Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Sansom, Lloyd
2013.
HTA AND VALUE - A COMMENTARY.
International Journal of Technology Assessment in Health Care,
Vol. 29,
Issue. 4,
p.
375.
Rawlins, Michael D.
2013.
HTA AND VALUE - A COMMENTARY.
International Journal of Technology Assessment in Health Care,
Vol. 29,
Issue. 4,
p.
374.
Husereau, Don
Henshall, Chris
and
Jivraj, Jamil
2014.
ADAPTIVE APPROACHES TO LICENSING, HEALTH TECHNOLOGY ASSESSMENT, AND INTRODUCTION OF DRUGS AND DEVICES.
International Journal of Technology Assessment in Health Care,
Vol. 30,
Issue. 3,
p.
241.
Price, Christopher P.
and
St John, Andrew
2014.
Anatomy of a value proposition for laboratory medicine.
Clinica Chimica Acta,
Vol. 436,
Issue. ,
p.
104.
Longson, Carole
2014.
HTAI POLICY FORUM: KEEPING HTA ON TRACK.
International Journal of Technology Assessment in Health Care,
Vol. 30,
Issue. 3,
p.
251.
Sampietro-Colom, Laura
Brugada-Terradellas, Josep
and
González-Juanatey, José R.
2015.
Introducción de innovaciones en el área de la patología cardiaca en España: InnovaSEC.
Revista Española de Cardiología,
Vol. 68,
Issue. 10,
p.
834.
Eichler, H‐G
Baird, LG
Barker, R
Bloechl‐Daum, B
Børlum‐Kristensen, F
Brown, J
Chua, R
Del Signore, S
Dugan, U
Ferguson, J
Garner, S
Goettsch, W
Haigh, J
Honig, P
Hoos, A
Huckle, P
Kondo, T
Le Cam, Y
Leufkens, H
Lim, R
Longson, C
Lumpkin, M
Maraganore, J
O'Rourke, B
Oye, K
Pezalla, E
Pignatti, F
Raine, J
Rasi, G
Salmonson, T
Samaha, D
Schneeweiss, S
Siviero, PD
Skinner, M
Teagarden, JR
Tominaga, T
Trusheim, MR
Tunis, S
Unger, TF
Vamvakas, S
and
Hirsch, G
2015.
From adaptive licensing to adaptive pathways: Delivering a flexible life‐span approach to bring new drugs to patients.
Clinical Pharmacology & Therapeutics,
Vol. 97,
Issue. 3,
p.
234.
Panteli, Dimitra
Eckhardt, Helene
Nolting, Alexandra
Busse, Reinhard
and
Kulig, Michael
2015.
From market access to patient access: overview of evidence-based approaches for the reimbursement and pricing of pharmaceuticals in 36 European countries.
Health Research Policy and Systems,
Vol. 13,
Issue. 1,
Agustí, Alvar
Antó, Josep Maria
Auffray, Charles
Barbé, Ferran
Barreiro, Esther
Dorca, Jordi
Escarrabill, Joan
Faner, Rosa
Furlong, Laura I.
Garcia-Aymerich, Judith
Gea, Joaquim
Lindmark, Bertil
Monsó, Eduard
Plaza, Vicente
Puhan, Milo A.
Roca, Josep
Ruiz-Manzano, Juan
Sampietro-Colom, Laura
Sanz, Ferran
Serrano, Luis
Sharpe, James
Sibila, Oriol
Silverman, Edwin K.
Sterk, Peter J.
and
Sznajder, Jacob I.
2015.
Personalized Respiratory Medicine: Exploring the Horizon, Addressing the Issues. Summary of a BRN-AJRCCM Workshop Held in Barcelona on June 12, 2014.
American Journal of Respiratory and Critical Care Medicine,
Vol. 191,
Issue. 4,
p.
391.
Sampietro-Colom, Laura
Brugada-Terradellas, Josep
and
González-Juanatey, José R.
