Meat and meat products, which concentrate and supply a large number of valuable nutrients (proteins, fats, vitamins and minerals), have traditionally been basic components of the human diet. However, epidemiological associations between consumption of meat and meat derivatives and some of the major degenerative diseases such as CHD, cancer, high blood pressure and obesity have influenced nutritional thinking and dietary guidelines over the last few years(Reference Jimenez-Colmenero, Carballo and Cofrades1). At present, the meat industry is introducing qualitative and/or quantitative modifications in meat and meat derivatives to create functional products(Reference Jimenez-Colmenero, Carballo and Cofrades1, Reference López-López, Bastida and Ruiz-Capillas2).
Functional foods can be obtained by combining products such as meat products with physiologically active substances (e.g. from plants). Marine algae, traditional components of the Asian diet whose consumption in the Western world has increased considerably over the last decade, are known to contain such substances(Reference Bocanegra, Bastida and Benedi3). Some species of Undaria and Porphyra contain high levels of fibre, several minerals and vitamins, and their lipid content is normally < 1·0 %. Furthermore, it has been reported that these algae contain several minor compounds with beneficial biological activities(Reference Bocanegra, Bastida and Benedi4).
Hypercholesterolaemia is associated with increased oxidative stress in animals(Reference Mahfouz and Kummerow5) and humans(Reference Napoli, Glass and Witztum6). Increased dietary intake of antioxidants may thus reduce the incidence and prevalence of CHD and other degenerative diseases(Reference Iwai7). Our group has tested the effects of diets containing supplements of Nori (N) and Konbu(Reference Bocanegra, Benedi and Sanchez-Muniz8) and those of N- and Wakame (W)-enriched meat products(Reference Schultz Moreira, González-Torres and Olivero-David9) on the antioxidant status of Wistar rats consuming high levels of cholesterol. It is well known that certain modifications of the lipoprotein profile increase the risk of developing atherosclerosis(Reference Barter and Rye10–Reference Wong, Sam and Cheung13). Moreover, increased lipoprotein oxidation constitutes one of the major emerging risk factors for atherosclerosis(Reference Ross14). Arylesterase (AE), one of the enzymatic activities of paraoxonase-1(Reference Canales and Sánchez-Muniz15), is known to play a protective role against peroxidation of LDL and other lipoproteins(Reference Canales and Sánchez-Muniz15–Reference Nus, Sánchez-Muniz and Sánchez-Montero18). However, the effect on the lipoprotein profile of diets containing a high percentage of meat and seaweeds, with or without supplementary cholesterol, has not been studied previously. The inclusion of alga in restructured meat (RM) could have a double-edged effect, as both meat and seaweeds are rich in Fe and high levels of this metal are known to increase oxidative stress(Reference Wright, Burden and Lee19). Due to the growing demand for alternative treatments for CHD, the present study aimed to investigate the effects of 5-week-long cholesterol-enriched and non-cholesterol-enriched diets that included restructured pork containing W or N on AE activity, lipaemia and lipoproteinaemia in growing Wistar rats.
In the present study, we hypothesise that certain seaweeds added to meat derivatives reduce the hypercholesterolaemic effect of cholesterol-enriched diets, partially normalising the lipoprotein profile. Moreover, alga-enriched meat increases AE activity, helping to maintain the antioxidant status of lipoproteins in rats fed diets that contain hypercholesterolaemic inductors.
Materials and methods
Restructured meat preparation
Meat raw materials (post-rigor pork and pork back fat), W (Undaria pinnatifida) and N (Porphyra umbilicalis) algae, and additives (NaCl, sodium tripolyphosphate and sodium nitrite) were used. Fresh marine seaweeds were collected on the Atlantic coast, dried in the shade and packed in polyethylene plastic bags for commercial distribution (Algamar C.B., Redondela, Pontevedra, Spain). These seaweeds were milled (Ultra Centrifugal Mill ZM 200; Retsch GmbH and Company, KG, Haan, Germany), passed through a 0·25 mm mesh sieve and stored in plastic flasks at 4 ± 2°C until used. Details of the RM preparation and composition have been published previously(Reference López-López, Bastida and Ruiz-Capillas2). In brief, the raw meat was homogenised and ground for 1 min in a chilled cutter (2°C; Stephan Universal Machine UM5; Stephan & Sóhne GmbH and Company, Stephanplatz, Hameln, Germany). All the fat and half of the seaweeds, NaCl (2·0 % for control samples and 0·5 % for samples with added seaweed), sodium tripolyphosphate and sodium nitrite were added to the ground meat and mixed together for 1 min; the rest of the ingredients were then added, and the mixture was homogenised for 1 min. The final mixture was homogenised under vacuum for 2 min. Each sample was prepared in duplicate. RM-N and RM-W contained less Na than RM-C (385·5, 626·7 and 873·8 mg/100 g RM, respectively; Table 1). As reported by López-López et al. (Reference López-López, Bastida and Ruiz-Capillas2), additional salt is required in the formulations without seaweed in order to overcome certain technological problems associated with low-salt products but was not necessary in the RM with algae(Reference López-López, Bastida and Ruiz-Capillas2).
