Introduction
A total of 25% of 10–11-year-old children in England are overweight or obese (Dinsdale and Ridler, Reference Dinsdale and Ridler2010). This has far-reaching physical and psychological consequences for the child (Rudolf, Reference Rudolf2004; Sabin et al., Reference Sabin, Ford, Holly, Hunt, Crowne and Shield2006), as well as financial implications for the National Health Service (NHS) and society in the future (Foresight, 2007).
While primary care has often been identified as a potential setting for managing simple childhood obesity (National Institute for Health and Clinical excellence (NICE), 2006; Scottish Intercollegiate Guidelines Network (SIGN), 2010), there is a lack of evidence-based guidelines to help general practitioners (GPs) assess the obese child.
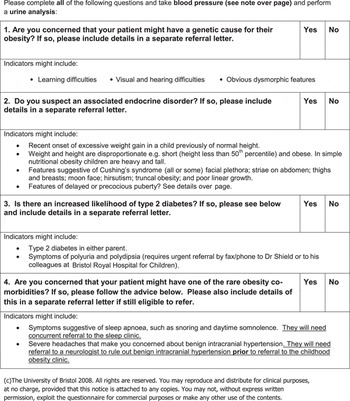
The ‘Bristol Obesity Online Screening Tool’ (BOOST – see Appendix 1) is an evidence-based tool that was developed in 2006 for use in a National Institute for Health Research (NIHR)-funded pilot trial ‘Evaluating the transferability of a successful, hospital-based, childhood obesity clinic to primary care: a pilot study (Hamilton-Shield, Reference Hamilton-Shield2006). It was designed for GPs to facilitate their assessment of obese children and to ensure that ‘red flags’ were not missed. These ‘red flags’ were to highlight either secondary causes of obesity or serious co-morbidities, necessitating specialist referral as opposed to recruitment into a practice nurse-led, primary care obesity clinic. It was completed in electronic format in GP surgeries and then downloaded for forwarding to the consultant paediatrician.
This paper reviews the evidence base to these guidelines and then assesses the appropriateness and safety of the 152 GP referrals made using BOOST during the care of childhood obesity (COCO) trial.
Evidence base and development of BOOST
BOOST provides a link to the ‘Health for all Children’ website (see Health for All Children, 2004), which gives clinicians a tool to calculate the child's body mass index (BMI) percentile, specific for age and sex. Childhood obesity, unlike in adults, cannot be based simply on a BMI score, as body composition varies between girls and boys and changes during childhood with respect to the proportion of fat and lean body mass (SIGN, 2010). The 1990 Body Mass Index Reference Curves for the UK (Cole et al., Reference Cole, Freeman and Preece1995) have therefore been traditionally used to define childhood obesity (Shield and Summerbell, Reference Shield and Summerbell2009; SIGN, 2010). Research studies tend to define obesity as above the 95th percentile as does the National Child Measurement Programme, whereas routine clinical situations recommend the 98th percentile, as used in our study (Rudolf, Reference Rudolf2004; NICE, 2006; SIGN, 2010). This is the level at which screening for co-morbidities or a secondary cause of obesity is recommended.
While the majority of childhood obesity can be ascribed to an imbalance between energy intake and expenditure (SIGN, 2010), the questions posed by BOOST aim to systematically review the child in order to exclude ‘red flags’.
Rare, but important, genetic causes of obesity exist (Shield and Summerbell, Reference Shield and Summerbell2009; Bochukova et al., Reference Bochukova, Huang, Keogh, Henning, Purmann, Blaszczyk, Saeed, Hamilton-Shield, Clayton-Smith, O'Rahilly, Hurles and Farooqi2010), including monogenic disorders such as melanocortin-4 receptor, leptin and its receptor gene mutations affecting the neuroendocrine regulation of satiety and eating. In significantly obese childhood cohorts, these mutations may account for up to 6% of cases (Farooqi and O'Rahilly, Reference Farooqi and O'Rahilly2006). In addition, there are multiple syndromic causes, the most familiar being the Prader–Willi syndrome (incidence estimated at 1:52 000; Whittington et al., Reference Whittington, Holland, Webb, Butler, Clarke and Boer2001). Recently, chromosomal copy number variants have been identified in association with profound obesity and autism (Bochukova et al., Reference Bochukova, Huang, Keogh, Henning, Purmann, Blaszczyk, Saeed, Hamilton-Shield, Clayton-Smith, O'Rahilly, Hurles and Farooqi2010). Dysmorphic features, learning difficulties or significant sensory or motor developmental delay should alert clinicians to the possibility of these underlying conditions, which are likely to be more frequently diagnosed as our understanding of childhood obesity improves.