2015.
Introduction of Innovations in Heart Disease in Spain: InnovaSEC.
Revista Española de Cardiología (English Edition),
Vol. 68,
Issue. 10,
p.
834.
Abrishami, Payam
Boer, Albert
and
Horstman, Klasien
2015.
How can we assess the value of complex medical innovations in practice?.
Expert Review of Pharmacoeconomics & Outcomes Research,
Vol. 15,
Issue. 3,
p.
369.
Ciani, Oriana
Armeni, Patrizio
Boscolo, Paola Roberta
Cavazza, Marianna
Jommi, Claudio
and
Tarricone, Rosanna
2016.
De innovatione: The concept of innovation for medical technologies and its implications for healthcare policy-making.
Health Policy and Technology,
Vol. 5,
Issue. 1,
p.
47.
Husereau, Don
Henshall, Chris
Sampietro-Colom, Laura
and
Thomas, Sarah
2016.
CHANGING HEALTH TECHNOLOGY ASSESSMENT PARADIGMS?.
International Journal of Technology Assessment in Health Care,
Vol. 32,
Issue. 4,
p.
191.
Migliore, Antonio
2016.
Technology assessment of innovative medical devices in Europe.
Expert Review of Medical Devices,
Vol. 13,
Issue. 3,
p.
217.
Antoñanzas, Fernando
Terkola, Robert
and
Postma, Maarten
2016.
The Value of Medicines: A Crucial but Vague Concept.
PharmacoEconomics,
Vol. 34,
Issue. 12,
p.
1227.
Gozzo, Lucia
Navarria, Andrea
Drago, Valentina
Longo, Laura
Mansueto, Silvana
Pignataro, Giacomo
Cicchetti, Americo
Salomone, Salvatore
and
Drago, Filippo
2016.
Linking the Price of Cancer Drug Treatments to Their Clinical Value.
Clinical Drug Investigation,
Vol. 36,
Issue. 7,
p.
579.
Wurcel, Victoria
Perche, Olivier
Lesteven, Daniel
Williams, Doris-Ann
Schäfer, Birgit
Hopley, Colin
Jungwirth, Rebecca
Postulka, Anne
Pasmans, Raf
Hermansson, Lisse-Lotte
Ott, Markus
and
Glorioso, Valeria
2016.
The Value of Companion Diagnostics: Overcoming Access Barriers to Transform Personalised Health Care into an Affordable Reality in Europe.
Public Health Genomics,
Vol. 19,
Issue. 3,
p.
137.
Maniadakis, N.
Kourlaba, G.
Shen, J.
and
Holtorf, A.
2017.
Comprehensive taxonomy and worldwide trends in pharmaceutical policies in relation to country income status.
BMC Health Services Research,
Vol. 17,
Issue. 1,
Liguori, Giorgio
Belfiore, Patrizia
D’Amora, Maurizio
Liguori, Renato
and
Plebani, Mario
2017.
The principles of Health Technology Assessment in laboratory medicine.
Clinical Chemistry and Laboratory Medicine (CCLM),
Vol. 55,
Issue. 1,
p.
32.
Wale, Janet
Scott, Anna Mae
Hofmann, Bjørn
Garner, Sarah
Low, Eric
and
Sansom, Lloyd
2017.
WHY PATIENTS SHOULD BE INVOLVED IN HEALTH TECHNOLOGY ASSESSMENT.
International Journal of Technology Assessment in Health Care,
Vol. 33,
Issue. 1,
p.
1.
The rapid development of new medicines, devices, procedures, and care pathways means that the range of treatment options continues to grow faster than the resources available to many patients and healthcare systems, particularly as the impacts of the global financial crisis are felt. Identifying treatment options that offer value and value for money is therefore becoming increasingly relevant (Reference Porter1–Reference Young, Olsen and McGinnis3).