Table 1 Some relevant components of control, Wakame and Nori restructured meats*
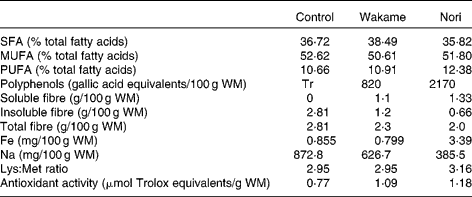
WM, wet matter.
* Data from López-López et al. (Reference López-López, Bastida and Ruiz-Capillas2)
Diet preparation and experimental design
A total of sixty male growing Wistar rats with a body weight of approximately 90 g at the outset were obtained from Harlan Laboratories Models (Harlan, SL, Barcelona, Spain). The animals were housed individually in metabolic cells in a temperature-controlled room (22·3 ± 1·8°C) with a 12 h light–12 h dark cycle. The present study was approved by the Spanish Science and Technology Advisory Committee (project AGL 2005-07 204-C02-01/ALI) and by an ethics committee of the Universidad Complutense of Madrid (Spain). All experiments were performed in compliance with Directive 86/609/EEC of 24 November 1986 for the protection of scientific research animals. The rats were fed commercial rat pellets (Panlab, Barcelona, Spain) during a 1-week period of adaptation to environmental conditions and then distributed into six groups of ten animals each, according to average body weight. The following six experimental semi-synthetic diets (Table 2) were prepared: (1) the control diet (C) without added cholesterol was composed of a homogeneous mixture of 85 % rodent diet (AIN-93M purified rodent diet; Dyets, Inc., Bethlehem, PA, USA) and 15 % freeze-dried restructured pork (with 4 % wet matter microcrystalline cellulose); (2) the W diet consisted of a mixture of AIN-93M no. 180729 feed (85 %) and freeze-dried, restructured W meat (15 %); (3) the N diet consisted of a mixture of AIN-93M no. 180729 feed (85 %) and freeze-dried, restructured N meat (15 %); (4) the cholesterol-enriched control (CC) diet was identical to the C diet but with 2·43 % cholesterol (95–98 % purity) and 0·49 % cholic acid (>98 % purity), substituting an equal amount of starch (AIN-93M no. 180730 diet); (5) the cholesterol-enriched W (CW) diet was the W diet enriched with cholesterol and cholic acid; (6) the cholesterol-enriched N (CN) diet consisted of the N diet enriched with cholesterol and cholic acid. All experimental diets contained approximately 20·7 % protein, 8·7 % fat and 4·4 % total dietary fibre. Water and food were provided ad libitum over the 5-week experimental period.
Table 2 Composition (g/kg) and energy content† of the control, Wakame- and Nori-enriched meat diets with and without supplementary cholesterol*
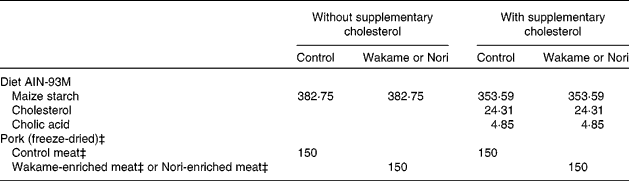
* Other ingredients: casein, 127·5 g/kg; soyabean oil, 34 g/kg; dyetrose (carbohydrate composition), 131·75 g/kg (monosaccharides, 10 g/kg; disaccharides, 40 g/kg; trisaccharides, 50 g/kg; tetrasaccharides and higher, 900 g/kg); sucrose, 85 g/kg; microcrystalline cellulose, 42·50 g/kg; salt mix§, 29·75 g/kg; vitamin mix∥, 12·16 g/kg; choline bitartrate, 3·06 g/kg; l-cystine, 1·53 g/kg; t-butylhydroquinone, 0·0068 g/kg.
† Diet energy content, considering as energy equivalent for monosaccharides; 15·69 kJ/g (3·75 kcal/g); polysaccharides, 16·73 kJ/g (4·0 kcal/g); fat, 37·65 kJ/g (9·0 kcal/g); protein 16·73 kJ/g (4·0 kcal/g); soluble fibre, 8·4 kJ/g (2 kcal/g); control diet without cholesterol, 16 587·7 kJ/kg (3964·6 kcal/kg); Wakame diet without added cholesterol, 16 790·9 kJ/kg (4013·1 kcal/kg); Nori diet without added cholesterol, 16 677·9 kJ/kg (3986·1 kcal/kg); control diet with added cholesterol, 16 296·6 kJ/kg (3895·0 kcal/kg); Wakame diet with added cholesterol, 16 106·0 kJ/kg (3849·4 kcal/kg); Nori diet with added cholesterol, 16 189·9 kJ/kg (3869·5 kcal/kg).
