Controversial results surround the prevalence of left-handedness in twins compared with singletons. Some studies have reported that left-handedness is more common in twins than in singletons (Annett, Reference Annett1994; Vuoksimaa et al., Reference Vuoksimaa, Koskenvuo, Rose and Kaprio2009), but others found no such differences (Medland et al., Reference Medland, Wright, Geffen, Hay, Levy, Martin and Duffy2003, Reference Medland, Duffy, Wright, Geffen, Hay, Levy and Boomsma2009). On average, twins are 1 kg lighter in birth weight, and are of shorter gestational age than singletons (Blickstein & Keith, Reference Blickstein and Keith2007): Twins, on average, have lower Apgar scores than singletons; the difference is greater in 1-min Apgar scores but remains evident in 5-min scores (Blickstein & Keith, Reference Blickstein and Keith2007); Apgar scores are summary assessments of the newborn's health (Finster & Wood, Reference Finster and Wood2005). While the difference in left-handedness between twins and singletons remains unclear, studies have not found difference between identical and fraternal twins (Medland et al., Reference Medland, Wright, Geffen, Hay, Levy, Martin and Duffy2003; Vuoksimaa et al., Reference Vuoksimaa, Koskenvuo, Rose and Kaprio2009).
A study of Finnish twins, born during 1984–1987, indicated that birth weight, gestational age, and Apgar scores were not significantly associated with handedness (Vuoksimaa et al., Reference Vuoksimaa, Eriksson, Pulkkinen, Rose and Kaprio2010), but another study did report an association between handedness and birth weight (Medland et al., Reference Medland, Duffy, Wright, Geffen, Hay, Levy and Boomsma2009). Among singletons, extremely low birth weight has been associated with increased prevalence of left-handedness (O’Callaghan et al., Reference O’Callaghan, Burn, Mohay, Rogers and Tudehope1993; Powls et al., Reference Powls, Botting, Cooke and Marlow1996).
The purpose of the present study was to evaluate whether left-handedness is more common in twins than in singletons, and if so, whether the higher prevalence of left-handedness in twins may be explained by birth- and pregnancy-related factors (birth weight, gestational age, and Apgar scores) known to differ between twins and singletons.
Materials and Methods
The twin data consisted of two population-based Finnish twin datasets. FinnTwin12 includes Finnish twins born during 1983–1987, and FinnTwin16 includes Finnish twins born during 1974–1979 (Kaprio et al., Reference Kaprio, Pulkkinen and Rose2002). Ascertainment was essentially exhaustive, and participation rates were very high. Information on birth weight, gestational age, and Apgar scores (1 and 5 min) of all twins was obtained as a part of postal questionnaires completed by mothers when the twins reached 12 years (FinnTwin12) or 16 years (FinnTwin16) of age. Both studies were initiated with a family questionnaire sent to the twins’ parents seeking their written permission to contact their twin children; the family questionnaire phase yielded maternal reports on pregnancy, birth weight, and Apgar scores of the twin children. The return of this family questionnaire prompted mailing individual questionnaires to twins, and in FinnTwin12, questions about handedness were included as part of the postal questionnaire data collection at the age of 14 years, with a response rate of 88.5%. One question asked whether the twin was right-handed, left-handed, or used both equally. A follow-up question asked whether the twin wrote with their right hand or not. Responses were obtained from 4,739 twins. Of these, four were missing writing-hand answers, and 48 twins were deleted because they inconsistently claimed to be right-handed but wrote with the left, or the other way round. The final number of twins in this dataset was 4,687 (Vuoksimaa et al., Reference Vuoksimaa, Eriksson, Pulkkinen, Rose and Kaprio2010).
A dataset of singletons (matched for birth year with the younger FinnTwin12 subjects) comprised the participants of the Helsinki Ultrasound Trial (born during 1986–1988 in the Helsinki region), in which there were 8,662 deliveries (Saari-Kemppainen et al., Reference Saari-Kemppainen, Karjalainen, Ylostalo and Heinonen1990). From these, we obtained handedness data from 4,150 subjects in a later questionnaire (Heikkilä et al., Reference Heikkilä, Vuoksimaa, Oksava, Saari-Kemppainen and Iivanainen2011). In that questionnaire, the parent was asked whether the child used left, both, or right hand for doing the following five tasks: writing name, throwing ball, using scissors for cutting paper, using knife, and eating with spoon. Details on the Ultrasound Trial data, including response rates, have been described earlier (Heikkilä et al., Reference Heikkilä, Vuoksimaa, Oksava, Saari-Kemppainen and Iivanainen2011; Saari-Kemppainen et al., Reference Saari-Kemppainen, Karjalainen, Ylostalo and Heinonen1990). For analyses reported here, when using merely the writing hand variable as the studied outcome, the number of subjects was 4,159. We omitted 34 twin individuals and eight others who were told to write with both hands. We also deleted 16 cases of inconsistent handedness in way similar to that described for FinnTwin12. The final sample for this non-twin dataset was 4,101 participants.
