Salmon is a good source of both long-chain (LC) n-3 PUFA and Se. The LC n-3 fatty acids, EPA and DHA, are well recognized for their protective effects against CVD(Reference Mozaffarian and Rimm1). Possible mechanisms include reductions in TAG, blood pressure, cardiac arrhythmias, platelet aggregation, the inflammatory response, growth of atherosclerotic plaque and improved endothelial function(Reference Kris-Etherton, Harris and Appel2). In addition, these fatty acids may play important roles in inflammatory disease(Reference Goldberg and Katz3) and in brain and retinal development(Reference Uauy and Dangour4). It may also be of benefit for cognitive decline during ageing(Reference Uauy and Dangour4), bone health(Reference Poulsen, Firth and Rogers5) and mood disorders(Reference Parker, Gibson and Brotchie6), but more research is still needed to elucidate the effects of LC n-3 fatty acids on these conditions. The ‘omega-3 index’, calculated as the percentage EPA and DHA of total erythrocyte fatty acids, has been introduced as a novel, physiologically relevant, modifiable and independent marker of risk for sudden cardiac death(Reference Harris and von Schacky7). Although very few data are available on the intakes of LC n-3 fatty acids by the New Zealand population, food disappearance data indicate that intakes are low, as in most other Western countries(Reference Hibbeln, Nieminen and Blasbalg8). Recommendations for intake of LC n-3 fatty acids for the prevention of CVD include the consumption of 500 mg LC n-3 fatty acids/d or one to two servings/week of fatty fish(Reference Gebauer, Psota and Harris9). This recommendation assumes equivalence between fatty fish intake and fish oil supplements with regard to bioavailability. However, the bioavailability of the fatty acids might be affected by the different matrices of fish and fish oil capsules but there are not many studies that have investigated the effects of fish v. fish oil on LC n-3 status. These studies had different study designs ranging in quality(Reference Arterburn, Oken and Hall10–Reference Visioli, Rise and Barassi13). Visioli et al. (Reference Visioli, Rise and Barassi13) and Elvevoll et al. (Reference Elvevoll, Barstad and Breimo11) demonstrated that n-3 fatty acids from fish were more effectively incorporated into plasma/serum lipids than when administered as capsules. However, two recent studies could not confirm this and concluded that n-3 fatty acids from fish or capsules were equally effective in enriching blood lipids with n-3 fatty acids(Reference Arterburn, Oken and Hall10, Reference Harris, Pottala and Sands12).
Consuming fish might be a more beneficial way of improving the LC n-3 status than consuming capsules because of its other nutritional properties; for example, it provides protein to the diet and it is also a good source of Se, a critical component of numerous selenoproteins in humans(Reference Mozaffarian14). Increased intakes of Se-rich foods are of particular importance to the New Zealand population, which has been shown to have a marginal Se status due to low concentrations of Se in the soil(Reference Thomson15). Marginal Se status has been associated with a number of chronic diseases such as CVD(Reference Flores-Mateo, Navas-Acien and Pastor-Barriuso16), some types of cancer (e.g. lung, prostate and colorectal and liver cancer)(Reference Rayman17), altered immune function, viral infections, miscarriage, reduced sperm motility, mood, hypothyroidism, inflammatory conditions, asthma and others(Reference Rayman18).
The objective of the present study was to compare the effects of consuming two servings/week of farmed New Zealand King Salmon with daily intakes of salmon oil capsules for 8 weeks in healthy men and women on erythrocyte levels of LC n-3 fatty acids, the ‘omega-3 index’ and Se status.
Experimental methods
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Massey University Human Ethics Committee (Southern A, Reference no. 07/72). Written informed consent was obtained from all subjects. The study was conducted between April and June 2008.
Subjects and study design
A total of forty-four healthy volunteers aged between 21 and 40 years, from the Auckland region in New Zealand, participated in the study. It was calculated that a sample size of ten per group would provide 80 % power to detect a significant difference (α 0·05, two-tailed) between groups of one unit in omega-3 index and in total erythrocyte n-3 fatty acid levels. Subjects (eleven per group) were recruited to allow for a 10 % dropout in each group. Subjects were eligible to participate if they had a low habitual intake of fatty fish (less than two servings/month), had not taken fish oil or Se supplements over the past 6 months, did not smoke, did not have allergies to seafood and did not have any known condition or disease or taking medication for any condition or disease.
