Trauma has become one of the leading causes of global mortality (10 %), whereas blood loss remains the major contributor to mortality after trauma(Reference Peden and Hyder1, Reference Mock, Joshipura and Goosen2). Systemic and local effects of resuscitated blood loss frequently include the systemic inflammatory response syndrome that may lead to the development of dysfunction or even failure of multiple organs(Reference Baue, Durham and Faist3, Reference Bogner, Keil and Kanz4). Systemic hypotension (low-flow hypoxia) followed by resuscitation compromises the integrity of several organs at the level of the microcirculation, an effect that is mediated – at least in part – via the activation of monocytes and polymorphonuclear leucocytes (PMNL) with subsequent release of inflammatory cytokines. In the clinical setting, a high activation state of PMNL is associated with the systemic inflammatory response syndrome(Reference Moore, Sauaia and Moore5, Reference Hietbrink, Koenderman and Rijkers6). Haemorrhage/resuscitation (H/R)-induced pre-activation or ‘priming’ of PMNL is an essential step for neutrophils to enhance their functional responses to pro-inflammatory cytokines and reactive oxygen species(Reference Hietbrink, Koenderman and Rijkers6, Reference Partrick, Moore and Moore7). Liver injury is associated with an increased PMNL accumulation in the liver following H/R(Reference Lehnert, Arteel and Smutney8, Reference Relja, Schwestka and Lee9). Furthermore, H/R increases the expression of endothelial adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) in the liver(Reference van Meurs, Wulfert and Knol10). Moreover, hepatic and serum IL-6 levels were elevated after H/R(Reference Relja, Schwestka and Lee9). IL-6-deficient mice did not show enhanced PMNL infiltration into the liver, increased hepatic necrosis and organ damage as opposed to wild-type mice following H/R(Reference Meng, Dyer and Billiar11). NF-κB was activated in the liver following H/R(Reference Meng, Dyer and Billiar11). Previous studies have shown that NF-κB activation up-regulated the gene expression of pro-inflammatory mediators that are associated with PMNL infiltration in the liver after H/R. These pro-inflammatory mediators include ICAM-1 and IL-6(Reference Roebuck and Finnegan12, Reference Gaddipati, Sundar and Calemine13). Activation and regulation of NF-κB are controlled by IκB proteins, which mask the nuclear localisation signal of NF-κB, thereby inhibiting its nuclear translocation(Reference Baeuerle and Baltimore14). Phosphorylation of inhibitor κBα (IκBα) at its regulatory N-terminus on serine 32 and 36 leads to subsequent conjugation with ubiquitin, and thereby degradation of the inhibitory unit with the following activation of the transcription factor NF-κB(Reference Baeuerle and Baltimore14).
Green tea (Camellia sinensis) extracts (GTE) contain high levels of polyphenols consisting mostly of catechin and catechin gallates including epicatechin, gallocatechin, epigallocatechin, epicatechin gallate and epigallocatechin gallate (EGCG) that are efficient free radical and singlet oxygen scavengers(Reference Hara15–Reference Chu, Wang and Chu18). GTE reduced hepatic ischaemia/reperfusion (I/R)-induced injury in various in vivo models of acute inflammation(Reference Zhong, Connor and Froh19–Reference Zhong, Froh and Connor21). Hepatoprotective effects of GTE after I/R were attributed to its antioxidative and anti-inflammatory properties(Reference Zhong, Connor and Froh19, Reference Zhong, Froh and Connor21). A recent study has reported that green tea consumption is associated with a reduced risk of total stroke incidence, cerebral infarction and cerebral haemorrhage in humans(Reference Tanabe, Suzuki and Aizawa22). However, the effects of green tea in H/R-induced liver injury as well as the underlying mechanism have not been studied yet. The objective of the present study was to evaluate the effects of GTE and the role of NF-κB in the pathogenesis of liver injury induced by H/R, and their effects on PMNL infiltration and ICAM-1 expression.