In FinnTwin16, the family baseline questionnaires were sent as the twins reached 16 years of age. Birth weights and other pregnancy data of 5,510 twins were returned by mothers. Self-reports on handedness were collected from 4,245 twins during 2010–2012 by an internet questionnaire (wave 5, mailed to 5,924 twins: response rate 72%). A handedness question asked whether the twin wrote with right or left hand, followed with a question about whether he/she could select the writing hand freely. Eighty twins were excluded because they reported they could not freely select their writing hand. An additional 66 had to be omitted because of missing data on handedness. The final number of twins in this data subset was 4,099, and the total number of twins was 8,786.
In FinnTwin16, partners of the twins were included in the wave 5 internet questionnaire data collection; this yielded 1,949 singletons. We omitted 57 persons who said they had a twin sister/brother, 77 respondents with erroneous or missing handedness values and 24 who could not freely select their writing hand. A total of 1,791 singletons remained for analysis.
The Ultrasound singleton data were administered and planned separately from the twin data, so the datasets lack some uniformity. The writing hand question was selected as the final most comparable variable (Perelle & Ehrman, Reference Perelle and Ehrman2005); in addition, all five handedness questions used in the Helsinki Ultrasound Trial were summarized using latent analysis to make a second handedness variable. This was to harmonize data with the FinnTwin12 dataset, which also included a second handedness variable (self-reported left-handedness/right-handedness/ambidextrousness). Latent analysis was done by submitting the five handedness questions to a polytomous latent class analysis of the package poLCA (Linzer & Lewis, Reference Linzer and Lewis2011) in program R (R Development Core Team, 2013). The resulting variable had three classes (left-handedness/right-handedness/ambidextrousness). In this way, we created a variable sufficiently consistent and comparable with the handedness variable of the FinnTwin12 twins. This second handedness variable was used for a consistency check of the writing hand in the same manner as the respective variable in FinnTwin12 (i.e., only individuals who reported consistent right- or left-handedness were included in the analyses).
The covariates are summarized in Table 1. These were not available for the partners of the FinnTwin16 twins. Gestational age was outside the expected range (22–44 weeks for singletons, 22–42 weeks for twins) for 29 twins and four singletons. The covariates of twins were collected during the initial rounds of the twin questionnaires and were reported by parents, while the covariates of the Ultrasound Trial singletons were collected from hospital records. Although birth- and pregnancy-related factors were based on parental reports in twins, we note that in Finland these measures are routinely included in hospital records and parents are given this information in a written format. Further, there is evidence that maternally reported birth covariates are reliable. For instance, in a study by O’Sullivan et al. (Reference O’Sullivan, Pearce and Parker2000), maternally reported birth weights of 649 children were noted to be comparable with hospital records. Similarly, according to Tate et al. (Reference Tate, Dezateux, Cole and Davidson2005), maternally reported birth weight had a high level of agreement with registries in 11,890 children in the United Kingdom. It has additionally been shown in the thesis of Pietiläinen (Reference Pietiläinen2004) in a 32-twin subset of Finnish monozygotic (MZ) twins that maternally reported birth weights are highly accurate among MZ twin pairs with large differences in birth weight, and the pairs with large differences are more important in the associations that we report here (Pietiläinen, Reference Pietiläinen2004; Pietiläinen et al., Reference Pietiläinen2001). Our analyses use the 5-min Apgar scores because they are more relevant to the potential effects of perinatal asphyxia as a risk factor for left-handedness. However, owing to common practice, the measurement at 5 min was not always done because labor had been smooth, and was mostly based on the measurement at 1 min. The missing dichotomous measure at 5 min was thus made to denote adequate status whenever the 1-min measurement also denoted an adequate status. There were additionally some subjects left with a missing 5-min measurement and a poor result from the 1-min measurement. These were likewise denoted to have a poor Apgar status.