‡ Protein, fat, minerals (ash) and fibre present in the 150 g of control, Wakame and Nori freeze-dried, restructured meats; control meat: 79·15, 52·95, 17·47 and 10 g (microcrystalline cellulose), respectively; Nori meat: 79·03, 47·82, 9·56 and 10·07 g, respectively; Wakame meat: 73·75, 52·98, 17·14 and 11·95 g, respectively.
§ Mineral mix contained AIN-93M mineral mix: calcium carbonate, 357·00 g/kg; potassium phosphate monobasic, 250·00 g/kg; potassium citrate.H2O, 28·00 g/kg; NaCl, 74·00 g/kg; potassium sulphate, 46·60 g/kg; magnesium oxide, 24·00 g/kg; ferric citrate U.S.P. (United States Pharmacopeia), 6·06 g/kg; zinc carbonate, 1·65 g/kg; manganous carbonate, 0·63 g/kg; cupric carbonate, 0·30 g/kg; potassium iodate, 0·01 g/kg; sodium selenate, 0·01 025 g/kg; ammonium paramolybdate.4H2O, 0·00 795 g/kg; sodium metasilicate.9H2O, 1·45 g/kg; chromium potassium sulphate.12.H2O, 0·275 g/kg; lithium chloride, 0·0174 g/kg; boric acid, 0·0815 g/kg; sodium fluoride, 0·0635 g/kg; nickel carbonate, 0·0318 g/kg; ammonium vanadate, 0·0066 g/kg; finely powdered sucrose, 209·806 g/kg.
∥ AIN-93VX vitamin mixture: niacin, 3·00 g/kg; calcium pantothenate, 1·60 g/kg; pyridoxine.HCl, 0·70 g/kg; thiamine.HCl, 0·60 g/kg; riboflavin, 0·60 g/kg; folic acid, 0·20 g/kg; biotin, 0·02 g/kg; vitamin E acetate (500 IU/g), 15·00 g/kg; vitamin B12 (0·1 %), 2·50 g/kg; vitamin A palmitate (150 000 μg retinol/g), 0·80 g/kg; vitamin D3 (10 000 μg/g), 0·25 g/kg; vitamin K1–dextrose mix (10 mg/g), 7·50 g/kg; sucrose, 967·23 g/kg.
At the end of the experiment, in order to avoid inter-assay variations that could affect the comparison of data from the different groups, animals in fasting conditions were anaesthetised and euthanised by extracting blood from the descending aorta with a syringe, taking one animal at a time, of each one of six groups.
Growth rate
The feed conversion ratio was individually tested relating food consumption (g) to body-weight gain (g).
Lipoprotein isolation
Blood from the descending aorta was collected into heparinised tubes. Plasma was separated from the whole blood within 30 min of collection by centrifugation at 2500 rpm (1500 g) for 20 min and kept at 4°C until lipoprotein isolation. A SW 50.1 rotor was used to separate the various classes of lipoproteins in 1 ml plasma samples. KBr (114 × 10− 3 g), sucrose (25 × 10− 3 g) and serum (1 ml) were added to the cellulose nitrate tube. The components were carefully mixed (final background density of d = 1·10 g/ml) and sequentially overlaid with 2·4 ml of a salt solution of d = 1·06 g/ml (11·42 × 10− 3 g NaCl and 75·98 × 10− 3 g KBr/ml) and 2·4 ml distilled water. After preparation, the gradients were spun for 7 h at 50 000 rpm (232 000 g) and 4°C, as indicated by Terpstra et al. (Reference Terpstra, Woodward and Sanchez-Muniz20).
Isolation of the lipoprotein fractions was performed taking into account the conventional boundaries for rats of the different lipoprotein classes(Reference Bocanegra, Bastida and Benedi4) (VLDL, ρ20 < 1·0063 g/ml; intermediate-density lipoprotein (IDL)+LDL, ρ20 = 1·0063–1·057 g/ml; HDL, ρ20>1·057 g/ml).
Determination of cholesterol, phospholipids and TAG in the lipoprotein fractions
Cholesterol, TAG and phospholipids were determined using standard enzymatic colorimetric tests (SpINREACT S.A., Sant Esteve de Bas, Girona, Spain). All intra-assay and inter-assay CV were < 5·5 %. Total lipids were calculated as the sum of cholesterol, TAG and phospholipids.