A randomised parallel study design was used. Subjects were matched for age and sex and then randomly assigned to one of four groups receiving one of the following treatments for a duration of 8 weeks: 2 × 120 g servings/week of farmed New Zealand King salmon fillets (Oncorhynchus tshawytscha) (The New Zealand King Salmon Company Limited, Nelson, New Zealand); 2, 4 or 6 capsules of salmon oil/d (Healtheries of New Zealand Limited, Auckland, New Zealand). Since the LC n-3 fatty acid content (specifically EPA and DHA) of salmon and salmon oil capsules is not equal and the quantities of EPA and DHA in different batches of salmon and with cooking may vary, the following method was used to compare intakes of LC n-3 fatty acids from salmon and capsules on n-3 fatty acid levels in erythrocytes. Data obtained from different dosages of capsules were used to create a linear predictive model from which changes in n-3 fatty acid levels in erythrocytes with intakes of LC n-3 fatty acids from capsules in amounts equivalent to that consumed from salmon could be predicted (this is explained in more detail in the Statistics section). To ensure that the most accurate value for LC n-3 fatty acids consumed from salmon could be determined, each subject received an additional portion of salmon for each meal, which they were requested to prepare in the same manner. For each salmon meal, one portion was served and eaten and the duplicate portion was stored in a coded plastic bag in a freezer. The duplicate cooked salmon portions were collected from subjects on a weekly basis and stored at − 80°C until the end of the study when the fatty acid and Se content of the portions were analysed. Subjects were also asked to return any unconsumed salmon in a separate coded plastic bag. The amount of unconsumed salmon was subtracted and the amount of LC n-3 fatty acids and Se consumed was calculated. The fatty acid and Se content of the salmon oil capsules were also analysed and the amounts consumed were calculated based on percentage compliance.
Demographic information including medical history, medication and supplement use and alcohol consumption habits were collected at recruitment using questionnaires. Fasting blood samples and anthropometric measurements (height, weight and BMI) were obtained at baseline and after 8 weeks, as any change may indicate a change in lifestyle. Subjects were requested not to consume any other fatty fish or supplements and to maintain their normal daily routines (e.g. eating patterns, physical activity, alcohol consumption, etc.) for the duration of the study. Food consumption pre- and post-intervention was assessed using a validated FFQ(Reference Russell, Parnell and Wilson19). Subjects completed a weekly compliance diary, including consumption of salmon and capsules, maintenance of normal daily routines, occurrence of any illnesses and any medication used during the study, in order to ascertain compliance to the study protocol.
Subjects received guidelines for the consumption and storage of salmon oil capsules and preparation of salmon. Guidelines for preparation of salmon were based on cooking methods shown to achieve optimal sensory qualities and preservation of n-3 fatty acid levels such as oven baking, pan frying and steaming(Reference Larsen, Quek and Eyres20). Subjects were advised to store the capsules in the refrigerator in opaque containers and to consume the capsules every day with a meal. Subjects were requested to return any left-over salmon and capsules, which was weighed and counted to determine the compliance. The oxidation levels (anisidine and peroxide values) of the salmon oil capsules were analysed. Fish oil, due to its high degree of unsaturation, is susceptible to oxidation, which results in decreased n-3 fatty acid levels and is associated with chronic inflammation and oxidative stress(Reference Kolanowski, Jaworska and Weissbrodt21).
At the end of the study, each subject completed a computer-based tolerance questionnaire. The first question was an open-ended question that asked about any side effects experienced during the study without any probing to ensure responses were not led by suggestion. Subjects were not able to go back to this question to change the answer. The subsequent questions, adapted from Freeman & Sinha(Reference Freeman and Sinha22), asked about specific side effects, including dizziness, diarrhoea, nausea, bloating, burping, heart burn/reflux, difficulty in swallowing the treatment, unpleasant breath and tiredness.