TABLE 1 Demographics of the Available Datasets With a Tabulation of Twin Status and Handedness
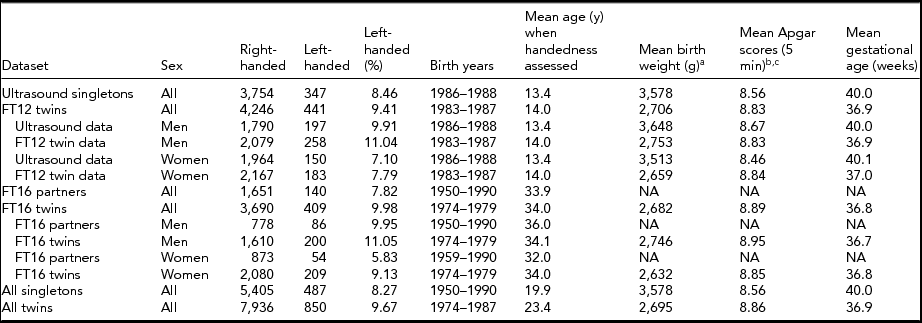
aBirth weight was available only for 4,098 singletons and 8,283 twins. bApgar scores were initially measured for 4,087 singletons and 7,035 twins. cApgar scores as continuous measure at 5 min were available only for those in need of a follow-up measurement, 564 singletons and 3,523 twins. Only non-missing values were summarized here.
Data Analysis
When performing twin versus singleton comparisons, we paired the two twin and singleton datasets according to their approximate ages: the FinnTwin16 twins were compared with their singleton partners, and the FinnTwin12 twins with the Ultrasound Trial singletons. The writing hand of singletons and twins was tabulated in a demographics table, separated by sex. The comparative proportions were analyzed using logistic regression, and the resulting odds ratios (ORs) were summarized. All logistic regression models were run using the cluster-option of Stata to adjust for the sampling of twin pairs in analysis (Stata Statistical Software: Release 12). We also used the Mantel–Haenszel approach for summarizing the two twin–singleton comparisons with the Stata program ‘cc’.
In a similar way, we also tested possible associations of handedness with covariates, including low birth weight and Apgar scores. Because of an expectation that only very low birth weight babies have more left-handedness (O’Callaghan et al., Reference O’Callaghan, Burn, Mohay, Rogers and Tudehope1993; Powls et al., Reference Powls, Botting, Cooke and Marlow1996), we also wanted to analyze the association of dichotomized birth weights and handedness. Dichotomized birth weights were used to separately evaluate associations with handedness in twins and singletons. A cut-off of 2,000 g was used for twins (Ooki, Reference Ooki2006). We turned to percentiles by sex, using 10% cut-off points for our twins, closely approximating what Ooki (Reference Ooki2006) used. The cut-off point selection for our singletons was the 2.5 percentile, near the original World Health Organization (WHO) standard of 2,500 g.
Apgar scores (American Academy of Pediatrics, 2006) were also dichotomized to lower (0–6) and normal (7–10) scores, as is done in healthcare. We used the 5-min Apgar measurement; if it was not obtained, then we assumed that measurements were stopped because the newborn's condition was healthy. Apgar scores had more missing values than other birth covariates, so replacement based on 1-min measurement had to be applied following the value of the 1-min measurement. Since birth weight and Apgar scores are associated with birth order, we tested for the birth order of twins, and, in addition, for gestational age. The final models were run to explain handedness with twin status, adjusting with covariates. A subsample of FinnTwin12 twins (806 subjects) was administered the Edinburg Handedness Inventory when they were aged 21 to 25 years (Oldfield, Reference Oldfield1971).
Results
Detailed demographics of twin and singleton comparisons with number of participants are shown in Table 1. Twins had on average 880-g lower birth weight than singletons (p < .001). Using dichotomized Apgar measure (0–6 vs. 7–10), twins had poorer Apgar status compared with singletons (χ2 = 92.9 p < .001). Twins had a significantly shorter gestational age than singletons (difference = 3.10 weeks, p < .001).
Differences between MZ and dizygotic (DZ) twins were more modest: Identical twins were slightly (132 g), although significantly (p < .0001), lighter than the fraternal ones. They also expectedly had a shorter gestational age (difference 0.21 weeks, p = .001). However, the dichotomous Apgar variable difference was not significant (these zygocity comparisons are not shown in the tables).