Health technology assessment (HTA) is used to ensure that healthcare decisions take account of relevant evidence in a systematic way (Reference Banta4). There is debate about how HTA can best assess the various aspects of value and allow these to be factored into decision-making processes, with particular interest in whether HTA and decision makers are taking appropriate account of what matters to patients and to society. Issues include variations in methods and decisions across systems, and the relationship between innovation and the assessment of value.
The Health Technology Assessment International (HTAi) Policy Forum discussed these issues in Barcelona in February 2013. This study describes some of the key themes from that discussion, and proposes areas where work is needed to improve methods, alignment or agreement.
METHODS
HTAi Policy Forum
HTAi is the international professional society for producers and users of HTA (5). The HTAi Policy Forum provides an opportunity for leaders and senior management of for-profit and not-for-profit organizations with strategic interests in HTA to meet with invited experts for in-depth discussions about issues of emerging international interest (6). A detailed description of the Forum can be found elsewhere (Reference Henshall7).
The Policy Forum met on February 3–5, 2013 to discuss the topic of HTA and value. The meeting included presentations and discussions among Forum members and guests invited because of their standing as researchers, or as patients or members of the public with relevant expertise and experience.
Development and analysis of the Forum discussion
The topic of HTA and value was chosen by Forum members in March 2012. A half-day scoping meeting was held at the main HTAi Annual Scientific Meeting in Bilbao in June 2012, open to Forum members and all those attending the main HTAi meeting. A background paper (8) was then developed by the HTAi Secretariat and Policy Forum Chair based on a review of key references and discussion points identified before, and during, the scoping meeting and by the Policy Forum Committee and Policy Forum members subsequently. The background paper was circulated to attendees before the meeting, together with a copy of the recent paper on value in health care by Porter (Reference Porter1).
This report presents the authors’ view of the background material and discussions at the meeting. It has been informed by comments on drafts by those present, but it is not a consensus statement from those at the meeting or their organizations, and cannot be taken to represent the views of any of those individuals attending or of the organizations they work for. Supplementary Table 1, which can be viewed online at www.journals.cambridge.org/thc2013XXX, lists all attendees at the meeting.
RESULTS
Defining value
Definitions and Stakeholder Perspectives
Definitions vary both for value in general and for value in health; value is to an important extent in the eye of the beholder and dependent on context. The meeting therefore focused initially on understanding different stakeholders’ perspectives on value.
Patient Perspective
For patients, the most important determinant of value is improvement in the length and/or quality of their life. Survival, freedom from pain, and the ability to undertake activities of daily living are therefore fundamental, but patients may also value choice; convenience; reduced financial and other burdens for them, their caregivers, family, or society; and increased certainty about diagnosis or outcomes. Patients’ views on value tend to reflect the whole of their care pathway and the way they experience a technology in their healthcare system, not the value of the technology in isolation. Patients generally want to be considered and treated as individuals, and to have access to the treatments they need. Patients may acknowledge that resource constraints mean that system-level decision makers have to consider the wider public good as well as individual patients’ wishes, although they may be distrustful of high-level decisions to restrict access to treatments, especially if they do not believe their voice has been heard.
General Public/Societal Perspective
Whereas the “societal perspective” may be constructed theoretically in terms of maximizing the “public good,” the actual views of the public are complex and challenging to define, may change over time, and may be affected by media coverage of particular issues. The case is often made to decision makers that the public supports giving increased weight in the determination of value to benefits for some groups of patients over others, for example favoring groups such as the young or those with serious or life-threatening illnesses (the latter sometimes called the “rule of rescue”). The Forum heard, however, that members of the general public can take widely differing views and can think both in a manner that reflects the broad interests of society and in one that is more driven by their personal interests, and may switch between these according to circumstances. Even the considered views of a group of informed members of the general public convened specially for the purpose of discussing and advising on citizens’ views of healthcare priorities may vary markedly between members and over time.