Materials and methods
Animals and experimental model
Female Lewis rats (n 24) weighing 180–250 g were obtained from Harlan (Borchen, Germany). At 5 d before H/R, rats received daily a diet containing 0·1 % polyphenolic extracts from C. sinensis (GTE; Sunphenon 90LB (low bitter), >80 % polyphenols and >80 % catechins, which contain >40 % EGCG, kindly provided by H. S.) or a regular chow diet (control, ctrl). After an overnight fast, rats were anaesthetised with isofluran (1·5 %), and the right carotid artery, the right femoral artery and the left jugular vein were cannulated with polyethylene tubing. Then, shock was induced over 5 min by withdrawing blood from the right carotid artery into a heparinised syringe (0·02 mg) to a mean blood pressure of 30–32 mmHg. Systemic blood pressure was monitored in the right femoral artery using a blood pressure analyser (BPA 400; Digi-Med, Louisville, KY, USA). Constant pressure was maintained by further withdrawal of small volumes of blood as necessary for 60 min. Then, rats were resuscitated by transfusion of 60 % of the shed blood and twice the shed blood volume as Ringer's lactate solution with a syringe pump over 30 min via the left jugular vein. After the end of resuscitation, the catheters were removed, the vessels were occluded and the wounds were closed. At 2 h after the end of resuscitation, the animals were re-anaesthetised. The cava was punctured, blood was collected and tissue was harvested. The liver was flushed with normal saline, infused and fixed with 10 % buffered formalin through the portal vein, embedded in paraffin and subsequently sectioned and stained with haematoxylin–eosin. Sham-operated animals underwent the same surgical procedures, but haemorrhage was not carried out (sham_ctrl and sham_GTE, six animals per group). Body temperature was measured in the colon and maintained at 37°C throughout the experiment with a heating pad. Animal protocols were approved by the Veterinary Department of the Regional Council in Darmstadt, Germany.
Examination of tissue injury
Sera were stored at − 80°C for later analysis of alanine aminotransferase using the Vitros 250 device (Ortho-Clinical Diagnostics, Neckargemünd, Germany). Determination of the histological damage was performed by an independent examiner who allocated the haematoxylin–eosin-stained liver sections to various experimental groups in a blinded manner as published previously(Reference Meng, Dyer and Billiar23).
Detection of polymorphonuclear leucocytes
Analysis of the hepatic infiltration with PMNL was performed by chloroacetate esterase staining (4 % pararosanilin, 4 % sodium nitrite and naphthol solution) for 30 min at room temperature (RT). Sections were counterstained with haematoxylin. PMNL infiltration was determined by counting the number of chloroacetate esterase-positive cells in a total of twenty-five high-power (400 × ) fields/liver section per rat in a blinded manner as described previously(Reference Lehnert, Relja and Sun-Young24). Data from each tissue section were pooled to determine mean values.
Quantification of cytokine levels
Concentrations of serum IL-6 were determined using a Quantikine Rat IL-6 ELISA kit of R&D Systems according to the manufacturer's instructions (Wiesbaden-Nordenstadt, Germany). ELISA ninety-six-well microtitre plates were analysed using a microplate reader (Bio-Tek Ceres UV900C; Bio-Tek, Winooski, VT, USA).
Western blotting
Liver tissue was homogenised in lysis buffer at 4°C, followed by centrifugation for 30 min at 4°C at 20 000 g. Supernatants were stored at − 80°C for later analysis. Lysates (50 μg protein) were separated by electrophoresis on 12 % polyacrylamide SDS gels and transferred to nitrocellulose membranes (Amersham-Buchler, Braunschweig, Germany). IκBα (phospho) was detected using a rabbit polyclonal IKB-α (phospho S32+S36) antibody (Abcam, Cambridge, UK). β-Actin, which served as a loading control, was determined using an anti-β-actin antibody (Sigma, Taufkirchen, Germany). Blots were blocked (10 % non-fat dry milk in 1 mm-Tris, 150 mm-NaCl, pH 7·4) for 1 h, incubated 1 h at RT with primary antibody (diluted according to the manufacturer's instructions in blocking buffer with 0·5 % Tween 20 and 0·5 % bovine serum albumin) and then incubated for 1 h with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:1000 in blocking buffer with 0·5 % Tween 20 and 0·5 % bovine serum albumin at RT. Proteins were detected with ECL™ Western blot detection reagents (GE Healthcare, Munich, Germany). Films were digitised, and the integrated density of individual bands was determined using MultiAnalyst software (Bio-Rad, Munich, Germany). The amount of protein expression was normalised to β-actin by densitometric measurements using the same software.
