Primary infection with varicella-zoster virus (VZV) causes varicella (chickenpox), a highly contagious disease that can result in outbreaks in susceptible populations. Following primary infection, VZV remains latent in the body and may reactivate years or decades later to cause herpes zoster (HZ). VZV transmission poses an occupational risk and a nosocomial risk for healthcare personnel and susceptible patients, respectively [Reference Gustafson1–Reference Gustafson, Shehab and Brunell6]. Current guidance indicates that VZV is transmitted person-to-person, primarily by inhalation of aerosols from or direct contact with vesicular fluid of acute varicella or HZ skin lesions. Transmission could also potentially occur if infected respiratory tract secretions were aerosolised [Reference Marin7]. The incubation period for varicella is 10–21 days. Prodromal symptoms may be present, particularly in older children and adults; fever, malaise, anorexia, headache and occasionally mild abdominal pain may occur 1–2 days before the rash appears. Patients with varicella are generally thought to be infectious for 1–2 days before rash onset until all lesions are crusted, typically 4–7 days after onset of rash [Reference Marin7].
Airborne transmission of VZV has been suggested by some epidemiologic evidence indicating varicella in susceptible persons who had no direct contact with a varicella or HZ index patient and no other potential exposures to the virus [Reference Gustafson1, Reference Leclair4, Reference Josephson and Gombert8, Reference Lopez9]. The mechanism by which VZV is spread from respiratory secretions and the relative importance of the respiratory route compared to spread from skin lesions, however, have been difficult to assess. VZV has long been a challenging virus to study in patients due to difficulty in propagating clinical samples in cell culture. Isolation of VZV from skin lesions was first accomplished by Weller and colleagues in 1956 [Reference Cheatham10]. Although difficult, it is possible to isolate VZV from patients with varicella, especially if skin vesicles are present. Interestingly, however, isolation of VZV from the respiratory tract is rare, even in patients with obvious varicella [Reference Gold11].
Implementation of effective infection control measures for varicella depends on understanding routes and timing of VZV transmission. We reviewed the medical literature to assess evidence for transmission of VZV before rash onset.
Methods
We searched Medline, Embase, Cochrane Library and CINAHL databases for articles published in any language, from database inception through 31 October 2019, using the search terms (‘chickenpox’ or ‘varicella’ or ‘herpes zoster’) and (‘transmission’ or ‘spread’ or ‘epidemic’ or ‘isolation’) and (‘respiratory’ or ‘airborne’ or ‘nasal/pharyngeal/throat swab’ or ‘before rash’). The complete search strategy is described in Supplementary Table. Two authors independently reviewed each title and abstract to determine if the article likely included information on transmission of VZV before rash onset. For articles that passed this initial screen, we reviewed the full text; the summary of the full text review was reviewed by a second reviewer. We also reviewed references that described transmission of VZV identified in the reference sections of papers retrieved by the database search. Only articles that included original reports of assessment of VZV transmission before rash onset were retained.
We describe the evidence by whether it was provided using epidemiologic or laboratory data.
Results
We screened 693 articles and identified 59 for full-text review; of these, 16 met inclusion criteria for our review (Fig. 1). Included articles originated from the United States (7), Japan (5), United Kingdom (2), Australia (1) and Czechoslovakia (1). Seven articles addressed VZV transmission before varicella rash onset using epidemiologic data [Reference Brunell12–Reference Schamberg and Kolmer18] and 10 with laboratory data [Reference Gold11, Reference Brunell12, Reference Asano19–Reference Nelson and Geme26]; one epidemiologic study had a laboratory component [Reference Brunell12].