Blood samples and biochemical analysis
Registered phlebotomists collected fasting venous blood samples from subjects between 07.00 and 09.30 hours after fasting overnight (at least 8 h with no food or beverage, except water). An EDTA blood sample was collected for the analysis of erythrocyte fatty acids, plasma Se and whole-blood glutathione peroxidase (GPx) activity. Blood was centrifuged for 10 min at 2000 g at 4°C within 2 h after blood collection, plasma was removed and the erythrocytes were then washed three times with saline (0·9 % NaCl). Aliquots of whole blood, plasma and erythrocyte were stored at − 80°C until analysis was carried out in one batch at the end of the study.
Erythrocyte fatty acids were analysed using a Shimadzu GC 2010 ported to a GCMS-QT2010 Mass Spectrometer (Shimadzu Corporation, Kyoto, Japan) as described previously(Reference Stonehouse, Kruger and Smuts23). The CV for EPA and DHA assays were 2·7 and 3·6 %, respectively. Erythrocyte fatty acids are expressed as weight percentage of total fatty acids. The omega-3 index was calculated as follows(Reference Harris and von Schacky7):
Plasma Se concentrations were analysed by graphite furnace atomic absorption spectroscopy with Zeeman background correction (Perkin-Elmer Model 3100; Perkin-Elmer, Norwalk, CT, USA), using a modified version of the method of Jacobson & Lockitch(Reference Jacobson and Lockitch24). Accuracy was assessed by analysing certified reference material with each batch (Seronorm Reference Serum, batch no. JL4409; Laboratories of SERO AS, Billingstad, Norway), with a certified Se concentration of 0·92 (95 % CI 0·84, 1·00) μmol Se/l, which gave a mean concentration of 0·88 (sd 0·04) μmol/l (CV 4·9 %, n 45). Whole-blood GPx activity was determined using a modification of the coupled enzyme procedure by Paglia and Valentine(Reference Thomson, Robinson and Campbell25) on the Cobas Fara autoanalyser (Hoffman-La Roche, Basle, Switzerland). Whole-blood GPx was assayed as a measure of erythrocyte GPx, which has been shown previously by us to constitute 95 % of total whole-blood activity using this assay method(Reference Thomson, Robinson and Butler26). Because no external controls were available at the time, pooled samples of whole blood were analysed with each batch, which gave a mean activity of 45·5 (sd 2·8) U/g Hb (CV 6·2 %, n 45).
Analysis of salmon and salmon oil capsules
The salmon samples from each subject (n 16) were thawed, pooled and homogenised (resulting in one sample per subject). In total, nine salmon oil capsules, previously stored at − 80°C, were thawed at room temperature in the dark and analysed.
The procedure for the analysis of fatty acids in salmon and salmon oil capsules was described by Larsen et al. (Reference Larsen, Quek and Eyres20). The CV′ values for EPA and DHA concentrations in oil were 2·52, 2·62 % and 1·74, 1·94 % for the salmon and capsules, respectively.
The Se content of the salmon and salmon oil capsules was analysed by inductively coupled plasma MS using a Perkin-Elmer Sciex Elan 6000 (Perkin-Elmer, Inc., Waltham, MA, USA). Approximately 0·5 g sample was weighed into 68 ml polypropylene tubes (Environmental Express, Mt Pleasant, SC, USA) with calibrated volume markings. Tetramethylammonium hydroxide solution (12·5 %; 5 ml) was added and digested on a hot block set (Environmental Express) to achieve a digest temperature of 90°C for 1 h. The digest was made to 50 ml in digest tubes, shaken and filtered through a 0·45 μm cellulose acetate syringe filter (Sartorius Minisart; Sartorius Stedim Biotech S.A., Aubagne Cedex, France). The isotope 82Se was used to monitor Se.
Oxidation values of capsules
At 0, 4 and 8 weeks, samples of salmon oil capsules (nine in total) were collected randomly during the study and stored at − 80°C until the end of the study for analysis of oxidation values (anisidine and peroxide values). Peroxide values were analysed using the AOCS Official Method Cd8-53 with modifications and anisidine was analysed using the AOCS Official Method Cd18-90(27).
