According to the WHO, infants with a birth weight <2500 g, irrespective of gestation, are defined as low birth weight (LBW)( Reference Jain and Singhal 1 ). Infants with LBW have high morbidity and mortality during the neonatal period as an outcome of intra-uterine growth restriction (IUGR) or preterm birth( Reference Lawn, Kerber and Enweronu-Laryea 2 ). Although great efforts have been put into nutritional management and clinical support for pregnant women, LBW infants still account for about 15 % of newborns( 3 ). Among all of the environmental factors leading to the occurrence of IUGR, maternal undernutrition has been recognised as the most important. Because of improper nutritional provision during the fetal period, a critical period according to the nutritional programming theory, LBW infants not only show growth failure during the neonatal period, but also lifelong metabolic disturbance( Reference Garite, Clark and Thorp 4 – Reference Claris, Beltrand and Levy-Marchal 6 ). Therefore, nutritional intervention of LBW infants during their neonatal stage has aroused great attention in recent years. Because of the ethical issues involved, animal models have been widely used to investigate the physiological differences between LBW offspring and normal ones as well as the nutritional support strategies( Reference Dinerstein, Nieto and Solana 7 ).
In this review, we will focus on discussing the physiological differences related to provision and digestion of nutrients between LBW infants and normal infants. Furthermore, we provide information about the current nutritional support given to these LBW infants, with a focus on the neonatal stage.
Consequences of being born with low birth weights
Catch-up growth and risks for the metabolic syndromes
The occurrence of IUGR reflects that the infant probably experienced undernutrition during its development within the uterus. In this case, as a protective mechanism, the fetus prefers to allocate the limited nutrients to vital organs (e.g. the brain) for survival and development, at the expense of somatic growth. Therefore, the growth-hormone system is down-regulated, as indicated by the lower serum concentrations of insulin, insulin-like growth factor (IGF) and insulin-like growth factor-binding protein 3 (IGFBP-3)( Reference Giudice, Dezegher and Gargosky 8 – Reference Kajantie, Dunkel and Rutanen 10 ). By using IUGR piglets as a model, Wang et al. ( Reference Wang, Zhang and Zhou 11 ) reported that IUGR piglets showed lower concentrations of insulin and IGF-I in the jejunal mucosa when compared with normal littermates. IGF and IGFBP-3 reflect the growth velocity in childhood, and several studies have reported that these two factors can be used as predictors of catch-up growth of LBW infants during the neonatal period( Reference Thieriot-Prevost, Boccara and Francoual 12 – Reference Leger, Noel and Limal 14 ).
Normally, catch-up growth in LBW infants is achieved by overnutrition compensation, and it is postulated to erase the growth deficit generated during the fetal period( Reference Jain and Singhal 1 ). However, given the fact that IUGR infants are born with lower concentrations of insulin, IGF-I and IGFBP-3, the sudden shift to a normal or overly compensatory diet after birth might increase these parameters during the first 3 months of life( Reference Leger, Noel and Limal 14 , Reference Cianfarani, Germani and Rossi 15 ), which will lead to insulin resistance in tissues to prevent hypoglycaemia( Reference Jain and Singhal 1 , Reference Cianfarani, Germani and Branca 16 ). Therefore, catch-up growth actually reflects an insulin-resistance state( Reference Dulloo, Jacquet and Seydoux 17 ). Meanwhile, preferential abdominal fat deposition, excess circulating lipids and ectopic fat storage were observed in the catch-up growth infants, all of which have been implicated in the risk for developing obesity, type II diabetes (T2D), hypertension and CVD( Reference Barker, Osmond and Forsen 18 , Reference Dulloo 19 ). Studies with rodents showed that an accelerated postnatal growth induced excessive adiposity, increased adipocyte sizes and glucose intolerance( Reference Crescenzo, Samec and Antic 20 – Reference Wang, Tang and Wang 23 ). Notably, some researchers stated that growth in different neonatal periods may have different effects on later T2D and CVD risks. It has been reported that catch-up growth that occurred during early infancy (the first 3 months) has a greater programming effect on adiposity and metabolism when compared with growth in later stages of infancy( Reference Jain and Singhal 1 ).
Furthermore, previous studies have suggested that small-at-birth people have higher fasting plasma cortisol concentrations in their adult lives and increased adrenal responsivity to adrenocorticotropic hormone stimulation, which can then reduce lean body mass and increase lipid accumulation( Reference Phillips, Barker and Fall 24 – Reference Phillips, Walker and Reynolds 27 ). As a consequence, the elevated cortisol levels may present a possible link between LBW and adult metabolic syndrome. During the neonatal period, cortisol might also play a role in limiting IGFBP-3 proteolysis and therefore reducing IGF bioavailability( Reference Cianfarani, Geremia and Scott 28 ) and leading to growth failure in LBW children.
Digestive function deficiency
Recently, by using different experimental models, it has been reported that LBW offspring such as LBW fetuses, neonates, children or young adults have a higher incidence of short- and long-term dysfunctions in several vital organs, as indicated in Table 1. For example, evidence has shown abnormal brain volumes( Reference Bjuland, Rimol and Lohaugen 29 ) and muscle fibre distributions( Reference Jensen, Storgaard and Madsbad 40 ) of young LBW adults, lower bone quality of preterm children( Reference Longhi, Mercolini and Carloni 41 ) as well as smaller thymic size of IUGR human fetuses( Reference Cromi, Ghezzi and Raffaelli 42 ). Besides lower tissue weight, dysregulated expressions of proteins were observed in the liver, skeletal muscle and small intestine of newborn IUGR pigs( Reference Wang, Chen and Li 31 ).