Staining of intercellular adhesion molecule-1
Cryosectioned liver samples (5 μm) were air-dried overnight, fixed in acetone for 10 min at RT and air-dried for 60 min at RT. Sections were washed with PBS and water. Then, endogenous peroxidase activity was blocked with H2O2 (for 10 min, RT, ready-to-use solution; Labvision, Fremont, CA, USA). Liver sections were washed and incubated with blocking solution (2 % bovine serum albumin in PBS) for 1 h at RT. Mouse anti-rat CD54 monoclonal antibody (BD Pharmingen, Heidelberg, Germany) diluted 1:150 in antibody dilution buffer (AB Dilution Buffer; Dako, Hamburg, Germany) containing 1 % bovine serum albumin was used as the primary antibody (overnight incubation, 4°C). Anti-mouse horseradish peroxidase-linked secondary antibody (30 min, RT, Histofine; Nichirei Biosciences, Tokyo, Japan) and diaminobenzidine (Labvision, Fremont, CA, USA) were used to detect specific binding. Sections were counterstained with haematoxylin. The immunostained tissue sections were captured at 400 × and analysed in a blinded manner. The extent of labelling in the liver lobule was defined as the percentage of the field area within a preset colour range determined by the software (Adobe Photoshop 7.0; Adobe Systems Inc., San Jose, CA, USA). Data from each tissue section (ten fields/section) were pooled to determine mean values.
Statistical analysis
Differences between groups were determined by one-way ANOVA using a multiple comparison procedure (Student–Newman–Keuls) and by ANOVA on ranks as appropriate. A P value of < 0·05 was considered significant. Data are expressed as means with their standard errors.
Results
Cell damage after haemorrhage and resuscitation in the liver
Serum alanine aminotransferase as a marker of hepatocellular damage increased to 1385 (sem 191) IU/l at 2 h after H/R compared with 97 (sem 14) IU/l after the sham operation (P < 0·001; Fig. 1(e)). In GTE pre-treated animals, alanine aminotransferase release decreased by 72 % after H/R (385 (sem 63) IU/l, P < 0·01; Fig. 1(e)) compared with the control group after H/R. GTE did not affect alanine aminotransferase levels after the sham operation compared with the sham_ctrl group (Fig. 1(e)).

Fig. 1 Green tea extract (GTE) reduces histological liver necrosis and serum alanine transaminase (ALT) levels at 2 h after resuscitation. At 5 d before haemorrhagic shock and resuscitation (H/R), rats received chow containing GTE (0·1 %, haemorrhage/resuscitation (H/R)_GTE) or regular chow (H/R_ctrl (control)). Sham-operated (■) animals underwent the same surgical procedures, but H/R (□) was not carried out (sham_GTE and sham_ctrl, respectively). Representative haematoxylin and eosin-stained liver lobes from vehicle-treated ((a) after sham operation and (c) after H/R) and GTE-treated ((b) after sham operation and (d) after H/R) rats are shown. Scale bar is 200 µm. Blood samples were collected at 2 h after resuscitation for the measurement of (e) serum ALT. Values are means with standard errors represented by vertical bars (six animals per group). * Mean values were significantly different from those of the sham-operated animals (P < 0·001). † Mean values were significantly different from those of the shock control group (P < 0·01).
Histopathological changes in liver tissue after haemorrhage and resuscitation
Evaluation of hepatic necrosis at 2 h after H/R in histological sections showed confluent, large areas of coagulative necrosis as indicated by cellular enlargement and dissolution in the H/R_ctrl group (Fig. 1(a–c)). Tissue damage occurred predominantly in the pericentral areas of liver sections. These changes were detected neither in control sham-operated nor in GTE pre-treated sham-operated rats, respectively (Fig. 1(a–c)). GTE chow substantially decreased hepatic necrosis after H/R (Fig. 1(d)) compared with vehicle-treated rats (Fig. 1(c)).