Statistical analysis
Data were analysed using the SPSS package version 16 (SPSS, Inc., Chicago, IL, USA). Normally distributed variables are presented as means and standard deviations and non-normally distributed variables as medians (25–75th percentiles). A P value of < 0·05 was considered to be statistically significant. The variables were tested for a normal distribution using Kolmogorov–Smirnov and Shapiro–Wilk tests, together with examination of normal Q–Q plots, box and stem and leaf plots. Differences within groups between baseline and end values were analysed using the paired-samples t test for parametric variables and the Wilcoxon ranked-sum test for non-parametric variables. One-way ANOVA with post hoc tests (Tukey's honest significance difference test) were used to determine the differences between groups for parametric variables. Differences between groups for non-parametric variables were analysed using the Kruskal–Wallis test with post hoc analysis and Bonferroni adjustments. Differences in plasma Se concentrations between the salmon and the capsule groups (three groups combined) were analysed while controlling for baseline Se using the ANCOVA test, while differences in whole-blood GPx were analysed using the Mann–Whitney U test. All statistical tests were two-sided. Linear regression analysis predictive models were fitted to the capsule data. All assumptions for linear regression analyses were met; residuals were normally distributed and independent (Durbin–Watson test) and variance was constant (as assessed by plotting standardised residuals against the predicted values). Linear regression analysis was carried out with a dummy variable to calculate the predicted change and 95 % CI in erythrocyte n-3 fatty acid levels to the intake of LC n-3 fatty acids from capsules in amounts equivalent to that consumed from salmon.
Results
Baseline characteristics and compliance
Of the forty-four subjects recruited, three subjects withdrew from the study, one due to illness (six-capsule group) and two for personal reasons (four-capsule group). Subject characteristics are described in Table 1. There were no differences between the intervention groups at baseline. Compliance to the interventions was 98 (sd 3·3) % and did not differ between the four intervention groups. Anthropometric measurements remained constant throughout the study.
Table 1 Baseline characteristics of the study subjects
(Mean values and standard deviations)

* Differences between the groups were determined by one-way ANOVA (two-sided).
Interventions
Cooked salmon contained on average 1·01 g EPA, 0·33 g docosapentanoic acid (DPA), 1·11 g DHA and 0·02 mg Se/100 g salmon. Each gram of salmon oil capsule contained on average 45 mg EPA, 14 mg DPA and 60 mg DHA. Se was not detected in salmon oil capsules. Mean peroxide values (3·30 (sd 0·06) meq/kg oil) and anisidine values (8·36 (sd 0·67)) of salmon oil capsules were below the maximum permitted levels during the study (5 meq/kg for the peroxide value and 10 for the anisidine value(28)).
The total daily average LC n-3 fatty acids and Se consumed from the different treatments by the subjects per day are summarised in Table 2. Analysis showed that the salmon oil capsules contained less LC n-3 fatty acids than claimed by the company supplying the capsules (0·045 g EPA and 0·06 g DHA/capsule v. 0·06 g EPA and 0·10 g DHA/capsule as claimed by the company). This resulted in the higher level of n-3 fatty acids supplied by six capsules being less than the n-3 fatty acids supplied by salmon, which could possibly affect the linear regression analysis. However, it can be argued that the linear regression analysis was still valid to use, based on evidence in the literature showing a linear relationship between dosage of n-3 fatty acids even higher than that used in the present study and erythrocyte n-3 fatty acid levels(Reference Brown, Pang and Roberts29, Reference Katan, Deslypere and van Birgelen30). In the present study, the relationship between intake of LC n-3 fatty acids from capsules and erythrocyte n-3 fatty acid levels was indeed linear and is described below.
Table 2 Daily consumption of long-chain (LC) n-3 fatty acids and selenium during the 8-week intervention with salmon and salmon oil capsules
(Mean values and standard deviations)
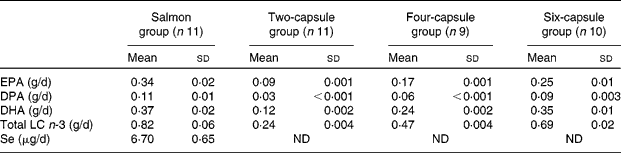
DPA, docosapentanoic acid; ND, not detected.