Table 1 Examples of epidemiological and animal studies that reveal alterations in vital organs of low birth weight (LBW) offspring compared with normal ones
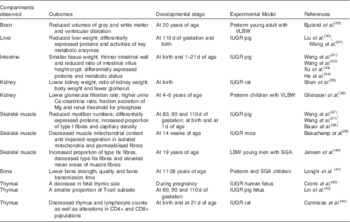
VLBW, very low birth weight, <1500 g; IUGR, intra-uterine growth restriction; SGA, small for gestational age.
Among these organs, the gastrointestinal tract (GIT) is of paramount importance in postnatal nutrient acquisition. The epithelial barrier of the GIT is involved in the first steps of postnatal immune system maturation, providing protection against food antigens and invasion of environmental micro-organisms( Reference Xu, Mellor and Birtles 33 , Reference D’Inca, Gras-Le Guen and Che 45 ). Most studies on the effect of LBW on GIT health were carried out in animal models, especially on piglets. LBW piglets normally show impaired gastrointestinal development, which further imposes limitations on postnatal body growth and development of other organs( Reference Xu, Mellor and Birtles 33 ). Compared with normal-birth weight (NBW) newborns, LBW piglets show a reduced small intestinal weight and a reduced small intestine:body weight ratio up to 21 d of age( Reference Wang, Chen and Li 46 ). The reduced ratio of intestinal weight:length in these LBW piglets indicates a thinner intestinal wall( Reference Wang, Lin and Liu 32 , Reference Wang, Wu and Lin 47 ). Differences in intestinal architecture between IUGR and NBW neonates were widely documented, indicating that the intestinal absorptive surface was smaller in IUGR piglets during the early days of life, as evidenced by the reduced ratio of intestinal villus height:crypt depth( Reference Xu, Mellor and Birtles 33 , Reference He, Ren and Kong 34 , Reference D’Inca, Gras-Le Guen and Che 45 , Reference Wang, Huo and Shi 48 – Reference D’Inca, Kloareg and Gras-Le Guen 50 ). This reduction of exchange surface is crucial because of its role in processing dietary nutrients into available molecules and regulating the flux of antigenic material( Reference D’Inca, Kloareg and Gras-Le Guen 50 ). Further proteome analysis of the jejunum of LBW piglets revealed that the expression of key proteins involved in major biological processes such as absorption, digestion and transport of nutrients, cell apoptosis, nutrient metabolism, cellular redox homoeostasis and stress response were affected by birth weight during the first 21 d of life( Reference Wang, Chen and Li 46 , Reference Wang, Wu and Lin 47 , Reference D’Inca, Kloareg and Gras-Le Guen 50 ). Moreover, He et al.( Reference He, Ren and Kong 34 ) have reported that IUGR piglets have a distinct metabolic status compared with NBW piglets at 21 d of age, with changes related to lipogenesis, lipid oxidation, energy supply and utilisation, amino acid and protein metabolism, and antioxidant ability.
Gut bacterial colonisation of LBW piglets is also altered during the early days of life( Reference D’Inca, Gras-Le Guen and Che 45 ). For example, preterm LBW infants had reduced population levels of strict anaerobes such as Bifidobacterium and Bacteroides, and had a high prevalence of Staphylococcus, Enterobacteriaceae, Enterococcaceae and other lactic acid bacteria including the genus Lactobacillus in a low-diversity bacterial ecosystem( Reference Jacquot, Neveu and Aujoulat 51 – Reference Arboleya, Solis and Fernandez 53 ). In summary, these results all suggest that LBW newborns are associated with both immediate and long-term altered intestinal adaptation during the neonatal period.
Possible nutritional interventions for improving growth and health of low birth weight infants
Appropriate patterns of nutrient delivery
Parenteral nutrition
During the initial days or weeks of life, GIT impairment in LBW infants usually induces an inability to tolerate enteral feedings, which can be referred to as ‘feeding intolerance’, as indicated by increased gastric residuals, abdominal distension and/or emesis( Reference Fanaro 54 ). In this case, parenteral nutrition (PN), supplying essential nutrients either by a central or peripheral intravenous injection( Reference Ziegler, Thureen and Carlson 55 , Reference Heird 56 ), is considered a useful strategy to avoid feeding intolerance until full enteral nutrition (EN) can be adopted. PN should be started either immediately after birth or within the first 2 h of life( Reference Prince and Groh-Wargo 57 ). In addition, Valentine et al.( Reference Valentine, Fernandez and Rogers 58 ) reported that when PN started within the first 24 h of life, these small infants had shorter durations of PN administration and achieved full enteral feedings earlier compared with those that started PN more than 24 h after birth. The early use of PN has been shown to reduce postnatal growth failure and mortality, prevent N imbalance, prevent essential fatty acid and trace mineral deficiency, and improve growth and neurodevelopmental outcomes( Reference Patel and Bhatia 59 ), without the associated short-term metabolic or clinical side effects( Reference Moyses, Johnson and Leaf 60 ). In early intravenous nutrition for very low birth weight (VLBW; birth weight <1500 g) infants, the recommended administrations of amino acids, glucose and lipids are 2·5–3·5, 12–18 and 3 g/kg per d, respectively. The reasonable levels of Na, K, Cl, Ca, P and Mg are assumed to be 3–5 , 1–2, 2–3, 75–90, 60–67 and 7·5–10·5 mg/kg per d, respectively( Reference De Curtis and Rigo 61 ).