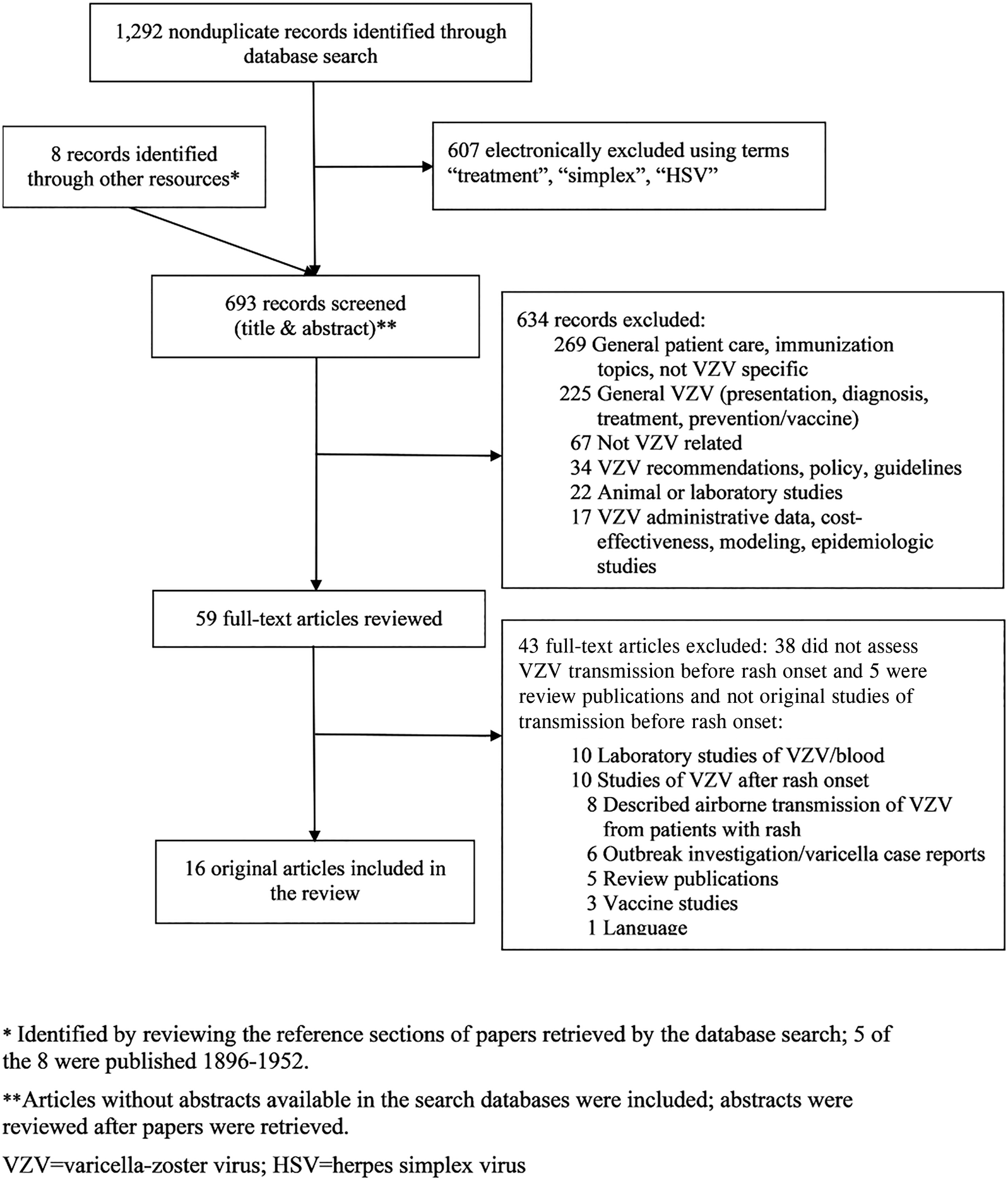
Fig. 1. Study selection.
Epidemiologic evidence
Of the seven articles that addressed VZV transmission before rash onset with epidemiologic evidence, four were published during 1896–1940 and three during 1989–2003. Three contained data from reports of varicella case series during outbreaks in hospitals, three included institutional outbreak investigations (two in schools and one in a jail) and one was a case report (Table 1). Each report indicated transmission from an index patient who exposed contacts before being diagnosed with varicella (i.e. before rash onset); no reports described transmission from a patient with HZ before rash onset.