Fatty acid status
Baseline erythrocyte fatty acid levels did not differ between the intervention groups (Table 3). As expected, erythrocyte EPA, DHA and omega-3 index levels increased and arachidonic acid levels decreased significantly from baseline to end with all treatments. Erythrocyte DPA levels increased significantly only in the salmon and six-capsule groups. The increase in erythrocyte EPA and omega-3 index were significantly higher in the salmon and six-capsule groups compared with the two-capsule group. This trend was also seen for erythrocyte DHA, but the differences were not significant. Although some small significant differences were seen within-group changes for some of the other fatty acids, there was no clear trend in changes in these fatty acids with the interventions (Table 3).
Table 3 Erythrocyte fatty acid levels (% of total fatty acids) during the 8-week intervention with salmon and salmon oil capsules
(Mean values and standard deviations)

DPA, docosapentanoic acid.
a,b Mean values with unlike superscript letters were significantly different for post hoc tests (P < 0·05).
* Salmon group, n 11; two-capsule group, n 11; four-capsule group, n 9; six-capsule group, n 10.
† Differences within groups (from baseline to end) were analysed using the paired-samples t test (two-sided).
‡ Differences between changes from baseline to end in groups were determined by one-way ANOVA and post hoc tests using Tukey's honest significance difference test (two-sided).
Increased intakes of LC n-3 fatty acids from capsules were linearly related and significantly predicted changes in total erythrocyte n-3 fatty acid, erythrocyte EPA, erythrocyte DHA and omega-3 index levels, but did not significantly predict erythrocyte DPA levels, which are as follows:
● Change in total erythrocyte n-3 fatty acids (%) = 0·36+2·39 × total LC n-3 fatty acid intake (g/d), n 30, F(1,29) = 12·7, P = 0·001, r 2 0·31, β = 2·39 (95 % CI 1·02, 3·76).
● Change in erythrocyte EPA (%) = 0·09+1·04 × total LC n-3 fatty acid intake (g/d), n 30, F(1,29) = 13·0, P = 0·001, r 2 0·32, β = 1·04 (95 % CI 0·45, 1·64).
● Change in erythrocyte DHA (%) = 0·28+1·02 × total LC n-3 fatty acid intake (g/d), n 30, F(1,29) = 5·10, P = 0·032, r 2 0·15, β = 1·02 (95 % CI 0·10, 1·95).
● Change in omega-3 index (%) = 0·38+2·07 × total LC n-3 fatty acid intake (g/d), n 30, F(1,29) = 12·71, P = 0·001, r 2 0·31, β = 2·07 (95 % CI 0·88, 3·26).
● Change in erythrocyte DPA levels: n 30, F(1,29) = 4·03, P = 0·05, r 2 0·13.
The changes and predicted changes in erythrocyte LC n-3 fatty acid levels and omega-3 index with intake of 0·82 g/d of LC n-3 fatty acids from salmon and salmon oil capsules are summarised in Table 4. Based on the large overlap in 95 % CI levels between salmon and salmon oil capsules, it can be concluded that the increase in erythrocyte total n-3 fatty acid levels, EPA, DHA and omega-3 index were similar to the intake of salmon and salmon oil capsules.
Table 4 Changes and predicted changes in erythrocyte long-chain (LC) n-3 fatty acids after an 8-week consumption of 0·82 g/d LC n-3 fatty acids from salmon or salmon oil capsules
(Mean values and 95 % confidence intervals)
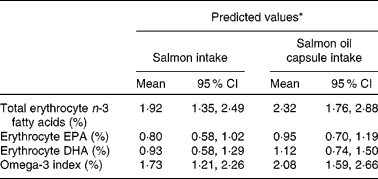
* Changes predicted from linear regression models.