In total PN of infants, glucose is the most widely used intravenous carbohydrate for neonates because it is a main energy source and is readily available to the brain. Many other non-glucose carbohydrates such as fructose, galactose, sorbitol, glycerol and ethanol have been used as sources of carbohydrates. However, their effects are considered inferior to glucose( Reference Patel and Bhatia 59 ). Commercial lipid emulsions generally include soyabean oil, mixtures of olive and soyabean oils and mixtures of olive and fish oils( Reference Patel and Bhatia 59 ); the fish oil-based lipid emulsion may be a more effective source( Reference Gura, Lee and Valim 62 , Reference de Meijer, Gura and Le 63 ).
Enteral nutrition
Enteral feeding is the preferred pattern of nutrition provision for LBW infants. Human milk is not only the paramount EN source but also a supplier of various bioactive compounds to infants, which play vital roles in regulating GIT development and protection from infections( Reference Wu, Wang and Wu 64 ). However, it can be accompanied by side effects including feeding intolerance and other aforementioned complications. A combination of PN and EN is commonly practiced after birth until full EN can be accomplished( Reference Prince and Groh-Wargo 57 ). Once full feedings have been established and PN has been terminated, EN is fully responsible for providing all nutrients to support normal growth( Reference Ziegler 65 ). Considering that the maternal milk from preterm mothers provides inadequate quantities of nutrients( Reference Kuschel and Harding 66 ), especially protein( Reference De Curtis and Rigo 61 , Reference Kuschel and Harding 66 ), targeted human-milk fortifiers are added to either the maternal or the donor milk to meet the nutritional needs of rapidly growing LBW infants( Reference Groh-Wargo and Sapsford 67 ). It can be advised to supply a fortifier content of up to 1·3 g of protein/100 ml for these small infants, beginning from the time they can tolerate 50–70 ml/kg per d of milk( Reference De Curtis and Rigo 61 ). Tolerance formulas including soya-protein, protein-hydrolysate and amino-acid-based formulas can be utilised to promote feeding tolerance in LBW neonates( Reference Prince and Groh-Wargo 57 ).
In preterm formulas, as a reference for LBW infants, the carbohydrate source is a combination of lactose and sucrose( Reference Hay and Hendrickson 68 ), considering the inadequate lactase activity of the GIT( Reference Tan-Dy and Ohlsson 69 ). A part of the sucrose or lactose in formula could be replaced by easily digestible glucose polymers to ensure low osmolality of formulas( Reference De Curtis and Rigo 61 , Reference Hay and Hendrickson 68 ). The protein sources are whey and casein derived from cows’ milk, and sometimes soya protein. In addition, the fat source is a mixture of vegetable oils containing 30–40 % medium-chain TAG in lipids to improve fat absorption( Reference De Curtis and Rigo 61 , Reference Hay and Hendrickson 68 ).
Continuous and intermittent bolus feeding
Compared with continuous feeding, intermittent bolus feeding is considered to be more effective in shortening the time to establish full enteral feeding, improving feed tolerance and accelerating weight gain in premature LBW infants( Reference Dollberg, Kuint and Mazkereth 70 , Reference Schanler, Shulman and Lau 71 ). Using the newborn NBW pig as a model, it has been demonstrated that intermittent bolus feeding increases protein synthesis to a greater extent than continuous feeding by improving activation of amino acids and insulin-induced translation initiation( Reference Gazzaneo, Suryawan and Orellana 72 , Reference El-Kadi, Gazzaneo and Suryawan 73 ). On the other hand, contradictory results have also been reported( Reference Premji and Chessell 74 , Reference Dsilna, Christensson and Alfredsson 75 ). This observation in pigs would also be useful to provide some implications for clinical practice in LBW infants. Dsilna et al.( Reference Dsilna, Christensson and Gustafsson 76 ) demonstrated that continuous feeding could contribute to reduced behavioural stress response compared with intermittent bolus feeding among premature VLBW infants. In spite of these data, it is still difficult to recommend either method of gavage feeding, and more trials in LBW infants or animals are needed to evaluate the benefits and side effects of both methods.
Macronutrients
LBW infants are generally fed high-protein/energy formulas to improve their growth rates and N retention( Reference Kashyap, Schulze and Forsyth 77 – Reference Thureen and Heird 81 ). For instance, Fenton et al. ( Reference Fenton, Premji and Al-Wassia 82 ) demonstrated that a higher protein intake (≥3·0 but <4·0 g/kg BW per d) could accelerate weight gain and N accretion in formula-fed hospitalised infants, which indicated by an enhancement of postnatal growth. Providing a nutrient-rich formula to preterm infants (20 % energy-enriched and 40–60 % more protein and minerals than term formula) increased body weight, length and head circumference growth during the first 18 months( Reference Young, Morgan and McCormick 83 ). Similar results were shown in a piglet study in which the LBW piglets had a comparable growth rate with the normal piglets when fed a high-protein content diet between 7 and 28 d of life( Reference Morise, Seve and Mace 84 ). Han( Reference Han 85 ) also reported that when LBW piglets received a high-nutrition-level diet with all nutrients at about 1·5-fold those of the control, they had markedly increased weight gain of the psoas major muscle. This was probably due to the enhanced gene expressions of IGF-I, IGF-I receptor and mammalian target of rapamycin (mTOR).