BOOST screens for endocrine causes of obesity, including hypothyroidism, Cushing's syndrome and growth hormone deficiency. These children tend to present with a weight that is disproportionate to their height. Other features and examination findings that might highlight these conditions include striae, truncal obesity and a general change in appearance (Cushing's syndrome) and either delayed or precocious puberty, alerting a clinician to a number of possibilities including underlying hypothalamo-pituitary, adrenal, chromosomal or central nervous system abnormalities. Overweight and obese children-initial assessment in primary care. Map of Medicine http://eng.mapofmedicine.com/evidence/map/obesity_in_children1.html.
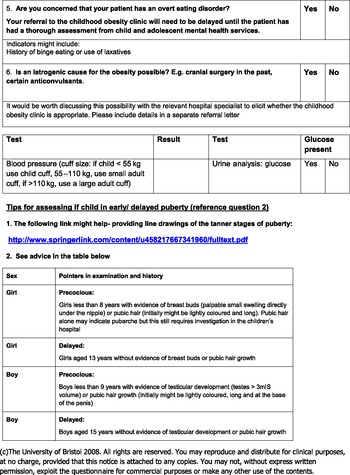
BOOST provides a web link to the Tanner definitions regarding pubertal development. While this specific link is no longer available, there are many alternative online resources available (eg, for boys http://bjsm.bmj.com/content/44/7/476/F5.large.jpg and for girls http://bjsm.bmj.com/content/44/7/476/F4.large.jpg) and BOOST provides a summary reference table regarding pubertal stages. Assessing puberty status can be a relatively subjective process, particularly in boys who lack the easily defined menarche stage. This is further complicated by the suggestion that increased adiposity might be linked to earlier onset of puberty (Lynn Ahmed et al., Reference Lynn Ahmed, Ong and Dunger2009). However, if a clinician had any suspicion of abnormal pubertal development, we recommended referral to secondary care.
As the incidence of childhood obesity rises, so does the incidence of type 2 diabetes, suggesting a causal relationship (Alberti et al., Reference Alberti, Zimmet, Shaw, Bloomgarden, Kaufman and Silink2004). BOOST screens for type 2 diabetes using both a urine dip as well as posing questions relating to possible symptoms and a family history. This multifactorial approach reflects findings from a Bristol study of 126 obese children, which identified that children with impaired glucose tolerance were more likely to have a parental history of type 2 diabetes (Sabin et al., Reference Sabin, Ford, Holly, Hunt, Crowne and Shield2006).
BOOST seeks to highlight obesity co-morbidities warranting a specialist referral. These include sleep apnoea and benign intracranial hypertension. Benign intracranial hypertension has an incidence up to 19/100 000/year in the high-risk group of obese women in the reproductive age range (Acheson, Reference Acheson2006), while identification in children and teenagers is less common. Interestingly, while in the adult population they tend to be female and obese, in children the relationship is less clear. One meta-analysis observes that in younger children the sex distribution is equal without any relationship to obesity. Adolescents showed the more typical pattern – with a propensity towards sufferers being overweight females (Genizi et al., Reference Genizi, Lahat, Zelnik, Mahajnah, Ravid and Shahar2007).
Adolescent children, similarly to adults, show an increased risk of having obstructive sleep apnoea syndrome with increasing weight (Kohler et al., Reference Kohler, Thormaehlen, Kennedy, Pamula, van den Heuvel, Lushington and Martin2009). Furthermore, at any level of sleep apnoea, obese children are more likely to experience excessive daytime sleepiness as compared with non-obese children (Gozal and Kheirandish-Gozal, Reference Gozal and Kheirandish-Gozal2009). This has obvious consequences for the physical, cognitive and mental well-being of the teenager.
BOOST also screens for overt eating disorders, which would need specialist review with the child and adolescent psychiatric team before an obesity clinic intervention. Similarly, obesity suspected to be secondary to iatrogenic causes such as anti-convulsants needs to be discussed with relevant specialists, to see whether adjustments to medication can be made. For example, unlike many of the other anti-convulsants used in childhood, topiramate has a weight-reducing effect in many patients (Sanker, Reference Sanker2004).
The only examinations required by BOOST are the urine dipstick analysis and a blood pressure measurement. Completed questionnaires were sent to a specialist paediatrician who used the age- and sex-specific blood pressure reference charts to determine whether there were concerns about hypertensive disease. It is known that obese children are likely to have higher levels of both systolic and diastolic blood pressure (Ribeiro et al., Reference Ribeiro, Guerra, Pinto, Oliveira, Duarte and Mota2003), but those with true hypertensive levels need to be referred to secondary care.
Assessment of referrals made using BOOST
Subjects and methods
Study recruitment was undertaken in Bristol between April 2008 and June 2009. A total of 152 referrals were made to the study from 50 general practices across the greater Bristol area using BOOST. The target children were those aged five to sixteen years with a BMI > 98th perpercentile. Before study recruitment initiation, three open meetings were held with GPs and other stakeholders to identify key requirements for primary care clinics and the referral tool to be used in the study.
During the study, all 152 referrals from general practice using BOOST were reviewed by a consultant paediatrician and the appropriateness of referral and likelihood of complicated obesity were assessed.