Selenium status
Changes in plasma Se concentrations and whole-blood GPx activities are summarised in Table 5. Plasma Se increased significantly with intake of salmon compared with salmon oil capsule intake (mean difference between the change in plasma Se with salmon and salmon oil capsules, 10·6 (95 % CI 3·44, 17·8) μg/l, F(1,36) = 9·04, P = 0·005). Whole-blood GPx did not change significantly in either the salmon or the salmon oil capsule groups.
Table 5 Plasma selenium concentrations and whole-blood glutathione peroxidase (GPx) activities during the 8-week consumption of salmon or salmon oil capsules
(Mean values and 95 % confidence intervals or medians and 25–75th percentiles)

* Adjusted mean and 95 % CI (adjusted for baseline plasma Se). Differences in plasma Se between the groups were analysed while controlling for baseline Se concentrations using ANCOVA (two-sided); differences in whole-blood GPx between the groups were analysed using the Mann–Whitney U test.
Food consumption
Food intake from the major food groups (including milk, bread, butter/margarine/spreads, breakfast cereal, starchy foods, meat, poultry, fish/seafood, eggs, legumes, cooking fat/oil, condiments, vegetables, fruit, beverages and takeaways) did not change during the study in any of the intervention groups and no differences were observed between the groups (data not shown). As expected, fatty fish intake increased significantly in the salmon group (from a median intake of 0·5 (25–75th percentile 0·3–0·8) to 3·0 (25–75th percentile 1·0–3·5) servings/week)) compared with the capsule groups (from a median intake of 0·6 (25–75th percentile 0·4–1·0) to 1·0 (0·5–1·3) servings/week) (P = 0·002; Mann–Whitney U test). Fatty fish intake did not differ between the three capsule groups. Since total fish and seafood intake did not change, we suspect that subjects in the salmon group exchanged their habitual intake of fish and seafood for salmon.
Tolerance of treatments
Unprompted, only six subjects reported side effects, all of them from the salmon oil capsule groups and included complaints about bloating (1 × ), nausea (2 × ), burping (2 × ), indigestion (1 × ) and stomach pain (1 × ). With regard to specific side effects, only one person in the salmon group reported one side effect (bloating), whereas more complaints were reported by subjects in the capsule groups (two-capsule group: nausea (2 × ), bloating (2 × ), burping (4 × ), unpleasant breath (1 × ) and tiredness (1 × ); four-capsule group: diarrhoea (2 × ), nausea (1 × ), burping (1 × ), heart burn/reflux (1 × ), difficulty in swallowing the treatment (2 × ), unpleasant breath (2 × ) and tiredness (4 × ); six-capsule group: nausea (2 × ), bloating (1 × ), burping (3 × ) and difficulty in swallowing the treatment (3 × ).
The number of capsules consumed did not seem to affect the number of side effects reported. Most of these side effects were experienced by the subjects as mild/insignificant.
Discussion
The present study showed that consumption of similar amounts of LC n-3 fatty acids either from two weekly servings of salmon or daily dosages of salmon oil capsules was equally effective in increasing LC n-3 status in healthy volunteers. However, consuming salmon had the added benefit of increasing plasma Se concentrations and was better tolerated than salmon oil capsules.