Health System Perspective
Decisions about the availability of treatments within a resource-limited system are typically the responsibility of government ministers or top managers. They generally aim to allocate resources to care of proven value, but can find the range of evidence and the range of views on value and on appropriate methods for assessing it challenging. As a result, they frequently seek advice from expert committees or bodies. Such bodies tend to see the benefit of a treatment to patients in the “real world” of their own healthcare system as key to its value, and they may consult or work with clinical experts, patients and patient organizations. They may also seek to balance patients’ views with those of the wider public, and to balance the value gained for patients receiving a treatment against the value foregone through opportunity costs, typically the value lost from treatments denied to other patients. The Forum also heard how health system decision makers may need to take account of political or commercial considerations when decisions become the focus of public attention. The discussion emphasized that system-level views on value involve complex judgments.
Industry Perspectives
The Forum meeting heard clearly from industry members that research-based companies aim to deliver value for patients and believe profits will follow success there. Thus, industry aims to focus on the value proposition for patients, and sees technological advance (e.g., the development of new molecules or devices) as the means to achieve that, rather than the end in itself. At the same time, industry is increasingly understanding and addressing the need to demonstrate value to payers. From the industry perspective, patient health outcomes are the core of the value proposition, but “wider aspects” of value for patients, caregivers, and society are also important (and viewed by some in industry as not always recognized and valued by payers). Innovative technologies may (also) deliver value to the system (e.g., cost savings or improvements in safety or reliability) that merits their introduction. Innovative technologies play a key role in improving health care, but the innovation process is risky and expensive, so it is important that innovative technologies are properly valued and rewarded. The Forum heard examples of how the current value or potential future value of an innovative technology cannot always be clearly understood at introduction. Industry members stressed the need to consider the “promise” of a technology and its likely value once fully developed and established in use, as well as current demonstrated value. This and other aspects of the definition and value of innovation and innovative technologies are discussed in more detail later in this study. Industry members also pointed out that a technology with multiple indications may have different values for each indication.
Elements of Value
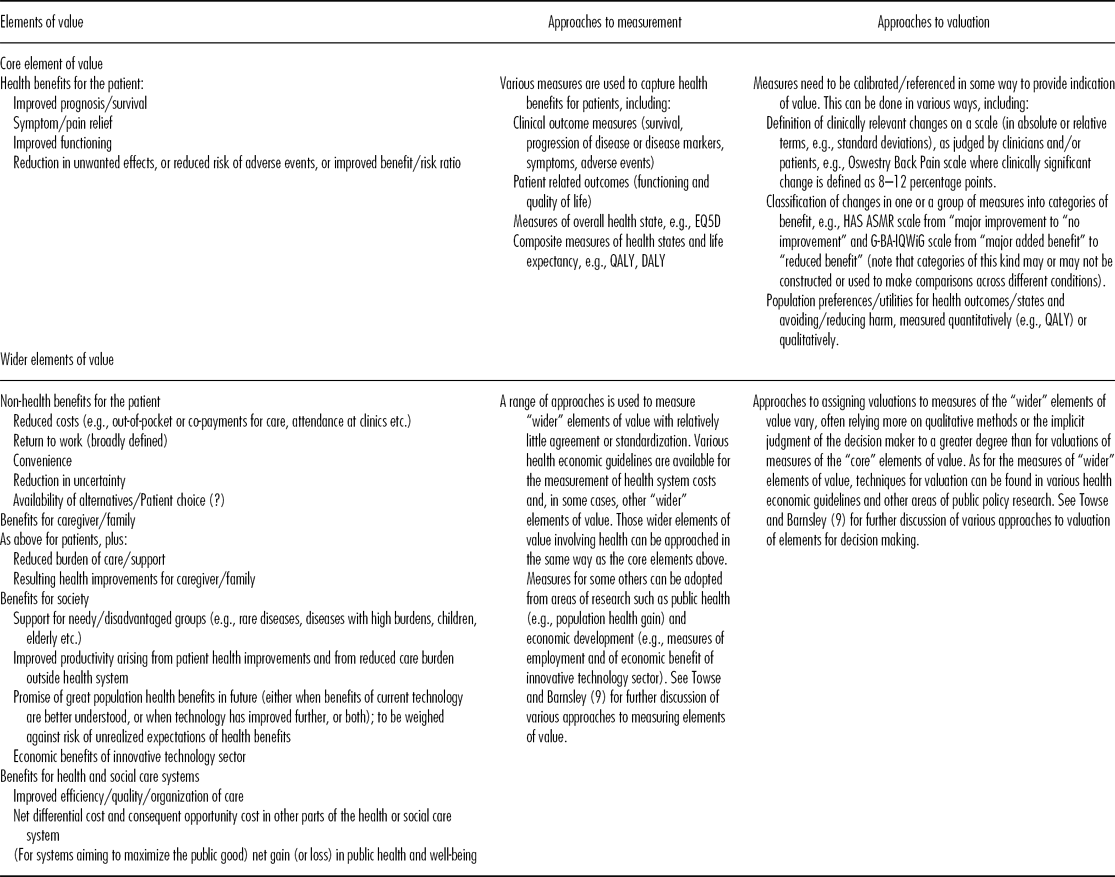