Arylesterase activity measurement
Rat plasma AE activity was measured according to Nus et al. (Reference Nus, Sánchez-Muniz and Sánchez-Montero16, Reference Nus, Sánchez-Muniz and Sinisterra Gago17). One unit of arylesterase was defined as the mmol phenol formed from phenyl acetate per min. Reaction rates were monitored at 270 nm in thermostated quartz cuvettes with a 10 mm light path, using a Shimadzu UV-2401 PC (Tokyo, Japan) spectrophotometer. Blanks without plasma samples were used to correct for the spontaneous hydrolysis of phenylacetate in the buffer. Each measurement was performed in duplicate.
Statistical analyses
Statistical analyses were performed using the SPSS version 15.0 statistical analysis package (SPSS, Inc., Chicago, IL, USA). The results are expressed as means and standard deviations or means with their standard errors. A two-way ANOVA (cholesterol and alga) was used. Pairwise comparisons of diet responses between groups were made employing the Bonferroni test. The effect of cholesterol consumption was evaluated using an unpaired Student's t test. The relationship between food intake and body-weight gain, and the decrease in plasma cholesterol and the decrease in AE were tested by Pearson's correlation test. Differences in growth rate induced by diets were assessed by the ANCOVA test. Differences were accepted as significant when P < 0·05.
Results
Restructured meat composition
Table 1 shows some main differences between the RM compounds. RM-N contains higher soluble fibre, polyphenols and Fe than RM-W and RM-C. Minor differences were found in SFA, MUFA and PUFA percentages and in the lysine:methionine ratio. Diets have similar energy contents (16 587·7–16 677·9 kJ/kg (3964·6–4013·1 kcal/kg) for non-added cholesterol diets and 16 106·0–16 296·6 kJ/kg (3849·4–3895·0 kcal/kg) for added cholesterol diets; Table 2).
Growth rate
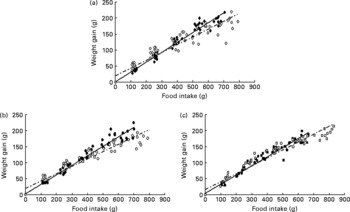
Fig. 1(a)–(c) shows the relationship between food intake and body-weight gain and the intercepts, slopes and significances found for the different groups. There were no significant differences (P>0·05) between the N and W diets, but the N diet induced a lower slope in body-weight evolution than the C diet (P < 0·05). Supplementary dietary cholesterol significantly affected all growth curves (CC v. C; CW v. W and CN v. N, all P < 0·001), but no significant differences (P>0·05) between the CN and CW diets were found.

Fig. 1 Growth rates in rats fed the control, Wakame- and Nori-enriched meat experimental diets with and without supplementary cholesterol. Y = (intercept with their standard error)+(slope with their standard error) × X, where Y is the body-weight gain and X is the food consumption. (a) ♦, control (C): Y = (1·042 ± 2·496)+(0·307 ± 0·006) × X; r 2 0·9762; ⋄, control with supplementary cholesterol (CC): Y = (19·44 ± 4·21)+(0·243 ± 0·010) × X; r 2 0·9039. (b) ●, Wakame (W): Y = (2·20 ± 2·68)+(0·296 ± 0·007) × X; r 2 0·9709; ○, Wakame with supplementary cholesterol (CW): Y = (19·10 ± 3·66)+(0·229 ± 0·009) × X; r 2 0·9245. (c) ■, Nori (N) Y = (4·86 ± 2·84)+(0·283 ± 0·007) × X; r 2 0·9667; □, Nori with supplementary cholesterol (CN): Y = (16·30 ± 3·62)+(0·246 ± 0·008) × X; r 2 0·9448. Mean values were significantly different (ANCOVA test) for C v. N (P <0·05). Mean growth rate values were significantly different (ANCOVA test) for CC v. C, CW v. W and CN v. N (P < 0·001). Mean growth rate values were not significantly different (ANCOVA test) for C v. W, N v. W, CC v. CW, CW v. CN and CC v. CN.
Plasma lipid concentrations and arylesterase activities
Plasma lipid data for the different groups are shown in Table 3. Significant cholesterol × type of diet interaction (P < 0·001) was observed for total cholesterol and AE activity and the AE:total cholesterol and AE:HDL-cholesterol ratios. There were no significant differences (P>0·05) between the N and W diets for total cholesterol, TAG, phospholipids, total lipids, cholesterol:phospholipids ratio, AE, AE:cholesterol ratio and AE:HDL-cholesterol ratio.
Table 3 Plasma cholesterol, TAG, phospholipids, total lipid, arylesterase (AE), and cholesterol:phospholipid, AE:total cholesterol and AE:HDL-cholesterol ratios in rats fed the control, Wakame- and Nori-enriched meat diets with and without the cholesterol supplement
(Mean values and standard deviations, n 10)

C, control; CC, cholesterol control; W, Wakame; CW, cholesterol-enriched Wakame; N, Nori; CN, cholesterol-enriched Nori.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05, Bonferroni's test).