Systemic pro-inflammatory changes after haemorrhage and resuscitation – serum IL-6 levels
The systemic immune response, a common sequela after resuscitated blood loss, was determined by serum IL-6 levels. Compared with the sham_ctrl group (15 (sem 12) pg/ml), serum IL-6 levels increased in the vehicle-treated group after H/R (1641 (sem 535) pg/ml, P < 0·05; Fig. 2). This increase after H/R was prevented by GTE chow (420 (sem 124) pg/ml, P < 0·05; Fig. 2). After the sham operation, serum IL-6 levels did not differ between the control and GTE groups (15 (sem 12) and 12 (sem 9) pg/ml, respectively; Fig. 2). These results indicate that systemic pro-inflammatory changes after H/R were substantially attenuated by GTE pre-treatment.
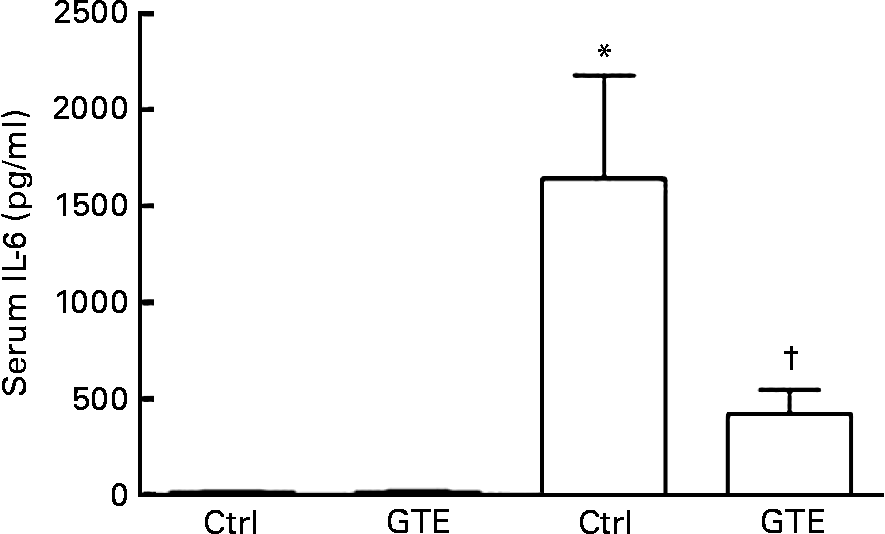
Fig. 2 Green tea extract (GTE) decreases serum IL-6 levels after haemorrhage and resuscitation in rats. Blood samples were collected at 2 h after resuscitation. Serum concentration of IL-6 was determined by ELISA. Ctrl denotes vehicle treatment, GTE denotes GTE administration. Values are means with standard errors represented by vertical bars (six animals per group). * Mean values were significantly different compared from those of the sham-operated animals (■, P < 0·05). † Mean values were significantly different compared from those of the H/R_ctrl group (□, n 6, P < 0·05).
Local pro-inflammatory changes after haemorrhage and resuscitation – intercellular adhesion molecule expression and hepatic neutrophil accumulation
The expression of ICAM-1, represented by strong brown staining of endothelial cells in the control group, increased 8·4-fold compared with the sham-operated control rats (76 (sem 2) and 9 (sem 2) %, respectively, P < 0·05; Fig. 3(a) and (c)). Hepatic ICAM-1 expression was markedly reduced in the group of animals fed with GTE chow (19 (sem 2) %; Fig. 3(d)).