When comparing the two independent dataset pairs, the FinnTwin16 twins had significantly (p < .01) higher prevalence of left-handedness (10%) compared with their partners (7.8%). The FinnTwin12 twins also had a higher prevalence of left-handedness than their age-matched singletons (9.4% vs. 8.5%) taken from the Helsinki Ultrasound Trial, but that difference was not statistically significant (p < .12). Jointly comparing all datasets, twins were significantly more likely left-handed than singletons (Table 2), OR = 1.19, p = .004, CI = [1.06, -1.34].
TABLE 2 Association Between Twin Status and Handedness
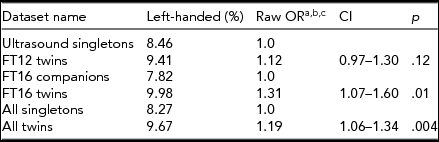
aThe odds ratios were given by cluster-corrected logistic regression.
bThe Mantel–Haenszel approach without twin pair correction gave essentially the same odds ratio that we show here, with closely similar confidence limits. The test for homogeneity was χ2 = 1.44, p = .23 and the test that combined OR would be unity resulting in a p = .005.
cThe results and significance were similar when birth year and sex were added as covariates.
There were gender differences as well: Men were more likely to be left-handed in all of the datasets shown in Table 1. However, there was no significant interaction of twin status and sex on left-handedness (p = .29). Although twins in our data were significantly more often left-handed than singletons, there was no difference between DZ and MZ twins (DZ 10.4% vs. MZ 10.2%, p = .74).
When covariates were introduced in analyses (Table 3), partners had to be omitted from models. In a simple model with sex included, twin status remained significant when only 4,098 singletons and 8,283 twins were available. There was no significant difference in the prevalence of left-handedness between twins and singletons when covariates (gestational age, birth weight, and Apgar scores) were introduced (Table 3). The pattern was similar when entering one covariate at a time (results not shown) and when entering all covariates simultaneously (Model 2). The significant result of Apgar scores resulted only when using values from the 1-min measurement, as explained above. Additional model results can be obtained from the first author on request.
TABLE 3 All Subjects Logistic Regression Analysis With Birth Weight Continuous (8,283 Twinsa and 4,098 Singletonsa)
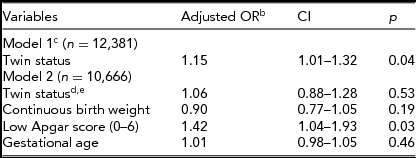
aFor the analysis of covariates we had to rerun the simple model of applicable participants only. bBoth models also had sex included, but it is not shown. cModel 1 was limited to those with non-missing birth weight, thus all partners were excluded. dTwin status had no interaction with covariates. eTwin status would become non-significant with any of the covariates alone in the model.
When we compared left-handedness with birth weight, gestational age, and Apgar scores, we did so for twins and singletons separately (Table 4; for birth weight see Table 5). The odds of left-handedness with respect to birth weight were significant for twins when birth weight was dichotomized (Table 5), but not with continuous measure of birth weight (Table 4). This significant association (Table 5) between birth weight and left-handedness survived the Bonferroni correction of four separate tests. In a follow-up study of a subsample of FinnTwin12 twins (n = 806) we could recognize one (0.1%) case of forced right-handedness, and three (0.4%) cases of left-handedness who became left-handed because of injury. The forced right-handed could already be excluded from analyses in the FinnTwin16 twin and companion datasets, but the inconsistent handedness in the FinnTwin12 and Ultrasound datasets left us uncertain of the amount of forced right-handedness in these two newer datasets; it may thus be negligible.
TABLE 4a Associations of Birth Weight, Gestational Age, and Dichotomous Apgar Scores With Handedness
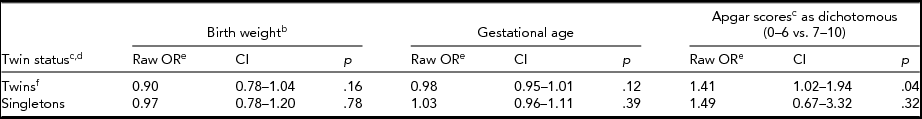
aTable of crude odds ratios for twins and singletons shown separately using cluster-corrected logistic regression. bContinuous birth weight. cThere was no interaction between Apgar scores and twin status. dThe combined results of twins and singletons were already in the multivariate models shown in Table 3. eThe odds ratios shown here would not have been different if run using sex and/or age as a covariate. fThe combined twin dataset showed a significant result for Apgar scores, as shown, but not the two twin datasets separately, the calculated odds ratios of which were however similar to each other and we thus decided to show only the combined result.