Results
Of the 152 referrals, two were rejected due to the children being outside the study age range. Thirty-two (21%) cases were ‘red flagged’ on scrutiny of the BOOST form. The most frequent reasons for red flagging were a history of parental type 2 diabetes (6%); potential endocrine disorders such as relative short stature for weight or concerns about pubertal development (6%); and children with a potential genetic disorder or associated learning difficulties (6%). The identification of potential co-morbidities (proteinuria/possible idiopathic intracranial hypertension and systemic hypertension) or overt eating disorders was less frequent causes for flagging (see Table 1).
Table 1 Causes for red flagging by BOOST

BOOST = Bristol Obesity Online Screening Tool.
Of those children identified as having a parent with type 2 diabetes, none had glycosuria reported from testing in general practice, although in two cases no details were provided on the form. None of these children had silent type 2 diabetes, as confirmed by oral glucose tolerance tests (OGTTs) conducted at the hospital.
In those in whom endocrine disorders were queried, no overt pathology requiring treatment was identified, although one female had adrenarche clinically and one boy had constitutional delay in growth and puberty that did not require a specific intervention.
Only 76% of children had their blood pressure recorded on the BOOST form, while 70% had a test for glycosuria or had a note that blood glucose was normal (2 cases). The GPs indicated on four forms that the family had declined these tests: in two cases for both blood pressure and urine testing and in one case each for blood pressure or urine. The majority of questionnaires provided no reason for absent data. In nearly half of the cases in which tests were missing, both urine analysis and blood pressure were omitted, while blood pressure was reported alone over twice as often as urine analysis.
Discussion
The Bristol Online Obesity Screening Tool was designed for two main purposes: to provide an accurate and user-friendly tool for GPs to rapidly assess a child's BMI percentile to diagnose obesity and allow referral into our study, and also to ensure that those children requiring secondary care assessment were easily identified and given hospital appointments. Primary care has been identified as a potential setting for managing childhood obesity (NICE, 2006; Reddy, Reference Reddy2006), but GPs feel they have neither the skills to assess obese children nor effective interventions to refer patients to (Turner et al., Reference Turner, Shield and Salisbury2009).
On the basis of our review of 152 referral forms, it would appear that the vast majority of children are suitable for interventions in primary care without recourse to secondary care assessment for underlying pathology. In reality, those cases ‘red-flagged’ for parental type 2 diabetes could be effectively screened by routine questioning and testing for glycosuria. Within this study and more importantly, within the childhood obesity clinic in Bristol, no case of silent diabetes has ever been identified from routine oral glucose tolerance tests (n > 300, paper in preparation). Adolescents with type 2 diabetes present with similar symptoms to children with type 1 or adults with type 2 diabetes: polyuria, polydypsia, weight loss and in some cases ketonuria (Haines et al., Reference Haines, Wan, Lynn, Barrett and Shield2007) and testing for glycosuria plus questions regarding possible symptoms should pick up those needing urgent referral. Therefore, while a screening tool should explicitly consider type 2 diabetes in obese children, especially in the context of parental diabetes, the need for secondary care referral and an OGTT is unlikely to be necessary with absent symptoms and a negative urine screen. While the tool seems to accurately direct appropriate children to the attention of secondary care, this study was not designed to examine whether other children might have benefited from a secondary care opinion, but were ‘missed’.
The tool was designed for use with children aged above five years. If used for younger children, it would be necessary to add a prompt about extreme weight gain in infancy or early years, alerting the clinician to the need for referral to exclude possible genetic causes of obesity (SIGN, 2010). We believe that our screening tool would enable >85% of obese children to receive their initial weight management safely in primary care. However, this figure takes into account that both blood pressure and a urine analysis are mandatory components of the primary care assessment, which was not the case in approximately 25% of the referrals in this study. GPs do not typically assess a child's blood pressure and so reference tables to interpret readings would need to be available (these are accessible online: www.rcpch.ac.uk/doc.aspx?id_Resource=1764). Furthermore, we suggest that a useful modification to the questionnaire would be to highlight the need for these tests to be performed before referral under a new heading in the form. In the current format, this mandatory component might simply go unnoticed by the referring doctor at the end of the consultation.
As the prevalence of childhood obesity continues to rise, with potentially serious physical, psychological and economic sequelae, it is vital to equip the GP with tools to assess obese children. We believe that BOOST is a useful adjunct to the sparse resources available in primary care, enabling GPs to rapidly calculate BMI, allowing comparison with national perpercentiles and ensures that those patients requiring secondary care assessment can be identified with ease and safety. Further evaluation is now needed in different centres to ensure the effectiveness, sensitivity and specificity of this new tool.
Acknowledgement
This paper presents independent research commissioned by the NIHR under its Research for Patient Benefit Programme (Reference no. PB-PG-0706-10090). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Appendix 1: Bristol Obesity Screening Tool