Another widely used strategy to promote the growth of LBW infants is increasing energy intake. However, it has been stated that the major effect of higher energy intake (594 v. 502 kJ/d (142 v. 120 kcal/d)) in LBW infants is an increase in fat accretion( Reference Kashyap, Schulze and Forsyth 77 ). Studies in experimental animals show that protein/energy malnutrition can affect the utilisation and deposition of protein and fat( Reference Weinkove, Weinkove and Pimstone 86 ). High nutrient intake in IUGR piglets led to abnormal immune function during the suckling period by lowering serum concentrations of cytokines such as TNF and IL-1β. Moreover, intense nutrient intake induces excessive oxidative stress( Reference Feillet-Coudray, Sutra and Fouret 87 – Reference Devaraj, Wang-Polagruto and Polagruto 89 ), which can impose a further burden on the immature antioxidant system in LBW offspring( Reference Hracsko, Orvos and Novak 90 – Reference Wang, Degroote and Van Ginneken 92 ).
Considering the potential risk for inducing metabolic problems, the intensive nutrition strategy might not be a proper nutritional intervention for LBW infants. Research on rats showed that postnatal energy restriction can be considered as an effective strategy to alleviate the metabolic syndromes in LBW offspring, like obesity and diabetes( Reference Desai, Gayle and Babu 93 – Reference Garg, Thamotharan and Dai 95 ). Che et al. ( Reference Che, Xuan and Hu 96 ) reported that restricting the intake of 7-d-old IUGR piglets (approximately 70 % of the control’s intake) can improve the antioxidant system at the expense of maintaining a low growth rate in the neonatal phase( Reference Hu, Liu and Yan 97 ).
It is worth noting that protein:energy ratio (PER) of diets will be important for the relative composition of net protein and fat stored in tissues while considering the different nutritional requirements of growth and maintenance( Reference Hay, Brown and Denne 98 ). In this case, therefore, an appropriate PER in infant formulas is necessary to maintain a positive N balance, ensure protein utilisation and prevent excessive fat storage( Reference Prince and Groh-Wargo 57 , Reference Kashyap 99 ). The PER of mature human milk ranges from 1·3–1·8 g/418 kJ (100 kcal), whereas the ratio ranges from 2·2–2·5 g/418 kJ (100 kcal) in standard formulas for normal infants( Reference Raiha, Fazzolari-Nesci and Cajozzo 100 ). However, a higher PER, approximately 3 g/418 kJ (100 kcal), is recommended for preterm LBW infants( Reference Prince and Groh-Wargo 57 , Reference Su 101 ), which would lead to increased lean mass with relatively decreased fat deposition( Reference Kashyap 99 ). Once protein intake is adequate to meet the needs of lean body accretion, excessive energy will primarily lead to relatively more fat deposition( Reference Hay, Brown and Denne 98 ), and then increase the risk for adult obesity( Reference Belfort, Gillman and Buka 102 ). Taken together, the optimal constitution and appropriate PER levels in formulas designed specifically for these LBW infants can be useful in achieving the desired growth rate while avoiding extra stress on their defective metabolic system.
Functional components applied to optimise nutritional support for low birth weights infants
Functional amino acids and derivatives
Epidemiological and metabolic studies have provided novel insights into alterations in the amino acid profiles in LBW fetuses and neonates. Reduction in the concentrations of the arginine (Arg) family of amino acids (Arg, proline, citrulline, glutamine (Gln)) have been reported in the umbilical vein plasma of fetuses or in the plasma of LBW newborns in humans( Reference Sanz-Cortes, Carbajo and Crispi 103 – Reference Tea, Le Gall and Küster 105 ), pigs( Reference Wang, Zhang and Zhou 11 , Reference He, Ren and Kong 34 , Reference Lin, Liu and Feng 106 , Reference Wu, Bazer and Johnson 107 ) and rats( Reference Alexandre-Gouabau, Courant and Le Gall 108 ). Branched-chain amino acids (BCAA) (leucine, isoleucine, valine) also show lower levels in the plasma of fetuses and neonates born with LBW( Reference He, Ren and Kong 34 , Reference Sanz-Cortes, Carbajo and Crispi 103 , Reference Tea, Le Gall and Küster 105 , Reference Lin, Liu and Feng 106 ). All of the above implicate that these functional amino acids could be used as potential biomarkers for designing effective strategies to improve the outcomes in LBW neonates.
l-Arginine
l-Arginine (Arg) is an essential amino acid for the maximal growth of young mammals( Reference Flynn, Meininger and Haynes 109 – Reference Southern and Baker 111 ). It is an essential precursor for the biological synthesis of important molecules such as glutamate, ornithine, proline, polyamines, creatinine, nitric oxide and agmatine( Reference Flynn, Meininger and Haynes 109 , Reference Wu, Knabe and Kim 112 – Reference Wu and Morris 114 ).