Table 1. Epidemiologic evidence of varicella-zoster virus (VZV) transmission before rash onset in patients with varicella, by timing of transmission in relation to rash onset
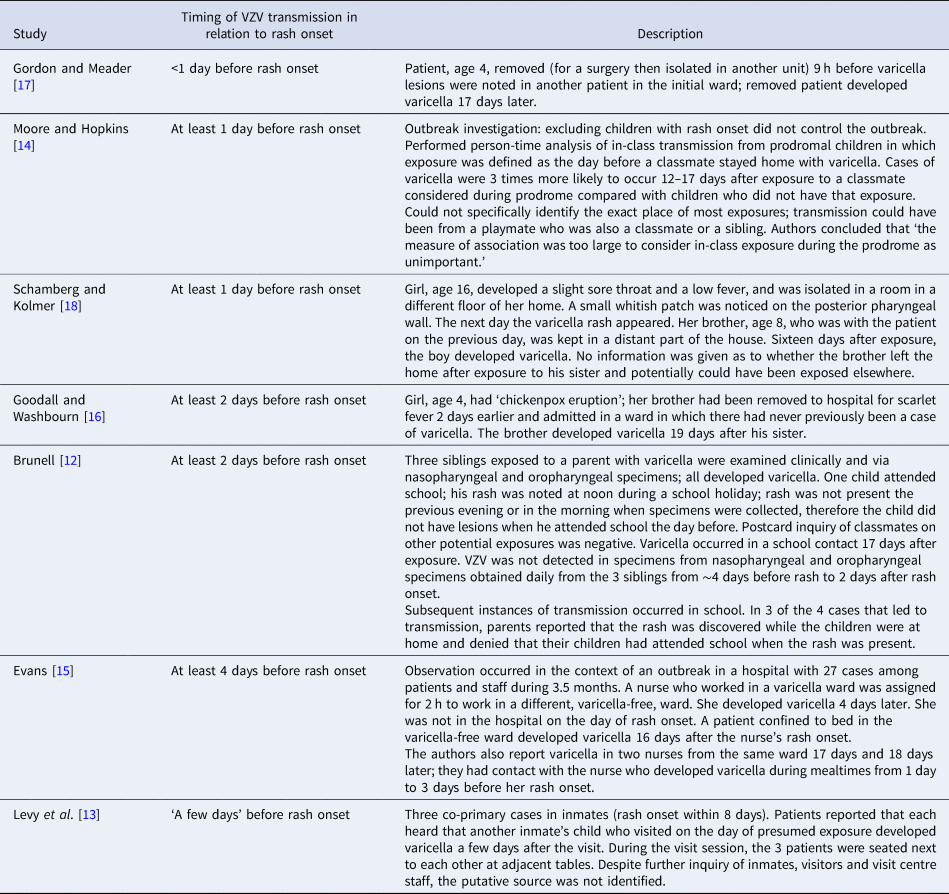
The authors reported that patients with varicella were infectious at various intervals before the rash appeared: <1 day (9 h) before (one report) [Reference Gordon and Meader17], at least 1 day before (two reports) [Reference Moore and Hopkins14, Reference Schamberg and Kolmer18], at least 2 days before (two reports) [Reference Brunell12, Reference Goodall and Washbourn16], at least 4 days before (one report) [Reference Evans15]; one report described potential infectiousness ‘a few days’ before rash onset, but no source of exposure could be identified [Reference Levy13] (Table 1). Exposure of contacts before rash onset in the index patient was determined based on reports of index patients and contacts being in the same setting a number of days or hours before rash onset in the index patient and not while the index patient had rash: two reports described transmission among siblings, two in hospitals (ward contacts), two in schools (in one report parents denied that the child with varicella attended school when the rash was present and in a second, a person-time analysis, exposure was defined as the day before a classmate stayed home with varicella) and one in jail (putative source not identified). In one report, a patient with varicella who was considered to have transmitted the virus to a schoolmate was monitored daily clinically and with laboratory studies [Reference Brunell12]. VZV was not detected in specimens of pharyngeal or nasal secretions obtained daily from ~4 days before to 2 days after rash onset (the same experience for his two younger siblings). However, VZV was detected (based on the characteristic cytopathic effect in cell culture) in vesicular fluid collected on days 2 and 3 of the rash in two of the three siblings [Reference Brunell12].