TABLE 5 Demographics of Low Birth Weight and Handedness Finntwin16 Twins, Finntwin12 Twins, and Ultrasound Singletons With Available Birth Weight
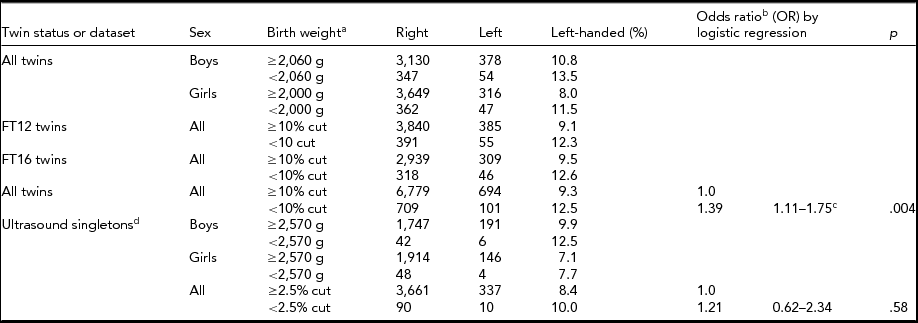
aBirth weight cut-off point for twins was 10% (2,060 g for boys and 2,000 g for girls) and birth weight cut-off point for singletons was 2.5% (2,570 g for both boys and girls). bLogistic regression was used to calculate OR of twins because of the cluster option to correct for twin pairs. cBonferroni correction would not remove significance. dThe companions of Finntwin16 twins did not have birth time data.
Discussion
Overall, our results showed a higher prevalence of left-handedness in twins than in singletons. However, after adjusting for birth and pregnancy-related factors known to differ between twins and singletons (Blickstein & Keith, Reference Blickstein and Keith2007; Medland et al., Reference Medland, Duffy, Wright, Geffen, Hay, Levy and Boomsma2009), no significant difference in the prevalence of left-handedness between twins and singletons remained. When comparing twins and singletons separately, the pregnancy- and birth-related factors included in our study were not generally related to handedness, although we observed a significant association between birth weight and handedness in twins when we used a dichotomized birth weight measure (contrasting males with birth weight lower than 2,060 g with those whose birth weight was >2,060 g, and for women respectively, with a cut-off at 2,000 g). Earlier studies have reported that extremely low birth weight and left-handedness are associated in singletons (Powls et al., Reference Powls, Botting, Cooke and Marlow1996; Saigal et al., Reference Saigal, Rosenbaum, Szatmari and Hoult1992), but we found this association only among twins.
Thus, our main result is a higher prevalence of left-handedness among twins than in singletons. It is consistent with a report by Vuoksimaa et al. (Reference Vuoksimaa, Koskenvuo, Rose and Kaprio2009), but not with that of Medland et al. (Reference Medland, Duffy, Wright, Geffen, Hay, Levy and Boomsma2009). We note that Vuoksimaa et al. (Reference Vuoksimaa, Koskenvuo, Rose and Kaprio2009) compared twins with unrelated singletons, while the singletons studied by Medland et al. (Reference Medland, Duffy, Wright, Geffen, Hay, Levy and Boomsma2009) were singleton family members of the twins. The latter is a more incisive comparison because it controls for environmental factors and shared genetic effects that make members of families similar to each other. Participants studied by Vuoksimaa et al. (Reference Vuoksimaa, Koskenvuo, Rose and Kaprio2009) were older (born before 1958) than our subjects, who were born in the late 1970s and 1980s. Vuoksimaa et al. (Reference Vuoksimaa, Koskenvuo, Rose and Kaprio2009) reported a prevalence of left-handedness in twins and singletons as 8.1% and 5.8% respectively; the corresponding rates in our current study were 9.7% and 8.3% respectively. The difference most likely reflects the fact that forced right-handedness has been decreasing during the 20th century (see Vuoksimaa & Kaprio, Reference Vuoksimaa and Kaprio2010; Vuoksimaa et al., Reference Vuoksimaa, Koskenvuo, Rose and Kaprio2009, for this trend in Finnish twins). Interestingly, we observed the significantly higher prevalence of left-handedness in twins compared with singletons only in the dataset that included participants from the older cohorts (twins born during 1974–1979), but not in the dataset of the participants from the younger cohorts (twins born during 1983–1987). Assuming that birth covariates are involved, improvements in twin pregnancy outcomes might be a natural explanation for diminishing difference over time. In the FinnTwin16 cohort, the twin–singleton difference in the prevalence of left-handedness was 2.2 percentage units whereas it was only 0.9 percentage units (and not statistically significant) in the comparison of twins from the FinnTwin12 study with singletons from the Helsinki Ultrasound Trial. Since birth year was not a significant covariate, one might think that there is no meaningful cohort effect between participants born in the 1970s and those born in the mid-1980s. We also note that in Finland, the routine use of ultrasound in twin pregnancies started at the time of the Ultrasound Trial in the latter half of the 1980s (Saari-Kemppainen et al., Reference Saari-Kemppainen, Karjalainen, Ylostalo and Heinonen1990); however, the prevalence of left-handedness in twins from the FinnTwin12 (9.4%) and FinnTwin16 (10%) was similar (p = .39). Perhaps the significant twin–singleton difference in the prevalence of left-handedness observed only in the FinnTwin16 datasets arises from the singletons: The group of singleton partners may not have been sufficiently age-matched with their twin partners; these partners were born during 1950–1991 (938 men, mean age 36.0 years) and 1959–1991 (981 women, mean age 32.0 years).