In two studies, it has been shown that fish consumption was more effective at increasing plasma/serum EPA and DHA levels than capsules(Reference Elvevoll, Barstad and Breimo11, Reference Visioli, Rise and Barassi13), whereas two other studies have shown no difference between fish v. capsules(Reference Arterburn, Oken and Hall10, Reference Harris, Pottala and Sands12). Visioli et al. (Reference Visioli, Rise and Barassi13) also used a dose–response model to compare the effects of daily portions of salmon with different daily dosages of fish oil capsules for 6 weeks. They added data from another study with a similar design to complete the dose–response curve, resulting in a less than optimal study design. They showed that, to obtain the same increment in plasma EPA induced by 0·38 g/d of EPA from salmon, a dose of 0·80 g/d (more than twofold) was needed when using capsules. For DHA, a dose almost ninefold was required to obtain the same increment in plasma DHA as with salmon intake. Furthermore, subjects had to consume 100 g of salmon every day, which might not be feasible in real life. Elvevoll et al. (Reference Elvevoll, Barstad and Breimo11) also showed that fish (salmon or cod) consumption was more effective in increasing serum EPA and DHA over a period of 8 weeks than supplementing the diet with cod-liver oil. In the present study, however, no attempt was made to match the EPA and DHA content of the fish and fish oil capsules or the type of fish and fish oil. Harris et al. (Reference Harris, Pottala and Sands12) demonstrated that consumption of equal amounts of EPA and DHA from oily fish (salmon and albacore tuna) on a weekly basis or from fish-oil capsules on a daily basis by premenopausal women for 16 weeks was equally effective in enriching erythrocyte and plasma phospholipids with n-3 fatty acids. Arterburn et al. (Reference Arterburn, Oken and Hall10) compared the availability of 600 mg DHA/d from algal oil capsules with equal amounts from cooked salmon. In this 2-week study, changes in DHA levels in plasma phospholipids and erythrocyte were similar between consumption of salmon and algal oil capsules. The authors from the first two studies(Reference Elvevoll, Barstad and Breimo11, Reference Visioli, Rise and Barassi13) speculated that the greater bioavailability of n-3 fatty acids from fish than from capsules could be due to capsules providing a small lipidic bolus following ingestion resulting in the processes of lipid absorption not being adequately activated. Fat from fish, on the other hand, is in a diluted emulsion after eating, which may result in higher surface interaction between food and the intestinal wall and more favourable secretion of drivers that aid digestion and absorption at the intestinal level(Reference Elvevoll, Barstad and Breimo11, Reference Visioli, Rise and Barassi13). In the present study, subjects were requested to consume the capsules with food, which could partly explain why salmon was not more effective at increasing LC n-3 status than the capsules. In addition, in the present study, the lipids in both salmon and salmon oil capsules were in the form of TAG, whereas in the study of Visioli et al. (Reference Visioli, Rise and Barassi13), EPA and DHA were provided as ethyl esters. On the other hand, Harris et al. (Reference Harris, Pottala and Sands12) also used n-3 fatty acids in the ethyl ester form. The strengths of the present study are that erythrocyte EPA and DHA levels were used as biomarkers, and subjects with high habitual intakes of fatty fish and n-3 fatty acid supplements were excluded and n-3 fatty acid intakes from fish and capsules were well matched, similar to the studies by Harris et al. (Reference Harris, Pottala and Sands12) and Arterburn et al. (Reference Arterburn, Oken and Hall10). In the present study, the n-3 fatty acid levels in salmon were analysed after cooking. Since cooking methods can result in varying amounts of n-3 fatty acid content(Reference Larsen, Quek and Eyres20), this would have resulted in more accurate assessment of actual intakes of n-3 fatty acids by each subject, whereas in other studies, the effect of cooking on n-3 fatty acid levels was not taken into account(Reference Elvevoll, Barstad and Breimo11–Reference Visioli, Rise and Barassi13).
It could be argued that 8 weeks were not a sufficient length of supplementation since erythrocyte n-3 fatty acids are considered a long-term marker of n-3 intakes and do not achieve a plateau until about 6 months(Reference Katan, Deslypere and van Birgelen30). However, 8 weeks were long enough to result in a significant increase in erythrocyte n-3 fatty acid levels and it was noted in previous studies that the greatest increases were achieved within the first 4–8 weeks of supplementation(Reference Katan, Deslypere and van Birgelen30, Reference Cao, Schwichtenberg and Hanson31). The study was powered to detect relatively large differences (13–17 %) in LC n-3 status and may not have been adequately powered to detect modest differences between salmon and salmon oil capsule intake. However, the large overlap between 95 % CI levels indicates that it is unlikely that LC n-3 fatty acid levels differed significantly between intakes of salmon and salmon oil capsules.