Mean values within a column for the same parameter were significantly different from their no supplementary cholesterol counterparts: *P < 0·05, **P < 0·01 and ***P < 0·001.
† To transform mg/l to mmol/l of cholesterol, TAG and phospholipids, divide data by 387, 890 and 750, respectively.
‡ Cholesterol+TAG+phospholipids. One unit of AE was defind as the mmol phenol formed from phenyl acetate per min.
The hypercholesterolaemic dietary agent significantly increased plasma cholesterol levels in CC (P < 0·001), CW and CN animals (both P < 0·01). However, CN rats showed significantly (P < 0·001) lower cholesterol levels than CC rats. Plasma TAG levels were significantly affected by dietary cholesterol (P < 0·001) and type of diet (P < 0·05), while only dietary cholesterol significantly influenced phospholipids, total lipids and the cholesterol:phospholipid ratio (all P < 0·001). CN rats presented lower (P < 0·05) phospholipid concentrations than N rats. CC rats had higher total lipid levels (P < 0·01) than C animals. The cholesterol:phospholipid ratio was significantly higher in CC, CW and CN animals than in their C, W and N counterparts (P < 0·001, <0·01 and < 0·001, respectively). For the total cholesterol, TAG, phospholipids, total lipids and the cholesterol:phospholipid ratio, no significant differences (P>0·05) between CN and CW rats were found.
The hypercholesterolaemic dietary agent significantly increased plasma AE activity in CC (P < 0·01) and CW animals (P < 0·001) but not in CN animals (P>0·05). The AE:total cholesterol ratio increased in CC (P < 0·05) and CN animals (P < 0·01) v. C and N animals, respectively. The N and W groups presented higher AE activity, AE:total cholesterol ratio and AE:HDL-cholesterol ratio than the C group (at least P < 0·05; Table 3). CW rats showed a significantly higher (P < 0·05) AE:HDL-cholesterol ratio than the CN and CC groups. CN rats showed a lower (P < 0·05) AE, AE:total cholesterol ratio and AE:HDL-cholesterol ratio than CW rats.
Lipoprotein profile
The lipid content of the different lipoprotein fractions is shown in Table 4. A significant cholesterol × type of diet interaction was observed for VLDL-cholesterol and (IDL+LDL)-cholesterol (both P < 0·05). Supplementary dietary cholesterol affected the cholesterol, TAG and phospholipid content of all the lipoproteins (P < 0·001) except VLDL-phospholipids and HDL-cholesterol (both P>0·05). The type of diet affected VLDL-cholesterol and (IDL+LDL)-cholesterol (both P < 0·05) and HDL-TAG (P < 0·01). When results were studied according to the cholesterol supplement status of each group, N rats displayed lower VLDL-cholesterol levels than W rats (P < 0·05), while the CN group had lower VLDL-cholesterol and HDL-TAG concentrations than CC animals.
Table 4 Lipoprotein lipid concentration in rats fed the control, Wakame- and Nori-enriched meat diets with and without cholesterol supplement
(Mean values and standard deviations)

C, control; CC, cholesterol control; W, Wakame; CW, cholesterol-enriched Wakame; N, Nori; CN, cholesterol-enriched Nori; IDL, intermediate-density lipoprotein.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05, Bonferroni's test).
Mean values within a column for the same parameter were significantly different from their no supplementary cholesterol counterparts: *P < 0·05, **P < 0·01 and ***P < 0·001.
† To transform mg/l to mmol/l of cholesterol, TAG and phospholipids, divide data by 387, 890 and 750, respectively.
‡ Cholesterol+TAG+phospholipids.
CC animals presented significantly higher levels of VLDL-cholesterol (P < 0·01), (IDL+LDL)-cholesterol (P < 0·001) and (IDL+LDL)-phospholipids (P < 0·001), and lower concentrations of VLDL-TAG (P < 0·01), (IDL+LDL)-TAG (P < 0·05), HDL-TAG (P < 0·001) and HDL-phospholipids (P < 0·001) than their C counterparts. Compared with N rats, CN animals had higher (IDL+LDL)-cholesterol (P < 0·01) and (IDL+LDL)-phospholipid (P < 0·05) levels and lower HDL-cholesterol (P < 0·05), VLDL (P < 0·01), (IDL+LDL)-TAG (P < 0·05) and HDL-phospholipid (P < 0·05) values. VLDL-cholesterol (P < 0·05), (IDL+LDL)-cholesterol (P < 0·001) and (IDL+LDL)-phospholipid (P < 0·01) concentrations were higher in W rats than in CW rats, while VLDL-TAG (P < 0·001), (IDL+LDL)-TAG (P < 0·05), HDL-TAG (P < 0·05) and HDL-phospholipids (P < 0·001) were lower.