Laboratory evidence
Of the 10 articles that addressed VZV transmission before rash onset with laboratory evidence, five were published during 1966–1989; they reported results of virologic techniques (culture) to identify virus in the oropharynx [Reference Gold11, Reference Brunell12, Reference Ozaki24–Reference Nelson and Geme26]. Five articles were published during 1991–1999 and examined evidence of VZV DNA presence in the oropharynx using polymerase chain reaction (PCR) [Reference Asano19–Reference Ozaki23]. Four studies included exposed siblings, three included exposed patients, one study included exposed daycare contacts and one included children and young adult participants in the clinical trial for acyclovir (specimens collected <24 h after appearance of skin lesions (most were maculopapular)) (Table 2).
Table 2. Laboratory evidence for VZV presence in the oropharynx before and after rash onset in patients with varicella
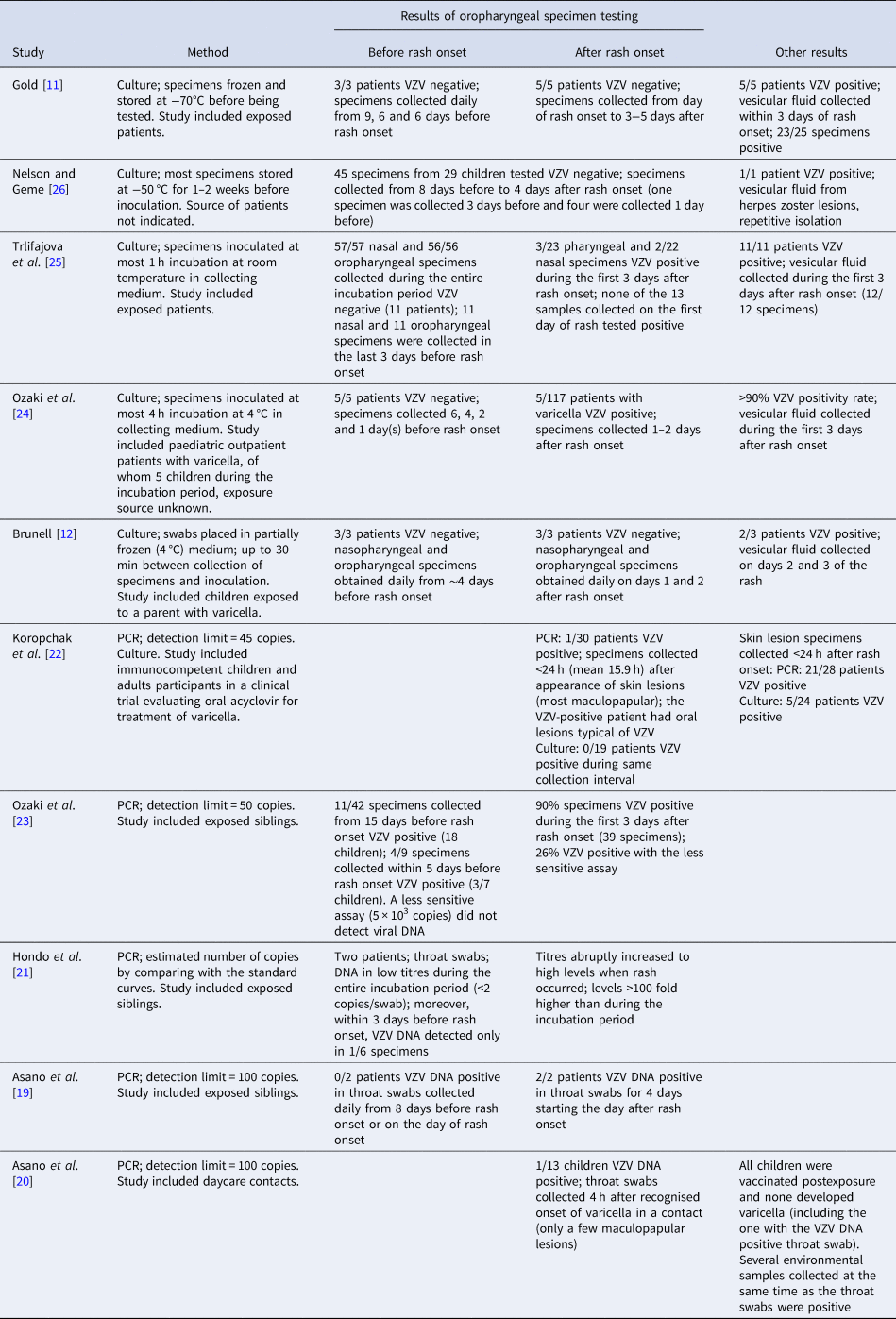
None of the studies that used culture to identify VZV in oropharyngeal specimens reported detection of the virus during the varicella incubation period. While these studies included a limited number of children who were tested before developing the characteristic varicella rash (between 23 and 27), the number of oropharyngeal specimens collected during the entire incubation period was ~160; ~47 were collected during the 3 days before rash onset (Table 2). These include the child mentioned above [Reference Brunell12] considered the source for a schoolmate who was VZV negative in culture of specimens of pharyngeal or nasal secretions collected before rash onset. Low levels of positivity (8–11%) [Reference Ozaki24, Reference Trlifajova, Bryndova and Ryc25] were reported in oropharyngeal samples collected after rash onset, with some children having lesions in the mouth [Reference Ozaki24]. Conversely, using the same methods, the studies reported positivity >90–100% in skin lesion specimens collected within the first 3 days after rash onset. Additionally, one study reported no positive cultures among 19 patients when oral specimens were collected <24 h (mean 15.9 h) after the appearance of skin lesions (most maculopapular) [Reference Koropchak22].
Positivity of oral specimens collected before rash onset was reported when VZV PCR was used for testing, although in rare instances (Table 2). On a limited number of patients or samples tested, Ozaki reported that three of seven patients and four (44%) of nine specimens collected within 5 days before rash onset were VZV DNA positive; two VZV-positive specimens were collected 5 days before rash onset and two were collected 2 days before [Reference Ozaki23]. One specimen collected 1 day before rash onset and another one collected 2 days before rash onset were negative. A less sensitive assay (5 × 103 copies) did not detect viral DNA in this study. Conversely, 90% of throat specimens (n = 