Missing 5-min Apgar scores limit our data, and differing definitions of handedness in older and younger datasets constrain direct comparisons across datasets. Although birth year was not significant as a covariate in our data, we suggest two cohort effects: First, that of forced handedness creating differences in comparison to earlier studies; and second, a recent effect of enhanced pregnancy monitoring, which would diminish differences between twins and singletons. A cohort effect has been noted in older populations by Vuoksimaa et al. (Reference Vuoksimaa, Koskenvuo, Rose and Kaprio2009), while Medland et al. (Reference Medland, Duffy, Wright, Geffen, Hay, Levy and Boomsma2009) found cohort effects in the Australian sample, but not in the Dutch one.
There were no differences between MZ and DZ twins in the prevalence of left-handedness. It is as expected in view of earlier studies (Medland et al., Reference Medland, Wright, Geffen, Hay, Levy, Martin and Duffy2003; Vuoksimaa et al., Reference Vuoksimaa, Koskenvuo, Rose and Kaprio2009), and as very recently reviewed by Ooki (Reference Ooki2014). According to Ooki (Reference Ooki2014), improvements in healthcare may be involved. We suggest that the order of magnitude of smaller differences in birth covariates would be an additional reason. We cannot tell for sure, but the fact of no difference remains between MZ and DZ twins.
Our study has several limitations. We lack birth covariates from the partner dataset. Moreover, the separate collection of the datasets yielded self-assessments of handedness in three twin and partner datasets but parental assessment of handedness in the data from Ultrasound singletons. Data collection in the Helsinki Ultrasound Trial and FinnTwin12 did not include a question on forced right-handedness, although a small subset of FinnTwin12 twins had forced right-handedness assessed in a later follow-up. However, we note that forced right-handedness is expected to be rare in the whole FinnTwin12 cohort as only 0.1% of the individuals in the representative subsample of 806 FinnTwin12 participants indicated that they were forced to use their right hand. Conversely, the FinnTwin16 twin and singleton partner samples were more similar to each other, with identical assessments of self-reported writing hand and forced handedness.
In summary, our main results are as follows: (1) Twins do exhibit an increased proportion of left-handedness compared with singletons in unadjusted analyses; (2) after controlling for Apgar scores, gestational age, and birth weight (factors that differ between twins and singletons), no difference in the prevalence of left-handedness between twins and singletons is observed. A significantly higher prevalence of left-handedness in twins compared with singletons is more pronounced in older cohorts, and no differences exist in younger birth cohorts of twins and singletons.
Acknowledgments
Data collection in FinnTwin12 and FinnTwin16 was supported by Grants AA-08315, AA-09203, and AA-00145 from the National Institute on Alcohol Abuse and Alcoholism to Richard J. Rose, and by Academy of Finland Center of Excellence in Complex Disease Genetics (Grants 213506 and 129680), the Academy of Finland (Grants 100499, 205585, 118555, 141054, 265240, 263278, and 264146 to Jaakko Kaprio). The Ultrasound data collection was funded by the Helsinki University Central Hospital and Rinnekoti Research Foundation. Eero Vuoksimaa has been supported by the Academy of Finland (Grant 257075).