Building on this discussion of different perspectives on value, the Forum considered the various elements that comprise value. Stakeholders appear to agree that patient health is central; it was therefore considered helpful to distinguish between Core Elements of Value comprising those elements relating to patient health improvement, and Wider Elements of Value comprising elements relating to other benefits for the patient, and to benefits for caregivers, the health and social care systems, and society more widely. Column 1 of Table 1 summarizes the Core and Wider elements of value identified in the Forum discussion and relevant background materials. The precise elements relevant to the value of a particular technology will depend on the nature of the technology and the perspective of the decision maker.
Table 1. Summary of Core and Wider Elements of Value, and Approaches to Measurement and Valuation
Assessing Value
Assessing value depends upon determining the elements to be considered, the scales to measure each relevant element, and how values will be attached to those scales. Columns 2 and 3 of Table 1 summarize the main approaches to measurement and valuation for the various elements of value identified in column 1. Towse and Barnsley (Reference Towse and Barnsley9) present approaches to measuring value for decision making.
Factoring Assessments of Value into Decisions
Nature of Decisions
Most health systems are subject to cost pressures. Health system decision makers therefore have to decide either (in systems that leave manufacturers free to set prices) whether a technology at a given price offers clear and sufficient value for money to justify coverage and for which patient groups, or (in systems that set prices) what price is justified by the level of value offered by a technology to a defined group of patients. In practice, the distinction between these two types of decisions is less clear-cut as there is increasing negotiation on price between manufacturers and decision makers in both types of systems. Those developing new technologies have to decide whether the likely value of the technology for a particular indication and/or patient group and health system will lead to a price and market share that will provide an adequate return on the investment required to bring the technology to market.
In all these situations, the decision maker is concerned with the real world of the healthcare system, and is therefore interested in the incremental value and value for money associated with the use of technology compared with current practice (even if this current practice is to offer no treatment).
Approaches to Value-Based Decision Making
The Forum heard examples of real-life HTA-based decision-making systems in the United States, Canada, France, and Germany. These vary considerably in the measures and methods they use, although most key aspects of value are considered in all systems and all involve a mix of formulaic and deliberative processes and judgment.
Forum members believed it was important to distinguish between two kinds of judgments in decisions: scientific judgments needed to interpret uncertainties and differing trends in the scientific evidence; and value judgments about the relevant elements of value for a technology, the relevance of the evidence to these, and the weightings to be attached to them (Reference Culyer10).