39) collected during the first 3 days after rash onset were PCR positive (100% of patients, n = 18). Asano reported VZV DNA in throat specimens of one child of 13 tested (susceptible), with the specimens collected 4 h after recognising onset of varicella in a contact who had only a few maculopapular lesions [Reference Asano20]. This result was considered indirect evidence of excretion of VZV from the index case, assumed to be from saliva. Lastly, in oropharyngeal specimens collected <24 h after appearance of the rash (most skin lesions were maculopapular), Koropchak reported VZV DNA present in one patient of 30 tested [Reference Koropchak22]. The VZV-positive patient had oral lesions typical of VZV mucosal infection. This compares with 75% PCR positivity rate in skin lesion specimens collected during the same period. Two other studies reported negative results in four patients with daily collection of oropharyngeal specimens within 5 days before rash onset [Reference Asano19, Reference Hondo, Ito and Inouye21].
Discussion
This review confirms the scarcity of evidence in the medical literature on transmission of VZV before varicella rash onset and substantiates limitations on reaching definitive conclusions about VZV transmission before appearance of skin lesions.
Few reports contributed epidemiologic evidence; no formal studies have been published, and most information was anecdotal and reported decades ago. The epidemiologic investigations reported that patients with varicella were infectious at various intervals before rash onset, ranging from <1 day to 4 days before, with only 1–2 patients identified for each interval. One study that included more cases concluded that transmission during the incubation period occurred based on person-time analysis of in-class transmission, where exposure was defined as the day before a classmate stayed home with varicella [Reference Moore and Hopkins14]. The epidemiologic reports are based on information from healthcare personnel and parental observations about when/if rash was present. Some rashes may not have been identified soon enough to rule out transmission from skin lesions. Additionally, observations occurred in the context of the wide circulation of the virus; exposures could have occurred from other sources. Studies that observed no evidence of pre-onset transmission have been reported, but we did not systematically review them for this report. Several outbreak investigations in healthcare facilities reported potential exposure prior to rash onset in a patient with varicella but no transmission among exposed staff and patients [Reference Haiduven-Griffiths and Fecko5, Reference Adler27]. A report that indicated infectiousness <1 day before rash onset also indicated that transmission did not occur if exposure was ⩾1 day before rash onset (18 susceptible children exposed 1–2 days before rash onset in contacts) [Reference Gordon and Meader17].
