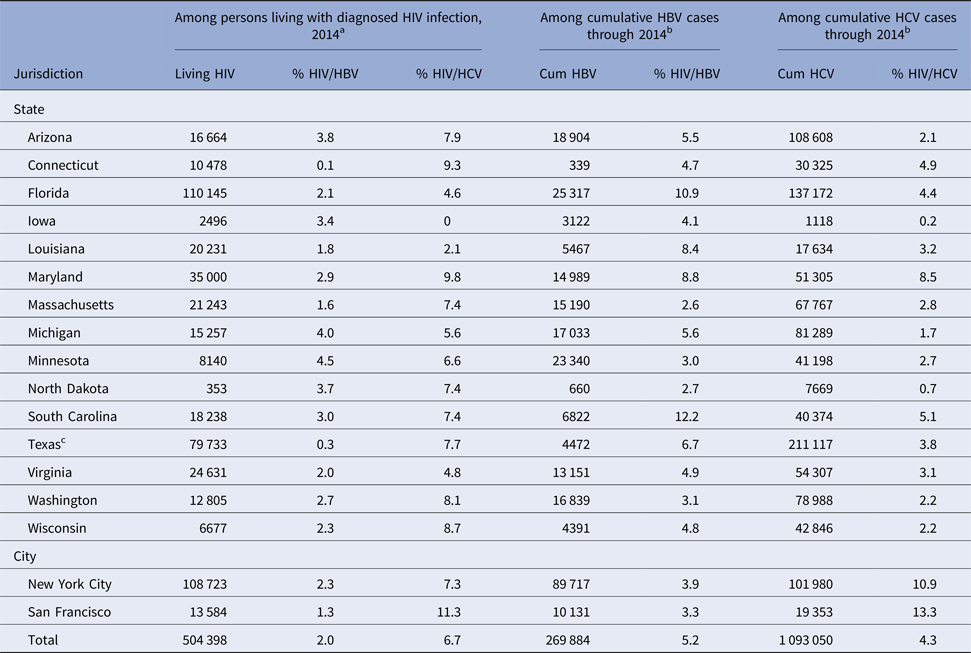
Cum, cumulative; HBV, hepatitis B virus (acute or chronic); HCV, hepatitis C virus (acute or chronic); HIV, human immunodeficiency virus.
a Includes persons living with diagnosed HIV infection, regardless of stage at disease diagnosis, whose most recently known address through 31 December 2014, was within the jurisdiction and who were presumed to be alive as of 31 December 2014. Coinfections refer to persons living with diagnosed HIV infection matched with a hepatitis infection event occurring through 31 December 2014.
b Includes persons reported with the hepatitis infection (acute or chronic) from the earliest event date included in the analysis for each jurisdiction (see Table 1) through 31 December 2014. Coinfections refer to persons reported with the hepatitis infection event date through 31 December 2014, matched with an HIV infection diagnosis through 31 December 2014.
c Houston, Texas (USA), independently reported to CDC the following coinfection information for this project:
(1) Among 23 272 persons living with diagnosed HIV in Houston, 396 (1.7%) were coinfected with HBV and 1126 were coinfected with HCV (4.8%).
(2) Among the 7884 cumulative persons with HBV in Houston, 573 (7.3%) were coinfected with HIV.
(3) Among the 27 769 cumulative persons with HCV in Houston, 1722 (6.2%) were coinfected with HIV.
(4) Variation in coinfection data is caused by differences in the hepatitis information reported to the Houston Health Department and the Texas Department of State Health Services. Houston HBV data include acute and chronic conditions, but Texas HBV data include only acute conditions.
Persons living with diagnosed HIV infection with or without HBV infection
Among persons living with diagnosed HIV infection, a greater proportion of those coinfected with HBV were black/African American (53.9%), and a lower proportion were Hispanic (14.2%), compared with persons living with diagnosed HIV infection without HBV (44.9% and 22.2%, respectively) (Table 4). The largest proportion of HIV/HBV coinfected persons were aged 40–49 years at the time of their second diagnosis (35.8%). A greater proportion of persons living with diagnosed HIV infection were male among those with HBV (82.9%), compared with those without HBV (74.0%). Among persons living with diagnosed HIV infection, a greater proportion of those with HBV were males with HIV infection attributed to male-to-male sexual contact (49.8%), compared with those without HBV (44.4%). A lower proportion of persons living with diagnosed HIV infection and coinfected with HBV were females with HIV infection attributed to heterosexual contact (8.6%), compared with those without HBV (13.8%). Among 74.4% of HIV/HBV coinfected persons, HIV diagnosis year preceded the HBV event year.