Most systems currently expect decision makers to consider both those core and wider elements of value that are seen as relevant to understanding the benefits gained and those displaced by the adoption of a technology. Decision makers may need to factor a wide range of disparate elements into a decision, and this can be challenging. Most systems seem to use a single measure to try to capture patient health gain, for example quality-adjusted life-years (QALYs) or the categorical scales of the French and German systems (Reference Henschke, Sundmacher and Busse11;12). Some systems are exploring numerical adjustments to these basic measures to take account of some “wider elements of value,” such as severity of illness (13). But, there are limits to the extent to which this can be done and there is increasing interest in Multi Criteria Decision Analysis (MCDA) (Reference Dolan14) as a tool to help decision makers define the elements to be considered and the relative weights to be given to each. MCDA can be applied in different ways; factors relevant to a decision may be combined mathematically and the product of numerical value scales and weights then used to inform a decision, or factors may simply be set out to allow them to be considered in a transparent manner without using formulas or numerical weights. Most health systems take an approach that falls somewhere between these two, by using formulas to combine some elements, and judgment to compensate for known inadequacies in the formulas and to factor in other elements to arrive at the overall decision.
Three main points emerged from the Forum's discussion of approaches to value-based decision making. The first is that most systems seek to take account of broadly similar elements of value (in particular, reductions in mortality and morbidity and improvements in quality of life), although they assess and combine them in different ways. The second is that systems vary in the extent to which they bring all the relevant data together for a decision at a single point (e.g., in the United Kingdom or Australia where all the relevant information is put on the table at a single meeting at which a decision is made on coverage and/or price), as opposed to making a decision in steps (e.g., in France and Germany where a decision is made on the added value which then informs a decision on coverage and/or price). This may be related to the involvement of single or multiple bodies in the overall decision, with the approach and methods used perhaps reflecting this, at least in part. The third, and perhaps most important, point is the need for judgment in decisions on value, and the need for those designing decision-making processes to accept this and find ways to help decision makers make better judgments, rather than try to replace judgment with rigid mathematical approaches that cannot adequately model the complexity of the various factors and weights.
Value, Innovation, and HTA
Given its interest to many Forum members, a section of the meeting was dedicated to an in-depth discussion of the relationship between value, innovation, and HTA.
It was believed important when considering innovation to distinguish between innovative technologies and the innovation processes involved in developing the technologies pre- and post-adoption, and in adapting the health system to them. Although the main focus of this discussion was innovative technologies, various examples presented showed clearly that the value of technologies can often only be understood by considering the way in which the health system adapts to their introduction, and the way the technologies themselves evolve in response to system needs and further scientific developments. Although there was no clear agreement on how an innovative technology should be defined, there was a detailed discussion of the value of innovation.
Value of Innovation
Well-conducted assessments addressing relevant elements of value should capture the immediate health and wider benefits arising from an innovative technology. Discussion focused on what (if any) value beyond this innovative technologies may bring patients and healthcare systems.
There was agreement that there is often uncertainty at launch about the benefit that a new technology may offer for patients and the system in the longer term. This led some to suggest that assessments should explicitly consider the “promise” of an innovation as well as the current value demonstrated. Promise may take the form of (a) benefits that could reasonably be expected from the current version of the technology but which cannot yet be demonstrated convincingly; (b) benefits that could reasonably be expected from further refinements in the use of a technology, or from developments in the technology itself; (c) benefits arising from new, previously unpredicted indications for the technology; and (d) benefits realized through accompanying structural or policy changes in the health system. Examples of (b) were provided by several medical devices, for example, LVADs, for which rapid improvements in pump and battery technologies appear to be leading to important improvements in clinical and cost effectiveness. It was argued that a similar case can be made for drugs where, for example, combination therapies developed for HIV/AIDS and some cancers have led to better outcomes (and cost-effectiveness) than could have been achieved by individual agents alone.
Time did not allow a detailed discussion at the meeting of the implications of considering the future value or “promise” of technologies. Health systems with limited resources would need to identify those technologies for which there are good grounds to expect significant future value. The challenge for HTA is to develop scientifically sound methods for estimating future benefits and risks. Managed entry and coverage with evidence development approaches (Reference Klemp, Fronsdal and Facey15–Reference Redberg and Walsh18) already attempt to address uncertainties in current value. It was noted in discussion that technology assessment as originally conceived (Reference Banta4) focused more on the technology and its likely developmental trajectory and impacts than is commonly found in HTA as currently practiced.