A systematic review derived from eighty-three human studies reported that the concentration of Arg was about 0·94 g/l in preterm transitional milk( Reference Zhang, Adelman and Rai 115 ), and the mean milk yield of preterm mothers at 6 weeks postpartum was approximately 541(SD460·9) ml/d( Reference Hill, Aldag and Chatterton 116 ). Therefore, provision of Arg from milk is far from adequate to meet the high requirements of growth and metabolic function in preterm newborns( Reference Tomlinson, Rafii and Sgro 117 ). Dietary supplementation of 0·6 % Arg to LBW piglets from 7 to 14 d of age resulted in increased average daily gain and daily DM( Reference Wang, Zhang and Zhou 11 ). The incidence of diarrhoea dropped by 61·5 %, accompanied by increased small intestine weight and mucosal villus height( Reference Wang, Zhang and Zhou 11 ). Notably, Arg supplementation was found to effectively reduce the incidence of necrotising enterocolitis (NEC) in premature infants with LBW( Reference Neu 118 – Reference Shah and Shah 120 ). In addition, a recent study observed daily dosing of Arg (145·0 mg/kg body weight per administration) to LBW piglets, from 1 to 17 d after birth, had an ability to revert some of the abnormalities involving amino acids, energy, lipid and nucleotide (NT) metabolism caused by LBW( Reference Getty, Almeida and Baratta 121 ). However, these effects appear to be independent of the growth-regulation system because reduced growth rate is still present in these piglets( Reference Getty, Almeida and Baratta 121 ). Therefore, optimisation of Arg dosage and timing should be investigated to achieve desirable effects in LBW neonates.
Glutamine
Gln plays vital roles in maintaining several important functions such as energy metabolism, immune response and cell signalling as well as the synthesis of Arg, NT, hexosamines and glycoproteins( Reference Wu and Morris 114 , Reference Wang, Qiao and Yin 122 – Reference Wu 125 ). The amount of Gln obtained from milk is far from sufficient in newborns to support the Gln requirements for growth( Reference Wu 126 ). Different studies have all shown that Gln supplementation (0·3 g/kg per d) in formulas can increase the growth rate in LBW infants( Reference Korkmaz, Yurdakok and Yigit 127 ), improve the tolerance to enteral feeding and decrease morbidity during the 1st month( Reference Neu, Roig and Meetze 128 – Reference Vaughn, Thomas and Clark 130 ). A previous study in IUGR piglets found that oral administration of Gln at 0·5 g/kg of body weight twice per d from days 0 to 21 of age could reduce amino acid oxidation, increase growth and reduce preweaning mortality( Reference Wu, Bazer and Johnson 107 ). Moreover, oral Gln (1 g/kg body weight every 12 h) during days 0 to 14 post weaning in LBW pigs induces an enhanced intestinal immunity by increasing heat shock protein 70 expression as well as the suppression of NF-κB( Reference Zhong, Li and Huang 131 ). Collectively, Gln is likely an effective amino acid to enhance the survival, immune response and postnatal growth of LBW infants.
Branched-chain amino acids
There are three amino acids recognised as BCAA: valine, isoleucine and leucine. They play vital parts in protein synthesis in skeletal muscle. The mechanisms that BCAA are involved in include the mTOR signalling pathway, decreasing rates of protein degradation( Reference Davis, Fiorotto and Burrin 132 ) and regulating cell differentiation and apoptosis( Reference Lei, Feng and Zhang 133 ). Importantly, BCAA are substrates for the synthesis of glutamate and Arg in the metabolic pathway of amino acids( Reference Rezaei, Wang and Wu 134 , Reference Yuan, Zhu and Shi 135 ). A recent study using weaned LBW pigs as a model showed that dietary supplementation with 0·35 % l-leucine improved the growth rate of LBW piglets by increasing the levels of phosphorylated mTOR and ribosomal S6 kinase 1, and also by reducing muscle atrophy F-box protein( Reference Xu, Bai and He 136 ). Similar results have also been observed in fetal( Reference Zheng, Huang and Cao 137 ) and postnatal LBW rats( Reference Teodoro, Vianna and Torres-Leal 138 ). Obviously, BCAA, particularly leucine, may have a potential effect on accelerating the early growth rate and protein synthesis in LBW offspring. Given the fact that BCAA play a major role in stimulating protein synthesis in skeletal muscle, the optimal BCAA supplement dosage should depend on whether it provides enough for maximum protein deposition in the skeletal muscle of LBW neonates.