Percentage contribution of lipids to lipoprotein composition
Fig. 2(a)–(c) shows the percentage contribution of the different lipids (% contribution) to the total VLDL, IDL+LDL and HDL lipid mass. A significant cholesterol × type of diet interaction was observed for the percentage of cholesterol in VLDL (P < 0·05). Cholesterol supplementation influenced the cholesterol, TAG and phospholipid composition of VLDL (all P < 0·001).

Fig. 2 Percentage contribution (%) of the different lipids to the total lipid mass of (a) VLDL, (b) intermediate-density lipoprotein (IDL)+LDL and (c) HDL in rats fed the control (C), Wakame (W)- and Nori (N)-enriched meat experimental diets and the cholesterol-enriched control (CC), Wakame (CW) and Nori (CN) meat experimental diets. Mean values were significantly different for cholesterol × diet interaction for VLDL-cholesterol and LDL+IDL-TAG (P < 0·05). a,b Mean values within the same lipoprotein lipid for C, W and N or for CC, CW and CN with unlike letters were significantly different (P < 0·05, Bonferroni's test), represented by vertical bar areas. Mean values within the same lipoprotein for C v. CC, W v. CW and N v. CN were significantly different (represented by vertical bar areas) (*P < 0·05, **P < 0·01 and ***P < 0·001). ![]() , Cholesterol; □, TAG;
, Cholesterol; □, TAG; ![]() , phospholipids.
, phospholipids.
CC, CW and CN rats presented VLDL and IDL+LDL particles enriched in cholesterol but impoverished in TAG (both P < 0·001) with respect to VLDL and IDL+LDL of C, W and N animals, respectively (Fig. 2(a) and (b)). CN rats had VLDL with less cholesterol (P < 0·05) than VLDL of CC and CW rats, and more TAG (P < 0·05) than VLDL of CW rats (Fig. 2(a)). HDL particles of W rats had less cholesterol and more TAG (both P < 0·05) than HDL particles of N rats (Fig. 2(c)). HDL particles of CC, CW and CN rats were impoverished in phospholipids with respect to HDL particles of C, W and N animals, respectively (P < 0·01, < 0·05 and < 0·05, respectively) (Fig. 2(c)). HDL particles of CC animals were enriched in cholesterol (P < 0·01) with respect to HDL of the C group, while HDL particles of CN rats were enriched in TAG (P < 0·01) with respect to HDL particles of N rats (Fig. 2(c)).
Discussion
The present study shows for the first time that how the consumption of seaweed-enriched meats, with or without cholesterol supplementation, influences rat plasma AE activity and the lipoprotein profile.
In the present study, diets containing seaweed-enriched RM were generally well accepted by growing rats, as corroborated by similar intake data from other studies(Reference Bocanegra, Benedi and Sanchez-Muniz8, Reference Viejo, García-Linares and Bastida21, Reference Sánchez-Muniz, García-Linares and García-Arias22). Rats given the N diet consumed less feed than those of the control group, probably due to the high soluble fibre content of the N alga. In agreement with Beynen et al. (Reference Beynen, Boogaard and Van Laack23), supplementary dietary cholesterol decreased the growing rate. Mahfouz & Kummerow(Reference Mahfouz and Kummerow5) found lower body-weight gain in rabbits but not in rats fed cholesterol-enriched diets.
Lipidaemia and lipoproteinaemia and plasma arylesterase activity
Several animal models have been used to study cholesterol and atherogenesis, but no single model is considered perfect for extrapolating results to humans(Reference Jacques, Sugano and Beynen24). Rats are the animals most commonly used in cholesterol metabolism studies and one of the most often used to study the cholesterolaemic effect of proteins(Reference Jacques, Sugano and Beynen24–Reference Sánchez-Muniz, Viejo and Medina27). Bovine bile or colic acid has been extensively used in animal studies to increase dietary cholesterol absorption and thus the hypercholesterolaemic effect of this sterol(Reference Jacques, Sugano and Beynen24–Reference Terpstra, Lapre and De Vrie28). Dietary casein does not induce hypercholesterolaemia when the diet contains no cholesterol supplement(Reference Viejo, García-Linares and Bastida21). The present study demonstrated that when cholesterol was not added to the diet, the consumption of C, W and N maintained normal levels of cholesterol, TAG and phospholipids in rats(Reference Bocanegra, Bastida and Benedi4, Reference Sanchez-Muniz and Bastida29–Reference Vázquez and Sánchez-Muniz31). In addition, the cholesterol:phospholipid ratio, used as a marker of hypercholesterolaemia(Reference Sanchez-Muniz and Bastida29), remained low in C, W and N rats, suggesting normocholesterolaemia(Reference Sanchez-Muniz and Bastida29). Inclusion of seaweed-enriched RM in the diet increased AE activity, suggesting an improvement in antioxidant status. AE, which binds to HDL and other lipoproteins(Reference Fuhrman, Volkova and Aviram32), is involved in lipoprotein metabolism and inhibits lipoperoxidation in LDL and HDL(Reference Aviram33). AE activity increases in rats consuming pomegranate polyphenols(Reference Rock, Rosenblat and Miller-Lotan34) or seaweeds(Reference Bocanegra, Bastida and Benedi4). The presence of antioxidants and other phytochemicals in algae(Reference Bocanegra, Bastida and Benedi4) at least partially explains the greater absolute AE activity in N and W rats than that observed in C animals. The same effect or tendency was observed when data were adjusted for cholesterol and HDL-cholesterol.