Table 4. Number and percentage of HIV and hepatitis coinfections among persons living with diagnosed HIV infection, by selected characteristics, 15 US states and two cities, 2014
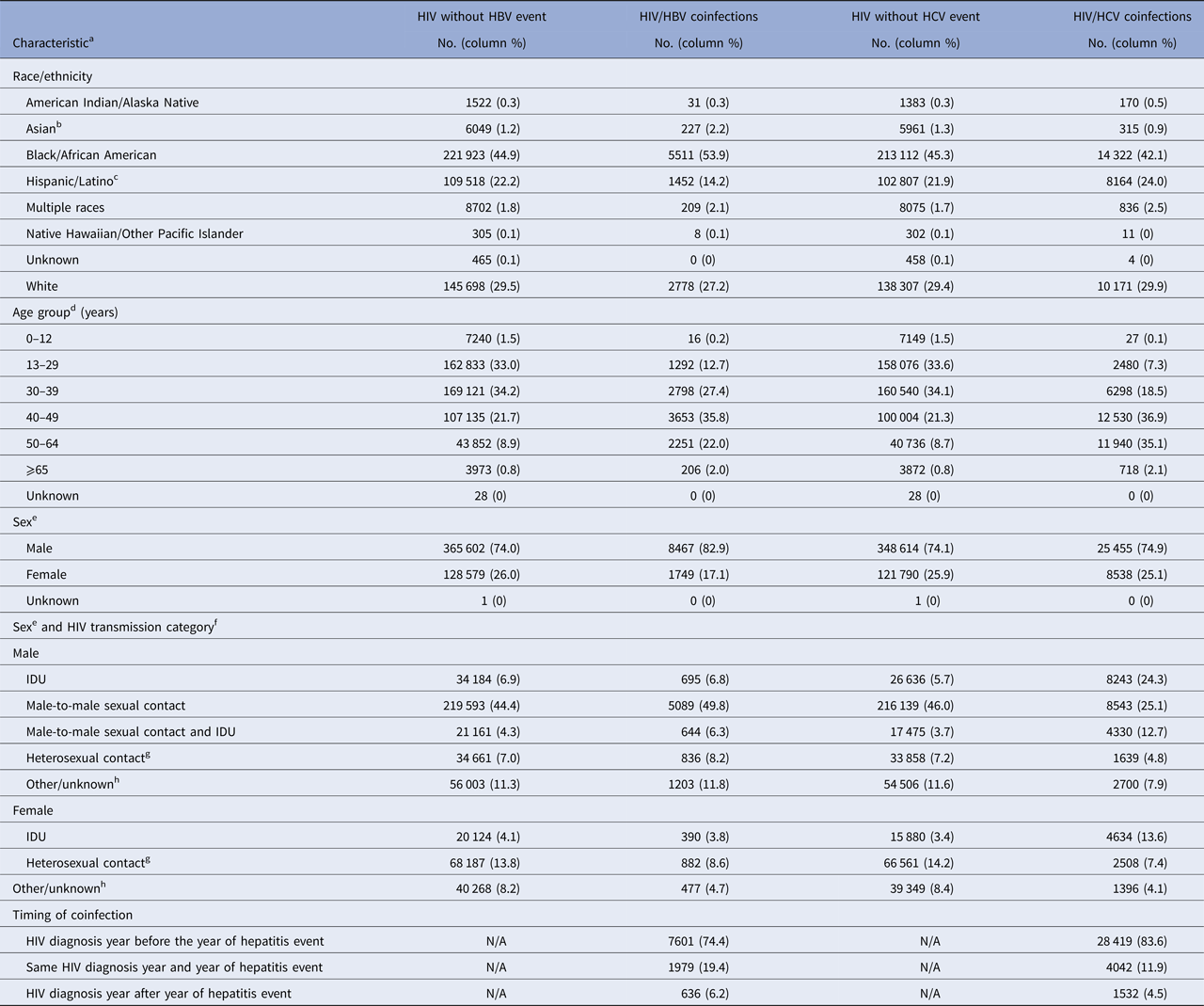
HBV, hepatitis B virus (acute or chronic); HCV, hepatitis C virus (acute or chronic); HIV, human immunodeficiency virus; N/A, not applicable; IDU, injection drug use.