l-Carnitine
l-Carnitine (3-hydroxy-4-N,N,N-trimethylaminobutyric acid) is a water-soluble quaternary amine essential for a series of indispensable functions in the intermediary metabolism of mammals. l-Carnitine serves as a shuttling molecule for the transportation of activated long-chain fatty acids from the cytosol into the mitochondrial matrix to produce energy( Reference Keller, Ringseis and Priebe 139 ). Preterm infants with LBW problems are at a high risk for carnitine deficiency because of an immature biosynthetic ability, insufficient transplacental transportation and exogenous supplementation( Reference Whitfield, Smith and Sollohub 140 ). A previous investigation implied that routine parenteral supplementation with l-carnitine had no demonstrable effect on growth, apnoea or length in LBW infants( Reference Whitfield, Smith and Sollohub 140 ). Nevertheless, evidence suggests that in piglets, adding l-carnitine to diets could accelerate the rates of protein and fat accretion( Reference Keller, Ringseis and Priebe 139 , Reference Owen, Nelssen and Goodband 141 , Reference Owen, Nelssen and Goodband 142 ) by stimulating IGF-I signalling, while inhibiting the expression of pro-apoptotic and atrophy-related genes or genes of the ubiquitin–proteasome system( Reference Keller, Ringseis and Priebe 139 , Reference Keller, Ringseis and Koc 143 ). In particular, Losel et al.( Reference Losel, Kalbe and Rehfeldt 144 ) reported that an oral administration of l-carnitine (400 mg/d) from 7 to 27 d of age resulted in an intensified myogenic proliferation in LBW suckling pigs, which demonstrated that increasing enteral l-carnitine could be considered as an effective method to improve growth outcomes of LBW neonates. Therefore, supplemental l-carnitine is recommended in LBW infants, but further clinical trials are needed to focus on the safe dosage and outcomes of l-carnitine usage.
PUFA
The major long-chain PUFA (LC-PUFA) such as arachidonic acid (ARA, 20 : 4n-6), EPA (20 : 5n-3) and DHA (22 : 6n-3) are essential nutrients for maintaining health, cognition and development during fetal as well as early postnatal life in humans( Reference Isganaitis, Jimenez-Chillaron and Woo 22 , Reference Innis 145 ). Previous evidence illustrated that neonates, including the LBW ones, can synthesise DHA and ARA from essential fatty acids such as linolenic acid (n-3 LC-PUFA) and linoleic acid (n-6 LC-PUFA)( Reference Salem, Wegher and Mena 146 – Reference Carnielli, Wattimena and Luijendijk 148 ). However, the LC-PUFA synthesis rate in these LBW infants was not enough to meet the requirement for optimal growth and development( Reference Salem, Wegher and Mena 146 , Reference Fleith and Clandinin 149 , Reference Uauy and Mena 150 ). The decreased proportion of ARA to linoleic acid as well as DHA to α-linolenic acid was seen in the fetal plasma of IUGR pregnancies( Reference Cetin, Giovannini and Alvino 151 ), indicating a deficit in LC-PUFA profiles in the IUGR fetus. Therefore, dietary LC-PUFA supplementation can be considered as an efficient strategy to counteract the defective fatty acid composition of IUGR neonates. In a clinical trial, preterm infants fed with a formula containing DHA (0·16 %)+ARA (0·42 %) for the 1st year had higher lean body mass and reduced fat mass at 1 year of age( Reference Groh-Wargo, Jacobs and Auestad 152 ). A systematic review reported that ω-3 LC-PUFA supplementation was found to reduce the incidence of NEC in extremely preterm infants (≤32 weeks)( Reference Zhang, Lavoie and Lacaze-Masmonteil 153 ). Notably, supplementing fish oil (rich in EPA and DHA) has been considered as a potential nutritional intervention to facilitate catch-up growth with normal body composition in preterm infants( Reference Yeung 154 ), because of its effect on suppressing the differentiation of fat cells and fat accumulation( Reference Azain 155 , Reference Ruzickova, Rossmeisl and Prazak 156 ). In addition, EPA and DHA play a key part in mediating inflammatory response, which can in turn improve insulin sensitivity( Reference Clifton and Nestel 157 , Reference Das 158 ).
Nucleotides
NT are a group of bioactive agents regulating nearly all biochemical processes including transferring chemical energy, biosynthetic pathways and coenzyme components( Reference Sauer, Mosenthin and Bauer 159 ). NT account for approximately 20 % of the natural non-protein fractions in milk( Reference Uauy 160 ) and play important roles in optimising intestinal and immunological function( Reference Sauer, Eklund and Bauer 161 ). De novo synthesis, salvage pathways and daily food are sources of NT in mammals( Reference Che, Hu and Liu 162 ). Cells of the intestinal mucosa have a limited capability for de novo synthesis( Reference Savaiano and Clifford 163 ). In a rapid growth stage, exogenous NT would become essential nutrients for optimal function especially when the mucosa has already been damaged, which is typically seen in LBW neonates. Recently, research using LBW pigs as a model showed that when LBW piglets received NT-supplemented formula from 7 to 28 d of age, intestinal villus height and lactase and maltase activity were improved, which led to a better growth rate( Reference Che, Hu and Liu 162 ).