The consumption of two portions/week of salmon or daily dosage of six capsules of salmon oil (providing 0·82 g and 0·69 g/d, respectively, of LC n-3 fatty acids) almost achieved omega-3 index levels of 8 %, which is considered to be cardioprotective(Reference Harris and von Schacky7). A longer duration would have resulted in an even greater increase. Cao et al. (Reference Cao, Schwichtenberg and Hanson31) showed that supplementation with approximately 2 g/d of LC n-3 fatty acids, a dosage much higher than in the present study, for 8 weeks nearly reached the optimal value of 8 %. The magnitude of increase is probably largely dependent on baseline levels, which in the present study ranged from 5·84 to 6·20 % compared with 4·3 % in the study of Cao et al. (Reference Cao, Schwichtenberg and Hanson31). Thus, the lower the baseline levels, the higher the dosage needed over 8 weeks to achieve an optimal omega-3 index level of 8 %. Furthermore, the predictive equation from the capsule data indicates that the omega-3 index will increase with 2·07 % (β-coefficient) with every 1 g/d increase in the intake of LC n-3 fatty acids. Taken together (from the present study and the study by Cao et al. (Reference Cao, Schwichtenberg and Hanson31)), every approximately 1 g/d intake of LC n-3 fatty acids may result in an approximately 2 % increase in omega-3 index over 8 weeks.
Salmon was consumed twice weekly without any tolerance problems. However, similar to other studies, the most frequent side effect reported for capsule intake (burping and unpleasant breath) was related to eructation(Reference Harris, Pottala and Sands12, Reference Freeman and Sinha22).
Mean plasma Se concentration at baseline (120 (sd 20·4) μg/l) was higher than that previously reported for New Zealand residents (ranging from 66 to 88 μg/l)(Reference Thomson15). These higher concentrations might be due to greater importation of Australian wheat and other cereal products especially in the North Island of New Zealand, where subjects in the present study resided, as well as due to increased Se concentrations in animal foods(Reference Thomson, McLachlan and Parnell32). Despite the relatively high baseline Se concentrations, the small amount of Se provided by consuming two portions of salmon/week (6·7 μg Se/d) resulted in a significant increase of almost 10 % in plasma Se after 8 weeks compared with capsule intake. A study by Thorngen & Akesson(Reference Thorngren and Akesson33) has shown a similar increase in plasma Se (13 %) with much greater amounts of Se from fish (herring, salmon or mackerel providing 40–50 μg Se/d for 6 weeks).
Whole-blood GPx, a functional measure of Se status, did not change during the study. This could be explained by plasma Se concentrations at baseline being higher than the levels needed for the full expression of GPx(Reference Thomson34).
In conclusion, consumption of similar amounts of LC n-3 fatty acids either from salmon or salmon oil was equally effective in increasing erythrocyte LC n-3 status. The n-3 status can be improved by either method according to consumer preference. However, consuming salmon had the added benefit of increasing Se status and was better tolerated than salmon oil capsule intake.
The model used in the present study could be used in future studies to compare different food matrices, e.g. fortified food v. natural sources, and different lipid forms, e.g. phospholipids v. TAG v. ethyl esters. These studies should include a larger sample size and be of longer duration (4–6 months), but good compliance should be ensured over this period.
Acknowledgements
We thank all the subjects who participated in the present study. We gratefully acknowledge Yan Wang for setting up and helping to analyse the fatty acid content of the salmon, salmon oil capsules and erythrocytes; Dr Karl Bailey and Ms Jody Miller, Department of Human Nutrition, University of Otago for Se and GPx analyses. The present study was funded by the Institute of Food, Nutrition and Human Health, Massey University, the Foundation for Research Science and Technology and the New Zealand King Salmon Company Limited, who supplied the salmon. None of the authors has any conflict of interest. W. S. is the main author of the paper and the project leader, involved in all aspects of the study, including conceptualising and design of the research project, the acquisition of the data and statistical analysis. M. R. P. is an MSc student in the project, involved in planning and conducting of the research project, the analysis and statistical analysis of the data and approving the manuscript. R. K. was involved in the planning of the study, the acquisition of the data, analysis of the data and revising and approving the manuscript. C. D. T. helped in the approval of the study design, analysis and interpretation of the results related to Se, revising and approving the manuscript. M. W. also helped in the approval of the study design, overseeing set-up and analysis of fatty acids in salmon, salmon oil capsules and erythrocytes, revising and approving of the manuscript. M. C. K. was involved in the conceptualisation and design of the research project, revising and approving the manuscript.