a For persons with coinfection, information comes first from the HIV surveillance system. If information was missing in the HIV surveillance system, information from the hepatitis surveillance system was used.
b Includes persons for whom the surveillance system did not differentiate between Asian and Native Hawaiian/Other Pacific Islander.
c Hispanics/Latinos can be of any race.
d For HIV cases without a hepatitis event, based on age at diagnosis of HIV. For coinfection cases, based on age at coinfection or second reported virus infection to the health department.
e From HIV surveillance system, sex indicates sex at birth. From hepatitis surveillance system, sex might indicate sex at birth, sex at the time of hepatitis event, or current sex at the time the data were extracted.
f Data have not been statistically adjusted to account for unknown transmission categories.
g Heterosexual contact with a person known to have, or to be at high risk for, HIV infection.
h Includes haemophilia, blood transfusion, or perinatal exposure, and persons with an unknown transmission category.
Persons living with diagnosed HIV infection with or without HCV infection
No differences were identified in the distribution of race/ethnicity by >5.0 percentage points among persons living with diagnosed HIV infection with and without HCV (Table 4). A greater proportion of persons coinfected with HIV and HCV were aged ⩾50 years (37.2%), compared with those coinfected with HIV and HBV (24.0%). Distributions by sex among persons living with diagnosed HIV infection with and without HCV were similar. Males and females with HIV infection attributed to injection drug use (IDU) (24.3% and 13.6%, respectively) represented a greater proportion of persons living with diagnosed HIV infection and HCV, compared with those without HCV (5.7 and 3.4%, respectively). Males with HIV infection attributed to male-to-male sexual contact and IDU (12.7%) represented a greater proportion of persons living with diagnosed HIV infection and HCV, compared with those without HCV (3.7%). In contrast, males with HIV infection attributed to male-to-male sexual contact (25.1%) and females with HIV infection attributed to heterosexual contact (7.4%) represented a lower proportion of persons living with diagnosed HIV infection and HCV, compared with those without HCV (46.0% and 14.2%, respectively). As with HIV and HBV coinfections, HIV diagnosis year preceded HCV event year among the majority (83.6%) of persons coinfected with HCV and HIV.
Persons ever receiving a diagnosis of viral hepatitis with and without HIV infection
Race/ethnicity was unknown for the majority of HBV monoinfected persons (53.1%), and comparisons with HBV/HIV-coinfected persons should be avoided (Table 5). The largest proportion of HBV/HIV-coinfected persons was those aged 40–49 years at the time of second diagnosis (35.9%). The proportion of males was higher among the HBV/HIV coinfected cohort, compared with the HBV monoinfected (83.4% vs. 53.8%). Among HBV/HIV-coinfected persons, the largest proportion was among persons with HIV infection attributed to male-to-male sexual contact (48.4%). Among the HBV/HIV coinfected population, HIV diagnosis year preceded HBV event year in 75.8% of all cases.