Vitamins and minerals
The augmented intakes of Ca, P, trace elements and vitamins in LBW infants are significantly beneficial in improving postnatal growth outcomes. For instance, bone mineralisation is obviously higher in preterm infants when fed Ca- and P-enriched formulas( Reference Lapillonne, Salle and Glorieux 164 ) compared with conventional preterm formulas. LBW formulas could be designed with much higher contents of Na and K in order to compensate for reduced kidney function( Reference Hay and Hendrickson 68 , Reference Silverwood, Pierce and Hardy 165 ). Because of a high incidence of vitamin A, D and E deficiency in VLBW infants( Reference Kositamongkol, Suthutvoravut and Chongviriyaphan 166 , Reference Agarwal, Virmani and Jaipal 167 ), addition of these vitamins is essential in parenteral or enteral feedings. Vitamin A supplementation in LBW neonates has the potential to improve lung and visual development( Reference Mactier and Weaver 168 ) as well as to reduce death and retinopathy( Reference Darlow and Graham 169 ). Similarly, a systematic review showed that, as an antioxidant agent, vitamin E supplementation in preterm infants was able to reduce the risk for retinopathy and intracranial haemorrhage( Reference Brion, Bell and Raghuveer 170 ). The addition of vitamin D contributed to higher levels of serum vitamin D( Reference Natarajan, Sankar and Agarwal 171 ), higher Ca retention( Reference Senterre, Putet and Salle 172 ), and therefore lower incidence of bone hypomineralisation( Reference Mathur, Saini and Mishra 173 ). More clinical trials are still required to determine the optimal regimens for these nutrients.
Probiotics and prebiotics
Compared with adults, the enteric microbiota of infants is extremely unstable in terms of composition because of the fast development of the GIT( Reference Palmer, Bik and DiGiulio 174 ). Lactobacillus supplementation remarkably depresses feeding intolerance( Reference Sari, Dizdar and Oguz 175 , Reference Oncel, Arayici and Sari 176 ) and increases growth velocity( Reference Hartel, Pagel and Rupp 177 ) in VLBW infants. Supplementing single-strain Bifidobacterium in early life is able to improve body weight gain of LBW infants( Reference Hartel, Pagel and Rupp 177 , Reference Yamasaki, Totsu and Uchiyama 178 ) and promote Bifidobacterium colonisation( Reference Li, Shimizu and Hosaka 179 ). Furthermore, a meta-analysis of twenty randomised and controlled trials showed reduced risks for NEC and mortality were achieved by Bifidobacterium or Lactobacillus supplementation in preterm VLBW infants( Reference Wang, Dong and Zhu 180 ). Accordingly, the use of nutritional intervention to foster a beneficial intestinal microbiota composition can be a good strategy to prevent potential health problems( Reference Marques, Wall and Ross 181 ). Multi-strain probiotic combinations have shown greater efficacy than single-strain probiotic supplementation for GIT and immune outcomes in animals and humans( Reference Chapman, Gibson and Rowland 182 ). A combination of Bifidobacterium and Lactobacillus supplementation is proven to reduce the occurrence of NEC( Reference Braga, da Silva and de Lira 183 , Reference Saengtawesin, Tangpolkaiwalsak and Kanjanapattankul 184 ), mortality( Reference Lin, Hsu and Chen 185 ) and to increase the growth rate( Reference Al-Hosni, Duenas and Hawk 186 ) in LBW infants.
Supplementation with prebiotics in formulas could promote the growth of beneficial microbes in LBW infants. Generally, prebiotics consist of one or more carbohydrates such as inulin, lactulose, fructo-oligosaccharides (FOS) or galacto-oligosaccharides (GOS). For example, supplementing the preterm formula with a mixture of FOS and GOS may stimulate the growth of Bifidobacteria ( Reference Boehm, Lidestri and Casetta 187 , Reference Westerbeek, van Elburg and van den Berg 188 ), whereas lactulose addition increases the growth of Lactobacillus ( Reference Riskin, Hochwald and Bader 189 ). Moreover, Dilli et al.( Reference Dilli, Aydin and Fettah 190 ) reported that adding a synbiotic (Bifidobacterium lactis plus inulin) to breastmilk or formula could decrease the risk for NEC in VLBW infants. Similar results were observed by supplementing another kind of synbiotic containing Lactobacillus, Bifidobacterium and FOS( Reference Nandhini, Biswal and Adhisivam 191 ). Overall, we can speculate that supplementing probiotics and prebiotics alone, or as a combination, might be useful for optimising the intestinal micro-ecology, GIT health and further stimulating the growth outcomes of LBW offspring.
Hormone regulation
Leptin
Leptin is a 16-kDa cytokine mainly produced by the adipose tissue and is responsible for the central regulation of food intake and energy balance as well as for enhancing the postnatal maturation of numerous peripheral organs. Its deficiency will lead to morbid obesity and diabetes as well as various neuroendocrine anomalies( Reference Gautron and Elmquist 192 – Reference Attig, Djiane and Gertler 194 ). Evidence illustrates that human newborns with LBW show significantly lower serum leptin levels than do normal newborns( Reference Jaquet, Leger and Levy-Marchal 195 ). Studies using pigs as a model confirmed that this reduction may be a result of abnormal hypothalamic distribution of leptin receptors( Reference Attig, Djiane and Gertler 194 ) and lower expressions of the leptin gene in perineal adipose tissue( Reference Morise, Seve and Mace 196 ). In piglets, leptin injection (0·5 mg/kg) from days 2 to 10 of age can improve body weight and lean mass of LBW piglets by increasing organ weights, like that of the pancreas, liver and lung( Reference Attig, Djiane and Gertler 194 ). Interestingly, leptin treatment can normalise the composition of the adipose tissue by decreasing white-adipocyte density while increasing the individual adipocyte size( Reference Attig, Djiane and Gertler 194 ). These findings suggest that leptin treatment in early postnatal life has the potential to correct abnormal fat deposition in LBW offspring through regulation of body weight gain, organ development and body composition.