Laboratory evidence supporting transmission of VZV before rash onset is also very limited. No culture-positive results were reported. PCR was a major advance for investigating disease caused by VZV, proving much easier, cheaper and more sensitive than culture of VZV. VZV DNA was found in the skin, saliva and respiratory tract of patients with varicella and HZ when rash was present [Reference Leung28, Reference Mehta29]. VZV DNA was identified by PCR during the incubation period in only one study [Reference Ozaki23]. While the proportion of detection appears high (44%) in that study, it was based on four of nine specimens collected from seven patients within 5 days before rash onset (VZV DNA identified in three patients). Two other studies reported a low PCR positivity rate when specimens were collected within 24 h of rash onset: two of 43 patients tested positive, one of whom had oral varicella lesions [Reference Asano20, Reference Koropchak22]. Methods for specimen collection and storage varied among studies and may have been suboptimal (collection using cotton swabs, freezing of specimens). It is critical to recognise, however, that presence of VZV DNA does not necessarily indicate infectivity or transmissible virus. For example, VZV DNA was found to persist in the environment of varicella and HZ patients’ rooms up to a month post rash onset [Reference Lopez9, Reference Yoshikawa30].
Varicella is a highly contagious viral disease. Many viral infections are spread by the respiratory route and virus can be readily identified in respiratory secretions of infected patients. Nevertheless, in patients with varicella, isolation of VZV in the respiratory tract has rarely been reported. Coughing and sneezing, associated with respiratory transmission of infectious diseases, are not characteristic of varicella. On the other hand, the pruritic vesicles characteristic of varicella contain high levels of well-formed, highly infectious virions [Reference Chen31] and itching of skin lesions is common during varicella. The virus was easily detected (75–100% for both culture and PCR) in skin lesion specimens collected within 3 days after rash onset. One study reported DNA values >100-fold higher in oropharyngeal samples when rash occurred compared to during the incubation period (titres during the incubation period were estimated at <2 copies/swab) [Reference Hondo, Ito and Inouye21]. These observations may indicate that failure to demonstrate VZV in respiratory secretions in most patients could result from lack of replication of the virus in the oropharynx at levels that enable identification and spread. However, low copy number during the incubation period does not preclude transmissibility.
Based on the available medical literature, VZV transmission seems unlikely prior to rash onset; however, it is impossible to prove it never happens. We did not find conclusive evidence to alter existing infection control guidelines. Providers should continue to implement appropriate infection control measures since the possibility of pre-rash, respiratory transmission of VZV cannot be entirely ruled out and sufficiently early identification of the rash, when only a few lesions are present, might be missed. Considering the scarcity and age of the existing evidence, additional evidence, using current laboratory assays would be beneficial. These studies can be undertaken in regions where varicella infections are still high; varicella rates are now low in the United States due to widespread use of the varicella vaccine [Reference Lopez, Zhang and Marin32] and situations of exposure occur less commonly today.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268821001102
Acknowledgements
We would like to thank Centers for Disease Control and Prevention colleagues Joanna Taliano, MA, MLS for performing the literature search and Mary Ann Kirkconnell Hall, MPH, for editorial review of the manuscript.
Author contributions
M.M, J.L. and A.A.G. designed the study. M.M., J.L., A.S.L. and L.S. reviewed the articles. A.A.G. and D.S.S. substantially contributed to interpretation of data. M.M. prepared the initial draft. All authors contributed to manuscript revisions.
Financial support
This work was supported by CDC.
Conflict of interest
Dr Gershon receives NIH funding (R01DK03094) and has a contractual relationship with Merck through the Varicella Zoster Virus Identification Program. All other authors: no reported conflicts.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, US Department of Health and Human Services.
Data availability statement
All data presented in this review are from previously published papers and available from the cited references.