Table 5. Number and percentage of HIV and hepatitis coinfections among persons with hepatitis B infection and hepatitis C infection, by selected characteristics, 15 US states and two cities, cumulative through 2014
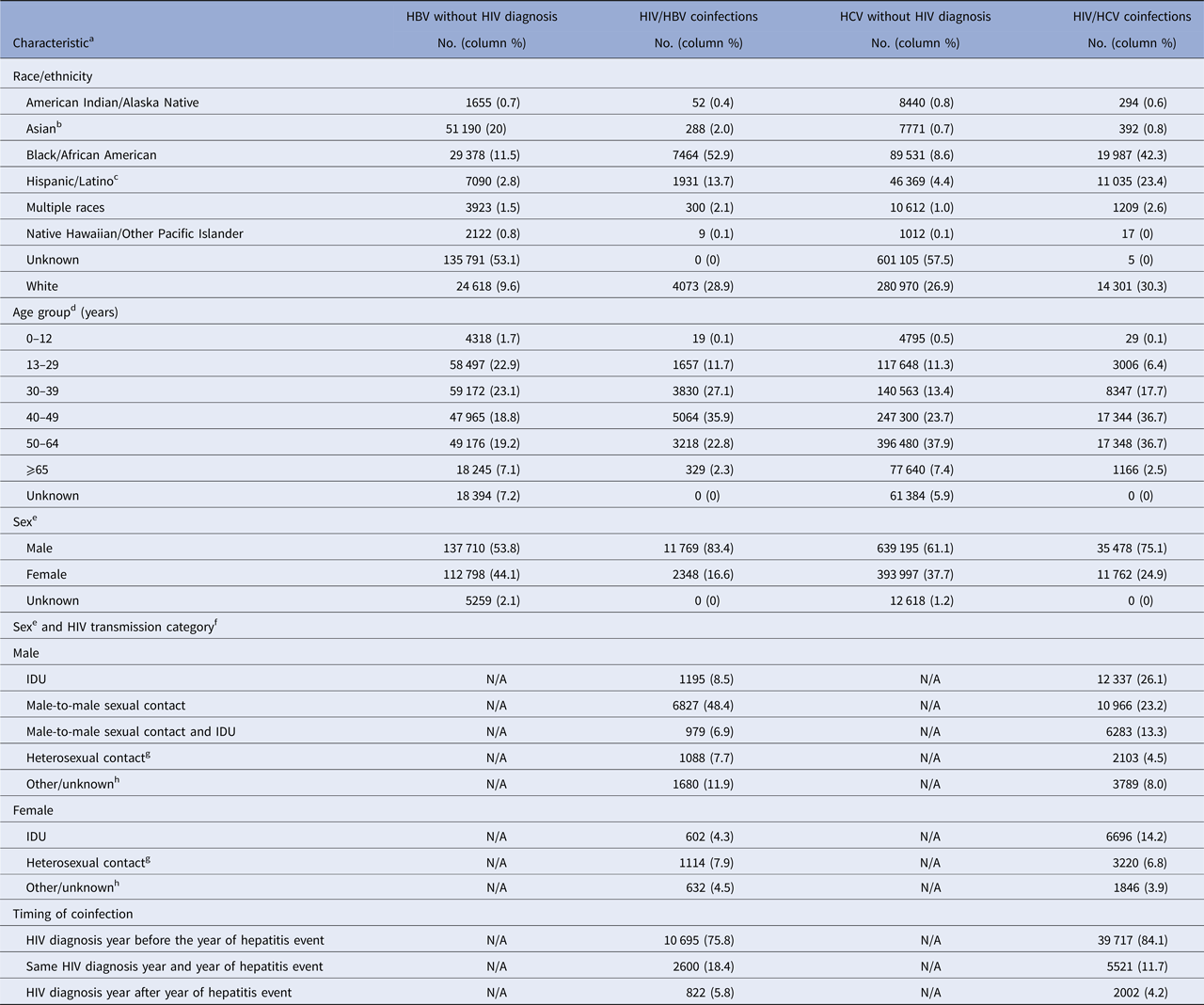
HBV, hepatitis B virus (acute or chronic); HCV, hepatitis C virus (acute or chronic); HIV, human immunodeficiency virus; N/A, not applicable; IDU, injection drug use.
a For coinfected cases, information comes first from the HIV surveillance system. If information was missing in the HIV surveillance system, information from the hepatitis surveillance system was used.
b Includes persons for whom the surveillance system did not differentiate between Asian and Native Hawaiian/Other Pacific Islander.
c Hispanics/Latinos can be of any race.
d For hepatitis cases without an HIV diagnosis, based on age at diagnosis of hepatitis. For coinfected cases, based on age at coinfection or second reported virus infection to the health department.
e From HIV surveillance system, sex indicates sex at birth. From hepatitis surveillance system, sex might indicate sex at birth, sex at the time of hepatitis event, or current sex at the time the data were extracted.
f Data have not been statistically adjusted to account for unknown transmission categories.
g Heterosexual contact with a person known to have, or to be at high risk for, HIV infection.
h Includes haemophilia, blood transfusion, or perinatal exposure and persons with an unknown transmission category.