Accurate predictions about the future value of technology have proved extremely challenging. Without clear methodologies for identifying technologies with significant “promise,” health systems are likely to remain reluctant to allocate scarce resources to technologies solely on this basis. Some also questioned why the healthcare system “customer” should pay for “promise” when in other technology sectors the manufacturer, capital markets, or both take the risk. Manufacturers and health systems could explore the development of a joint investment approach to adopting and developing promising technologies in the healthcare system (some proposing the term “pro-imbursement” for this).
HTA, Reimbursement/Procurement, and Innovation
The meeting discussed ways in which HTA, reimbursement, and procurement can affect innovation. Industry delegates emphasized that technology developers work on long timescales, with the consequent need for clear and consistent signals about how value will be assessed and rewarded.
There was extensive discussion of innovation in medical devices and procedures. Patent exclusivity and some aspects of the regulatory and HTA systems work less effectively to reward and promote innovation in medical devices than for medicines (Reference Drummond, Griffin and Tarricone19). For example, new medicines in the same class as a licensed product generally have to provide similar evidence as first in class whereas, for devices, some HTA and payer bodies allow follow-on products to demonstrate benefits and value by reference to evidence collected for the first in class. This can significantly reduce the development costs of follow-on products and, in some cases, may lead to a rapid downward price spiral shortly after the introduction of a new device. While this may promote value for patients and the system, it can dis-incentivize disruptive innovation by reducing the lead manufacturer's opportunity to recoup major development costs. Another challenge of innovation in medical devices is the rapid and ongoing technological development and improvement of devices and equipment after initial launch. This makes the issues discussed in the previous section on technology “promise” particularly relevant to devices and equipment.
While this discussion focused mainly on medical devices, similar concerns can arise in relation to drugs. In particular, concerns were noted about incentives for innovations addressing unmet medical needs when the main current treatment, and therefore comparator, is a low cost generic. In this situation (and particularly in systems that base decisions on assessments of incremental cost-utility), a new treatment offering worthwhile improvements in outcomes may not be seen to justify a price that would allow a manufacturer to make an adequate return on investment.
The Forum considered how innovation of value can be incentivized and supported. Governments support technology innovation in many ways, most importantly through publicly funded research and development and legal protection for intellectual property.
A development that could have a major positive impact on innovations of value in health care is improved alignment of the evidence expectations of different HTA and coverage bodies and, in so far as it is possible, the alignment of HTA/coverage bodies’ expectations with the requirements of regulatory bodies. Various initiatives, such as EUnetHTA (20) and the Green Park Collaborative (Reference Eichler, Oye and Baird21) are attempting to align expectations and requirements around the core of value: the elements concerned with health outcomes for patients. These developments were seen as important, but some wondered if there are ways to accelerate, focus, and improve coordination of work in this area.
The discussion also covered what might be done to improve alignment of evidence requirements for the “wider elements” of value. Values and culture vary across countries and health systems, so that systems will take different views on which “wider elements” of value are important and how they should be weighted in decisions. It was believed there should nevertheless be scope to improve alignment of definitions, evidence requirements and methods for these wider aspects, as well as for the core elements.
Thinking more radically, many believed that it was important now to move from conceiving and practicing HTA and reimbursement as one-off events (snapshots), to seeing them as ongoing processes aiming to provide greater certainty and increasing clarity on appropriate use (and price) as real-world evidence is collected and analyzed. “Progressive health system decision making” of this kind could align well with thinking in the regulatory community on “progressive” or “adaptive” licensing (Reference Eichler, Oye and Baird21), and build on existing approaches to managed entry or access with evidence development to create a system that better reflects the technology and evidence lifecycle. There are many practical problems to be addressed in putting such systems in place on the ground. These include agreeing responsibilities for funding products and information collection, developing fit-for-purpose low cost systems to collect key information routinely, managing the demands on HTA capacity and efficient deployment of that capacity, and agreed approaches to handling price adjustments when value is shown to be higher or lower than expected, and to managing unsuccessful products out of the system (disinvestment) (Reference Henshall, Schuller and Mardhani-Bayne22).