Insulin and insulin-like growth factor-I
In neonatal miniature pigs, oral insulin administration can stimulate ileal growth and enhance the specific activities of lactase and maltase( Reference Shulman 197 ). Several studies demonstrated that an extra addition of IGF-I in infant formula might improve GIT growth and function in newborn colostrum-deprived pigs( Reference Xu, Mellor and Birtles 198 – Reference Burrin, Wester and Davis 200 ). Infusion of IGF-1 (4 μg/h) to IUGR piglets aged 3–10 d evidently increased the circulating concentration of IGF-I and the rate of weight gain by approximately 10 %, because of the increase in protein and fat accretion levels( Reference Schoknecht, Ebner and Skottner 201 ). The potential mechanisms of this enhancement contain a stimulated cell proliferation in the GIT( Reference Xu, Mellor and Birtles 198 ), increased brush-border disaccharidase activity( Reference Houle, Schroeder and Odle 199 ) and increased intestinal weight and ileal villus height( Reference Burrin, Wester and Davis 200 ). In VLBW infants, continuous insulin infusion (0·05 units/kg per h) from 24 h after birth to 7 d of age led to an increase in IGF-I concentrations in the serum at 28 d and therefore, an increase in both body weight and head circumference( Reference Habbout, Li and Rochette 21 ). Similar insulin therapy (0·025 units/kg per h) reduced the incidence of hyperglycaemia in VLBW infants( Reference Beardsall, Ogilvy-Stuart and Frystyk 202 ). On the basis of this information, IGF-I and insulin could be two potential growth promoters in LBW offspring during the early postnatal period.
Conclusions and perspectives
In addition to the reduced growth rate after birth, LBW infants are also born with abnormalities in hormone regulation and nutrient utilisation, all of which might have adverse effects on lifelong health. Considering the physiological defects of LBW infants, nutritional interventions during the neonatal stage should focus on promoting the postnatal growth rate without causing potential metabolic problems. Available nutrition strategies based on the preceding information have been summarised in Table 2. First, the best pattern of nutrition supply is transitioning from a combination of PN and EN to full enteral feeding during the early life of LBW infants. The benefits and shortcomings of continuous v. intermittent bolus feeding needs further consideration. Next, the optimal protein and energy contents in the formulas for LBW infants, based on an appropriate PER, should be adopted for preventing metabolic problems caused by high protein or energy levels. Specifically, some functional components (see Table 2), such as functional amino acids and its derivatives, LC-PUFA, NT, vitamins and minerals, probiotics and prebiotics as well as hormonal manipulators could be used as additives in the formulas and HMF. They could also be used as parenteral nutrients to compensate for congenital physiological defects and improve postnatal outcomes in LBW infants. We believe that a combination of these functional components will contribute to an extra-positive effect on LBW neonates’ health and growth. More research is also needed to better understand the molecular and cellular mechanisms by which the mentioned nutrients regulate the short- and long-term growth of LBW infants. Another suggestion for further studies would be identifying the differences in metabolism and nutritional requirements between preterm and term LBW infants, and then designing the corresponding HMF and formulas for these two types of neonates. In addition, when the use of LBW infants is limited, a piglet model would be more suitable for the investigation of clinical nutrition because of high similarity in terms of anatomy, genetics and physiology( Reference Fritz, Desai and Shah 203 , Reference Meurens, Summerfield and Nauwynck 204 ), compared with rodent models.
Table 2 Examples of nutrition strategies for improving growth, development and health of low birth weight (LBW) infants
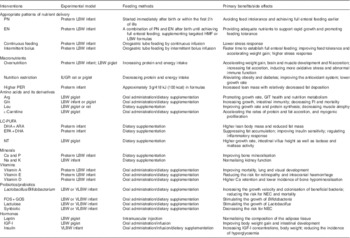
PN, parenteral nutrition; EN, enteral nutrition; HMF, human-milk fortifiers; IUGR: intra-uterine growth restriction; PER, protein:energy ratio; Arg, arginine; GIT, gastrointestinal tract; Gln, glutamine; LC-PUFA, long-chain PUFA; ARA, arachidonic acid; NT, nucleotides; VLBW, very low birth weight, <1500 g; NEC, necrotising enterocolitis; FOS, fructo-oligosaccharides; GOS, galacto-oligosaccharides; ; IGF-I, insulin-like growth factor.
Acknowledgements
The authors thank Mr Daniel Long and Dr Ying Wang for assistance in manuscript preparation.
This work was supported by the National Natural Science Foundation of China (nos 31272449, 31422052, 31572412 and 31630074), the National Key Research and Development Program of China (2016YFD0500506), the ‘111’ Project (B16044), Jinxinnong University Animal Science Developmental Foundation, Hunan Co-Innovation Center of Animal Production Safety (CICAPS) and Agriculture and Food Research Initiative Competitive Grants (2014-67015-21770, 2015-67015-23276 and 2016-67015-24958) from the United States Department of Agriculture (USDA) National Institute of Food and Agriculture, and Texas A&M AgriLife Research (H-8200).
The authors’ contributions are as follows: J. W. designed the framework of the draft. N. L. and W. W. collected the literature and drafted the manuscript. J. W., G. W. and W. W. revised and finalised the draft.
The authors declare that there are no conflicts of interest.