Similar to HBV/HIV coinfections, the greatest proportion of persons coinfected with HCV and HIV were black/African American (42.3%) (Table 5). The proportion of HCV/HIV-coinfected persons aged ⩾50 years at the time of the second diagnosis was 39.2%. A greater proportion of HCV/HIV coinfected patients were male than those only infected with HCV (75.1% vs. 61.1%). Among HCV/HIV-coinfected persons, the largest proportion was among persons with HIV infection attributed, at least in part, to IDU (53.6%). Among the HCV/HIV coinfected population, HIV diagnosis year preceded HCV event year in 84.1% of cases.
Discussion
We report here on a multijurisdictional HIV and viral hepatitis coinfection match conducted by using routinely collected nationally notifiable disease surveillance data in the USA. The project summarised results from >500 000 persons living with diagnosed HIV infection, >250 000 persons reported with HBV, and >1 million persons reported with HCV from 15 states and two cities. Overall, among persons living with diagnosed HIV infection, we determined that the proportion coinfected with HBV was 2.0% and HCV was 6.7%. Among persons ever reported to be infected with HBV, 5.2% were ever reported to be infected with HIV, whereas among persons ever reported to be infected with HCV, 4.3% were ever reported to be infected with HIV. Differences in the number of coinfections between the two analytic methods are the result of differences in the inclusion of decedents and those with an out-of-jurisdiction residency between the two methods. These proportions represent reported coinfections among participating jurisdictions. Infected persons who were never tested for HIV or viral hepatitis or who were identified as infected but never reported to public health are not represented in these data. Because HIV and viral hepatitis might be undiagnosed, estimates of viral hepatitis coinfection among persons with HIV are often higher than reported here [Reference Thio10–13].
The demography of the cohort of coinfected persons in our study matched that of other US studies regarding race and sex [Reference Sanchez23–Reference Prussing27]. HIV transmission categories were correlated with the most common viral hepatitis transmission risks in the USA (sexual transmission for HBV and IDU for HCV) [Reference Edlin3, Reference Atkins and Nolan31, Reference Suryaprasad32]. Identified coinfections are not necessarily recent infections, but rather new diagnoses, at least some of which must be of historical acquisition. HIV diagnosis often preceded the viral hepatitis event date in our study. Because the timing of coinfection in our analysis is based on surveillance data, HIV diagnosis preceding the viral hepatitis event date does not necessarily reflect the order in which each infection was acquired, but rather the timing of the diagnoses. Recommendations for testing persons living with HIV infection for HBV and HCV might explain the substantial proportion with an HIV diagnosis year before the hepatitis event year [33]. A public health need exists for screening all persons at risk for viral hepatitis infection, in addition to those with diagnosed HIV.
Our results are subject to certain limitations. First, viral hepatitis and HIV are chronic and often asymptomatic infections, and event year might not be consistent with the year of exposure or infection. Because our results were ascertained from surveillance data, persons with undiagnosed infection or diagnosed infection not reported to public health are not included in our analysis. Underreporting of viral hepatitis cases has been documented and might vary by jurisdiction or over time [34, 35]. Participating jurisdictions included 15 states and two cities, and therefore, our results might not be representative of the entire USA. Data from the various jurisdictions were not homogenous, particularly with regard to viral hepatitis. Although HIV surveillance is fairly similar across jurisdictions, interjurisdictional viral hepatitis surveillance activities, de-duplication efforts and data quality differ, and these differences might have confounded estimates of proportions of coinfected persons. Moreover, each jurisdiction sets its own priorities for viral hepatitis surveillance on the basis of state or local funding, regulations and resources. National definitions for viral hepatitis case surveillance have evolved and implementation of these definitions has not necessarily been uniform across jurisdictions [29]. Jurisdictions were encouraged to include data that they believed were reasonably valid; therefore, conditions and timeframe for which data were included varied by location. National surveillance for viral hepatitis infections is founded on an incident disease surveillance paradigm. The majority of jurisdictions do not track viral hepatitis cases prospectively, and therefore, cumulative viral hepatitis cases might include persons who cleared infection spontaneously (HBV or HCV) or through treatment (HCV). Finally, minor inaccuracies might have occurred during the matching process, affecting the results.