Fig. 3 Green tea extract (GTE) decreases intercellular adhesion molecule-1 (ICAM-1) expression and hepatic neutrophil infiltration after haemorrhage and resuscitation in rats. ICAM-1 (CD54)-stained, representative liver sections from sham-operated rats are shown: (a) regular chow, sham_ctrl (control)) and (b) 0·1 % GTE chow, sham_GTE. ICAM-1-stained, representative liver sections from rats after haemorrhage/resuscitation (H/R) are shown: (c) regular chow, H/R_ctrl and (d) GTE chow, H/R_GTE. Scale bar is 80 μm. Liver sections were stained for (e) chloroacetate esterase (CAE) cytochemistry. ■, Sham; □, H/R. Positively stained neutrophils were counted in twenty-five randomly chosen high-power fields (at 400 × ) of each liver specimen and blindly scored for the number of intensely staining CAE-positive PMNL. Ctrl denotes vehicle treatment, GTE denotes GTE administration. Values are means with standard errors represented by vertical bars (six animals per group). * Mean values were significantly different from those of the sham-operated animals (P < 0·05). † Mean values were significantly different from those of the H/R_ctrl group (n 6, P < 0·05).
Hepatic neutrophil infiltration increased to 8 (sem 1) cells/high-power field at 2 h after resuscitation compared with the sham operation (1 (sem 1) cells/high-power field, P < 0·05; Fig. 3(e)). GTE significantly diminished hepatic neutrophil infiltration after H/R (3 (sem 1) cells/high-power field, P < 0·05; Fig. 3(e)).
These results indicate that local pro-inflammatory changes after H/R were markedly reduced by GTE chow administration.
Western blot analysis of the NF-κB inhibitor IκBα after haemorrhagic shock and resuscitation
To analyse the mechanism influenced by GTE, detection of phosphorylated IκBα was performed by Western blot in liver homogenates collected at 2 h after resuscitation. Densitometric analysis of protein content related to β-actin content showed an increase in phosphorylated IκBα after H/R of 47 (sem 4) % compared with 6 (sem 1) % in sham-operated rats (P < 0·05, Fig. 4(a) and (b)). GTE administration prevented the increase in the amount of phosphorylated IκBα protein compared with vehicle-treated control rats after H/R (12 (sem 4) %, P < 0·05; Fig. 4(a) and (b)). These results indicate that H/R induced NF-κB activation, an effect that was strongly inhibited by GTE pre-treatment before H/R.

Fig. 4 Green tea extract (GTE) modifies IκBα phosphorylation after haemorrhage and resuscitation. Vehicle (control, ctrl)- or GTE-treated rats were subjected to haemorrhage/resuscitation (H/R, □) or a sham (■) operation. At 2 h after the end of resuscitation, liver tissue was harvested and Western blotting was performed. (a) Lanes 1–6 were liver protein extracts from rats after the sham operation (sham, lanes 1–3: ctrl rats; sham, lanes 4–6: GTE-treated rats) or haemorrhage/resuscitation (H/R, lanes 7–10: ctrl rats; H/R, lanes 11–14: GTE-treated rats). (b) Densitometric measurements after normalisation to β-actin staining are plotted. Ctrl denotes vehicle treatment, GTE denotes GTE administration. Values are means with standard errors represented by vertical bars (six animals per group); representative gel from three experiments is shown. * Mean values were significantly different from those of the sham-operated animals (P < 0·05), represented by vertical bars. † Mean values were significantly different from those of the ctrl H/R group, represented by vertical bars (P < 0·05, n 6 (representative gel from three experiments)). IκBα (p), IκBα (phospho).
Discussion
In the present study, haemorrhage followed by resuscitation induced liver injury, the release of pro-inflammatory cytokine IL-6, the enhanced expression of ICAM-1 as well as hepatic infiltration with PMNL (Figs. 1–3). These changes were associated with IκBα activation, suggesting that the activation of NF-κB is involved in the pathogenesis of liver damage induced by H/R (Fig. 4). In addition, administration of GTE with its high content of polyphenols prevents liver injury, the release of systemic pro-inflammatory cytokine IL-6, neutrophil infiltration and ICAM-1 expression after H/R (Figs. 1–3). These beneficial, anti-inflammatory effects are associated with decreased IκBα activation induced by GTE before H/R (Fig. 4). The present study demonstrates the anti-inflammatory and protective role of GTE under conditions of H/R, suggesting that GTE prevents liver damage in this model of acute inflammation through a NF-κB-dependent mechanism.