Possible actions
What could be done to address the challenges identified and to improve alignment in definitions and assessments of value, and improve assessment and decision making around innovative technologies? The following five actions were identified. Time did not allow discussion of who should be responsible for each, but there is need for the active involvement of HTA and payer bodies, regulators, industry, patients, and other relevant stakeholders:
1. Promote the development of a general framework for the definition and assessment of value by HTA/coverage bodies and regulators, recognizing that different HTA/coverage bodies work in different systems with different values. Key components would include: (a) Work to improve convergence on definitions, measures, and methods to assess health benefits for patients; (b) Start discussion of definitions and measures of wider elements of value; and (c) Building on and supporting the work of European Network for Health Technology Assessment (EUnetHTA) and the European Medicines Agency (EMA) in these areas, and the Green Park Collaborative (GPC).
2. Promote development of disease-specific guidance for industry from regulators and HTA/payer bodies on measures to capture value for specific conditions, building on GPC Pilot Alzheimer's Guidance (23) and EUnetHTA proposed work in Joint Action 2 (24). Although the general framework proposed above would help to frame and focus discussion, much of the challenge will be in agreeing the detail of measures for specific conditions.
3. Further develop joint scientific advice for industry from HTA/payer bodies and regulators. This needs to take place at appropriate steps in the product development pathway (including before phase 2/3 trials). There is also a need for feedback in both directions from discussions so each party can learn about the challenges for the others and consider how it might develop its current requirements and processes to reduce these. All parties have limited resources and the process must be run efficiently.
4. Explore development of a framework for progressive licensing, usage, and reimbursement, including: (a) Methods to assess the trajectory and expected future performance of a technology, and hence its future potential; (b) Practical approaches to challenges such as progressive pricing, withdrawal of technologies not meeting promise, etc.; (c) Ways to improve and use registries and routine health information collection systems; (d) Methods for analysis and use of observational data in decisions; and (e) Development of systems to promote appropriate use of a technology at all stages in the process. This work should build on the experience of current approaches to managed entry and coverage with evidence development.
5. Promote work to better adapt HTA and coverage and procurement approaches to medical devices.
CONCLUSIONS
For many of those present, the key outcome of the discussion was a clearer understanding of the issues related to value and what might be done to move forward.
There is much common ground between industry and HTA and coverage bodies, but there are also some important areas of disagreement that require further discussion and, where possible, resolution. Toward the end of the meeting, Forum members from HTA and coverage bodies were asked if they believe that they consider all relevant elements of value in their assessments, and coverage, and pricing decisions; most believed they did. Forum members from industry were asked if they believed that all relevant elements of value were considered by HTA and coverage bodies, and most indicated they did not. The actions suggested by the Forum should help to address this gap and hopefully ensure that valuable innovations are incentivized, made quickly available to patients, and appropriately rewarded.
SUPPLEMENTARY MATERIAL
Supplementary Table 1: www.journals.cambridge.org/thc2013XXX
CONTACT INFORMATION
Chris Henshall, PhD HTAi Policy Forum Chair and Honorary Fellow ([email protected]), Centre for Health Economics, University of York Tara Schuller, MSc Manager of Policy Programs, Health Technology Assessment International Secretariat
CONFLICTS OF INTEREST
Chris Henshall has received funding from HTAi for the work reported in this paper, and consultancy fees from several medical companies for chairing Advisory Board on specific technologies and advice on global HTA developments and strategy. Tara Schuller is employed by the Health Technology Assessment International Secretariat.