Our findings highlight key public health opportunities. Racial disparities exist with regard to the populations affected by HIV and viral hepatitis. Blacks/African Americans comprise approximately 12% of the US population, but in our analysis represented >50% of persons coinfected with HIV/HBV and 42% of persons with HIV/HCV coinfection. Male-to-male sexual contact was the predominant risk factor for HIV and HBV coinfections, whereas IDU was more common among persons coinfected with HIV and HCV. Efforts to reduce coinfections (e.g., safe sex, preexposure prophylaxis and syringe service programmes) should target gay, bisexual and other men who have sex with men and persons who inject drugs, respectively. National guidelines recommend that, at an entry to care, all HIV-infected persons be tested for HBV, vaccinated for HBV if susceptible and screened for HCV infection with annual retesting of HCV-uninfected persons thereafter [33]. Automated electronic medical record orders can provide testing reminders in accordance with published guidelines and help remove barriers to patient screening, testing and vaccination. Health departments might consider potential benefits of co-locating and integrating HIV and viral hepatitis testing and prevention services, which can help patients navigate care for HIV or viral hepatitis infection or both.
Shared social factors that place persons at risk for acquiring HIV and viral hepatitis along with some shared transmission routes for these conditions make coinfections more likely. Assessing coinfection trends provides important information about clinical care needs (e.g., linkage to care and treatment) and for public health intervention (e.g., preexposure prophylaxis or syringe service programmes). Using surveillance data to assess coinfections is crucial for monitoring health status and measuring benchmarks to eliminate HIV and viral hepatitis infections [Reference Van Handel28, 34, 36]. Our analysis demonstrated that a standardised approach for assessing coinfections can be applied to surveillance data from different systems and jurisdictions. However, limitations of the surveillance systems might have affected the results of this analysis and resulted in an underestimation of coinfections. The ultimate goal of identification is early intervention to decrease morbidity and mortality associated with these conditions, improve clinical outcomes and limit viral transmission to susceptible persons [Reference Van Handel28, Reference Purcell, McCray and Mermin37].
Acknowledgements
Included in the authorship are one or two authors from each participating jurisdiction, although we recognise that more persons might have contributed to the success of this project in all of the jurisdictions. In particular, we acknowledge the contributions of the following persons: Arizona Department of Health Services: Shane Brady, Rick DeStephens, Susan Robinson; Houston (Texas, USA) Health Department: Raouf R. Arafat, Biru Yang; Iowa (USA) Department of Public Health: Joanne Mostrom, Shane Scharer, George Walton; Louisiana (USA) Department of Health: Jessica Fridge, Megan Jespersen; Maryland (USA) Department of Health: Mary Kleinman, Dale Rohn, Stephen Stanley, Carly Stokum; Massachusetts (USA) Department of Public Health: Kevin Cranston, Alfred DeMaria Jr., R. Monina Klevens, Lawrence C. Madoff; New York City Department of Health and Mental Hygiene (New York, USA): Angelica Bocour, Sarah L. Braunstein, Kevin Guerra; Philadelphia (USA) Department of Public Health: Kathleen A. Brady, Melissa Miller; San Francisco Department of Public Health (California, USA): Sharon Pipkin, Melissa Sanchez; South Carolina (USA) Department of Health and Environmental Control: Terri Stephens, Claire Youngblood; Tennessee (USA) Department of Health: Jennifer Black, Samantha A. Mathieson; Texas (USA) Department of State Health Services: Charles Cohlmia, Sarah Norkin; and Washington (USA) State Department of Health: Tom Jaenicke.
Funding
This work was supported as public health surveillance by the Centers for Disease Control and Prevention.
Declaration of interest
All health departments receive funding from CDC to support public health surveillance. However, the funding was not specific to this analysis. C.F. reported receiving payment for lectures at John Hopkins University outside of the submitted work and reported his institution received funding through other grants from CDC and the Health Resources and Services Administration. L.H.A. reported receiving payment for writing and reviewing the manuscript as part of job responsibilities as an employee of the Texas Department of State Health Services. L.H.A. serves as a board member for Neuropathy Alliance of Texas. L.H.A. reported her institution received funding from CDC and that Neuropathy Alliance of Texas receives funding from various sources, including St. David's Foundation and Athena Health. B.J. reported her institution received funding for surveillance activities not directly related to activities of this project. S.K. reported her institution received funding to support travel to meetings for other purposes. No other reported conflicts are relevant to the content of the manuscript.
Disclaimer
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.