Previous studies have identified numerous mediators such as IL-6, TNF-α, ICAM-1, endothelin 1 and haem oxygenase-1 involved in the pathogenesis of liver damage induced by H/R(Reference Relja, Schwestka and Lee9, Reference Kubulus, Mathes and Reus25–Reference Yang, Hu and Chen27). Most of these mediators are regulated by NF-κB. The rapid response transcription factor NF-κB is maintained in the cytoplasm and consists of p65 and p50 subunits bound to an inhibitory protein IκB(Reference Baeuerle and Baltimore14). The phosphorylated IκB is tagged by ubiquitin for subsequent proteolytic degradation that enables the NF-κB complex to translocate into the nucleus(Reference Baeuerle and Baltimore14). In the nucleus, NF-κB transactivates target genes(Reference Jobin, Hellerbrand and Licato28, Reference Shakhov, Collart and Vassalli29). NF-κB is activated in the lung, heart and liver after haemorrhagic shock followed by resuscitation(Reference Hierholzer, Harbrecht and Menezes30, Reference Meldrum, Shenkar and Sheridan31). Consistent with these observations, we detected an activation of the NF-κB inhibitory IκBα unit in the liver after H/R (Fig. 4). Activated NF-κB induces the expression of pro-inflammatory mediators, such as IL-6(Reference Zingarelli, Sheehan and Wong32). Haemorrhage causes an oxidative stress response that activates hepatic NF-κB with subsequent IL-6 gene expression(Reference Gaddipati, Sundar and Calemine13). These results are further substantiated with the present study, where IκBα activation was accompanied by enhanced serum IL-6 levels (Figs. 2 and 4). The harmful role of IL-6 in H/R was demonstrated by IL-6 knockout mice undergoing H/R that were protected from hepatic neutrophil infiltration and liver injury(Reference Meng, Dyer and Billiar11). Cell adhesion molecule ICAM-1 is inducible by both NF-κB activation and inflammatory cytokines such as IL-1β and TNF-α(Reference Zingarelli, Sheehan and Wong32, Reference Wertheimer, Myers and Wallace33). Expression of ICAM-1 on hepatocytes correlates with the degree of hepatic inflammation(Reference Matsutani, Kang and Miyashita34). Transendothelial migration and the adherence of neutrophils to parenchymal cells require the expression of ICAM-1. Neutrophil infiltration decisively contributes to liver injury in models of H/R and hepatic I/R(Reference Jaeschke35). Resistance of ICAM-1-deficient mice to the lethal effects of a high dose of endotoxin correlated with a significant decrease in neutrophil infiltration in the liver(Reference Xu, Gonzalo and St Pierre36). In the present study, release of the pro-inflammatory cytokine IL-6 and ICAM-1 up-regulation may lead to an increased adhesion and infiltration with neutrophils into the injured liver, thereby enhancing liver damage after resuscitated blood loss.
In the present study, we used a GTE; Sunphenon 90LB. Major polyphenol species in the extract includes EGCG (>40 %). EGCG posseses the highest reducing potential among catechins. Previous studies have shown that various GTE increase antioxidant capacity in animals and humans under conditions of oxidative stress as demonstrated by electron-spin resonance spectroscopy or detection of oxidative damage to proteins, lipids or DNA(Reference Hara15–Reference Zhong, Connor and Froh19, Reference Zhong, Froh and Connor21). Frei & Higdon(Reference Frei and Higdon17) provided a detailed review of biomarkers for GTE efficiency. Beneficial effects of GTE are most probably due to polyphenols and their ability to scavenge free radical and singlet oxygen(Reference Hara15, Reference Zhao, Li and He16). Catechins administered orally to pregnant rats were also able to penetrate in all fetal organs, whereas EGCG had the highest level of uptake(Reference Chu, Wang and Chu18). The authors have suggested EGCG as a potential candidate for antioxidant supplementation of the fetus in utero (Reference Chu, Wang and Chu18). Feeding with a diet supplemented with 0·2 % Sunphenon BG (bitter epigallocatechin gallate) (91·3 % polyphenols and 76·6 % catechins, which contains 9·6 % epigallocatechin, 45·9 % EGCG, 5·3 % epicatechin, 8·6 % epicatechin gallate and others) enhanced aflatoxin B1 detoxification(Reference Tulayakul, Dong and Li37). The protective effect of another