The absolute lipid content and composition of IDL+LDL and HDL in the C group were comparable with those reported in other studies(Reference Wong, Sam and Cheung13, Reference Garrido-Polonio, García-Linares and García-Arias35). HDL-cholesterol accounts for 85 % or more of total cholesterol. Rats, moreover, display a very effective uptake of VLDL and a low transference of apoB from VLDL to LDL(Reference Sigurdsson, Nicoli and Lewis36), which explain the low levels of LDL found in the present study and in previous investigations(Reference Wong, Sam and Cheung13, Reference Sanchez-Muniz and Bastida29, Reference Garrido-Polonio, García-Linares and García-Arias35, Reference Sánchez-Muniz, García-Linares and García-Arias37).
In general terms, lipoprotein fraction composition was similar in C, W and N rats. Nonetheless, the VLDL lipid mass (cholesterol+TAG+phospholipids) was higher in rats on the W diet and lower in those fed the N diet, suggesting that the former diet increased the production of lipoproteins while the latter decreased it. According to their lipid composition, the HDL of N rats appears to be more anti-atherosclerotic lipoproteins than the HDL of W rats(Reference Sánchez-Muniz, García-Linares and García-Arias37). Differences in lipid and lipoprotein levels between groups without the cholesterol supplement must first be attributed to the different total fibre and soluble fibre contents of the diets and, second, to other compounds, such as minerals, which may also affect lipaemia(Reference Bocanegra, Bastida and Benedi3, Reference Bocanegra, Bastida and Benedi4, Reference Gueux, Mazur and Rayssiguier38). N contains more viscous-soluble fibre and polyphenols than W, partially explaining why lipaemia values in W rats were higher than those in their N counterparts. A previous publication of our group(Reference Bocanegra, Nieto and Blas39) has reported that algal remains were found in the caecum of rats fed Konbu seaweed, but not in animals fed N, suggesting clear differences in gastric emptying time and digestion speed between the two algal diets.
Consistent with the results of other studies(Reference Chiang, Chen and Huang25–Reference Sánchez-Muniz, Viejo and Medina27, Reference Krauss, Eckel and Howard40, Reference Fukushima, Ohhashi and Ohno41), the CC diet induced hypercholesterolaemia and decreased triacylglycerolaemia(Reference Bocanegra, Bastida and Benedi4, Reference Viejo, García-Linares and Bastida21, Reference Sánchez-Muniz, García-Linares and García-Arias37), producing very large amounts of β-VLDL and large quantities of LDL, and reducing the amount of total lipids transported by the HDL fraction. The CN diet partially blocked the hypercholesterolaemic induction observed in the CC and CW diets. As commented earlier, the higher soluble fibre and polyphenols of N than W(Reference López-López, Bastida and Ruiz-Capillas2) may be involved in the hypocholesterolaemic effect of the CN diet. These results were similar to those observed after adding N (7 %)(Reference Bocanegra, Bastida and Benedi4) or fried sardines(Reference Sánchez-Muniz, Higón and Cava26) to cholesterol-enriched diets.
The hypercholesterolaemic treatment increased AE activity in CC and CW rats, but unexpectedly, this was not observed in rats consuming the CN diet. Some algal compounds could be involved. RM-N contains three times more Fe than RM-W or RM-C; thus, a higher pro-oxidant effect in the CN group than in the other groups could be expected in the framework of cholesterol-enriched diets. Cholesterol is mainly eliminated from the body via bile acid production. Biosynthesis of cholic acid via cytochrome P450 increases the production of free radical substances. Thus, AE activity could be used to avoid lipoprotein peroxidative damage(Reference Reilly, Pratico and Delanty42, Reference Aviram, Rosenblat and Bisgaier43), acting as a suicide enzyme in CN animals(Reference Aviram, Rosenblat and Bisgaier43). In fact, a positive and significant correlation (P = 0·030) was found between the decrease in cholesterol and that in AE in the CW+CN groups with respect to the CC group (data not shown).
These results differ from those found by Bocanegra et al. (Reference Bocanegra, Bastida and Benedi4), as AE decreased in non-fasted rats fed the cholesterol-enriched control diet. However, similar data were obtained in rats fed the diet enriched in N and cholesterol. Thus, fasting and/or certain meat compounds, such as Fe, may explain differences between control diets of both studies. Nonetheless, the results on AE are difficult to be explained and deserve further studies to be confirmed.
TAG levels of N rats tended to be lower than those of C rats, while those of W animals were higher. Bocanegra et al. (Reference Bocanegra, Bastida and Benedi4) observed that postprandial TAG levels in control rats were similar to those given N for 3 weeks, while Murata et al. (Reference Murata, Sano and Ishihara44) reported that plasma and liver cholesterol levels decreased in rats given a diet containing a mixture of fish and W for 4 weeks.
Plasma cholesterol levels rose in all rats consuming cholesterol-enriched diets, but particularly in CC and CW rats due to an increase in cholesterol-enriched VLDL(Reference Bocanegra, Bastida and Benedi4, Reference Beynen, Boogaard and Van Laack23, Reference Chiang, Chen and Huang25, Reference Sánchez-Muniz, García-Linares and García-Arias37). VLDL-cholesterol concentrations of CN animals decreased, while those of CW rats resembled those of CC animals, suggesting that the N diet decreased β-VLDL levels, partially normalising the VLDL fraction of CN animals. Some researchers have reported that the water-soluble fractions of seaweeds or isolated algal polysaccharides showed hypocholesterolaemic and anti-hypertensive properties in experimental animals, a finding that would explain the hypocholesterolaemic effect of the CN diet in rats of the present study(Reference Wong, Sam and Cheung13, Reference Jimenez-Escrig and Sanchez-Muniz45).
The lipid content of the IDL+LDL fraction increased several-fold in animals given the cholesterol-enriched diets. However, this increase in CN rats was of lower magnitude than in CC or CW rats. Rats, moreover, display a very effective uptake of VLDL and a low transference of apoB from VLDL to LDL(Reference Sigurdsson, Nicoli and Lewis46). According to Havel(Reference Havel47), large VLDL contributes less to the formation of LDL than small VLDL in rats and other animals.
HDL-cholesterol levels were lower in CC, CW and CN animals than in their C, W and N counterparts. The hepatic scavenger receptor B-I plays an essential role in the hepatic uptake of plasma HDL-derived cholesterol and cholesterol esters for excretion into the bile(Reference Acton, Rigotti and Landschultz48–Reference Zhou, Li and Silver50). Scavenger receptor B-I deficiency results in a significant increase in plasma HDL-cholesterol and increased atherosclerotic lesions in a mouse(Reference Braun, Trigatti and Post51). Combined deficiencies of scavenger receptor B-I and apoE profoundly alter lipoprotein metabolism, plasma cholesterol in VLDL-sized and in abnormally large HDL-like particles(Reference Braun, Trigatti and Post51). This may explain the lower HDL-cholesterol in rats given the cholesterol-enriched diets than in those on the non-cholesterol-enriched diets.
In conclusion, diets including seaweed-enriched meat were well accepted and resulted in acceptable growth ratios and increased AE activity. The present results suggest the convenience of including only some specific seaweeds in meat derivatives to obtain functional meats, as N but not W partially blocked the hypercholesterolaemic effect of dietary cholesterol and partially normalised VLDL and LDL lipid levels and composition. Future studies are needed to understand the relationship between AE and cholesterol changes. The convenience of studying the effect of seaweed-enriched RM on lipid metabolism in different models, including human subjects, is emphasised.
Acknowledgements
The present study was supported by the Spanish projects AGL2005-07204-C02-01/ALI, AGL-2008 04892-C03-02 and Consolider-Ingenio 2010 project no. CSD2007-00016. We gratefully acknowledge the predoctoral fellowship of the Fundación Gran Mariscal de Ayacucho (FUNDAYACUCHO) from the Bolivarian Republic of Venezuela to R. O.-D., that of the Universidad Complutense, Madrid, Spain to A. S.-M. and the foreign fellowship for graduate studies granted by the Consejo Nacional de Ciencia y Tecnología (CONACYT) of México to L. G.-T. All authors have significantly contributed to the manuscript and agree with the present version of the paper. F. J. S.-M. contributed to the study design, data discussion and writing of the manuscript. R. O.-D., A. S.-M., L. G.-T. and S. B. contributed to the data acquisition and analysis and writing of the manuscript. J. B., M. J. G.-M., M. V.-V. and M. I. S.-R. contributed to the data discussion and made a critical review of the manuscript. The authors declare that there are no conflicts of interest.