GTE, Sunphenon (2·9 % catechins, 16·5 % epigallocatechin, 21·3 % EGCG, 6·8 % epicatechin, 6·6 % epicatechin gallate, 12·8 % gallocatechin and others) in a dose-dependent manner on renal cell viability after FK-506-induced cytotoxicity has been reported previously(Reference Hisamura, Kojima-Yuasa and Kennedy38). Moreover, the authors have shown that EGCG and epigallocatechin also reduced the cytotoxicity, whereas epicatechin and catechins had no beneficial effects(Reference Hisamura, Kojima-Yuasa and Kennedy38). Besides the results referring to EGCG specifically, several studies have indicated that the combination of polyphenols is more efficacious than a single chemically defined polyphenol(Reference Zhong, Connor and Froh19, Reference Zhong, Froh and Connor21, Reference Guo, Zhao and Li39). Nevertheless, further studies are required to determine which component or combination of polyphenols is responsible for the beneficial effects. In hepatic I/R models, 0·1 % dietary GTE, Sunphenon DCF-1 (decaffeinated), containing 85 % polyphenols and 47·2 % EGCG, decreased free radical formation, TNF-α pro-inflammatory cytokine formation, NF-κB activation and liver injury(Reference Zhong, Froh and Connor21). However, the authors could not exclude the possibility that GTE inhibited NF-κB activation by a non-antioxidant mechanism(Reference Zhong, Froh and Connor21). Because H/R models an acute inflammatory condition as does hepatic I/R, the present study has been designed to test for the first time the hypothesis that 0·1 % GTE, Sunphenon 90LB, containing >80 % polyphenols and >40 % EGCG, will exert its protective, anti-inflammatory effects in this model of acute inflammation and to reveal the mechanisms of protection. In support with this hypothesis, H/R-induced liver injury accompanied by enhanced serum IL-6 levels, hepatic ICAM-1 expression and neutrophil infiltration was significantly prevented by GTE administration before H/R (Figs. 1–3). Several studies have attributed liver injury to both apoptotic and necrotic cell death after H/R(Reference Lehnert, Relja and Sun-Young24). Hepatocellular necrosis after hepatic warm I/R occurs partly due to reactive oxygen species from activated neutrophils(Reference Gujral, Bucci and Farhood40). Reduced hepatocellular necrosis after H/R by GTE is accompanied by reduced neutrophil infiltration (Figs. 1 and 3(e)). Furthermore, protective effects of GTE are associated with reduced hepatic activation of NF-κB via the stabilisation of IκBα (Fig. 4). Reduction of NF-κB activation down-regulates the expression of ICAM-1, which is accompanied by decreased neutrophil infiltration into the liver (Figs. 3 and 4). Although this down-regulation of activated NF-κB in the liver is associated with a blunted inflammatory response after H/R, the precise mechanism by which GTE treatment modulates NF-κB activation in the liver still remains unknown.
In conclusion, activation of NF-κB plays an important role in the pathogenesis of liver injury after H/R by the up-regulation of systemic IL-6, hepatic ICAM-1 expression and neutrophil infiltration. GTE reduced neutrophil infiltration, hepatic ICAM-1 expression, IL-6 release and liver damage by the inhibition of IκBα activation. Hence, the present results suggest for the first time hepatoprotective, anti-inflammatory effects of GTE in a model of H/R and, furthermore, the stabilisation of NF-κB as a potential cause for its beneficial effects.
Acknowledgements
We thank Kerstin Wilhelm and Minhong Wang for outstanding technical assistance and Heinz Schneider for providing our group with GTE. The present study was supported by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) MA 1119/3-3. The authors' contributions were as follows: B. R. was involved in the study design, experimental work, data interpretation and manuscript writing; E. T. and L. B. were involved with experimental and analytical aspects of the manuscript; H. S. provided scientific expertise on GTE; D. H. and I. M. provided scientific expertise and were involved in the study design and manuscript editing; M. L. conceptualised the study, and provided significant scientific advice and consultation throughout the study. The authors declare that they have no conflicts of interest.






