Dyslipidaemia is generally due to abnormal lipid metabolism, which results in increased concentrations of total cholesterol (TC), LDL, TAG and/or reduced HDL(Reference Mancini, Hegele and Leiter1). Clinical studies also suggest that this condition elevates the risk of CVD mortality by facilitating the onset and progression of atherosclerosis(Reference Peng, Luo and Ruan2). In fact, prolonged exposure to even moderately elevated cholesterol concentrations is known to increase the risk of CVD later in life(Reference Ashtary-Larky, Bagheri and Ghanavati3–Reference Asbaghi, Choghakhori and Ashtary-Larky5). Therefore, identifying effective interventions for the improvement of lipid profile is an important public health concern.
There are several approaches for controlling dyslipidaemia, including safe and secure methods such as dietary interventions, and unlike pharmacological therapies, they do not cause any long-term side effects(Reference Kelly6–Reference Asbaghi, Fouladvand and Moradi9). Interventions that improve dietary soluble fibre intake, in particular, have received considerable interest due to their hypolipidaemic effects and effectiveness in reducing the prescribed dose of statins(Reference Surampudi, Enkhmaa and Anuurad10–Reference Ballesteros, Cabrera and Saucedo13).
Guar gum is a rich source of soluble fibre(Reference Yoon, Chu and Juneja14) and is derived from the seeds of the Cyamopsis tetragonoloba plant(Reference Whistler and Hymowitz15). Numerous clinical trials have examined the effects of guar gum supplementation on various diseases, such as diabetes, irritable bowel syndrome and dyslipidaemia(Reference Aro, Uusitupa and Voutilainen16–Reference Groop, Aro and Stenman19). Animal studies suggest that guar gum supplementation has hypolipidaemic effects(Reference Saeed, Mosa-Al-Reza and Fatemeh20–Reference Frias and Sgarbieri22). While numerous human studies have been performed to investigate the effects of guar gum supplementation on lipid profile, results have been contradictory. Indeed, some studies have shown that guar gum supplementation effectively improves lipid profile(Reference Aro, Uusitupa and Voutilainen16,Reference Groop, Aro and Stenman19,Reference Khan, Khan and Mitchel23) , while others do not show such an effect(Reference Tuomilehto, Voutilainen and Huttunen24,Reference Makkonen, Simpanen and Saarikoski25) . Recently, a meta-analysis has been published on the topic by Wang et al. (Reference Wang, Pan and Guo26), which revealed that guar gum supplementation significantly decreased serum concentrations of LDL and TC but did not affect TAG and HDL. However, two critical limitations make it difficult to draw meaningful conclusions from their investigation. The first is the failure to incorporate six relevant studies on the topic(Reference Dall’Alba, Silva and Antonio18,Reference Groop, Aro and Stenman19,Reference Farrell, Owens and Tomkin27–Reference Ebeling, Yki-Järvinen and Aro30) that meet the inclusion criteria set forth by Wang et al. (Reference Wang, Pan and Guo26). The second limitation is that four studies without a placebo/control group were incorporated in the analysis(Reference Cicero, Derosa and Manca31–Reference Vaaler, Hanssen and Dahl-Jørgensen34), and therefore their outcomes may not account for some unspecific effects of observation and expectation (i.e. the Hawthorne and placebo effects). Consequently, the overall effects of guar gum supplementation on lipid profile remain unclear. Thus, we sought to conduct a systematic review, meta-regression and dose–response meta-analysis of randomised placebo-controlled trials (RCT) to quantitatively assess the effects of guar gum supplementation on lipid profile markers in adults based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines(Reference Stroup, Berlin and Morton35).
Experimental methods
Search strategy
We searched PubMed, Embase, Scopus and Web of Science databases from inception through September 2021, by using the following terms: Guar OR Guaran, which was paired with the following words: Intervention OR ‘Intervention Study’ OR ‘Intervention Studies’ OR ‘controlled trial’ OR randomised OR random OR randomly OR placebo OR ‘clinical trial’ OR Trial OR ‘randomized controlled trial’ OR ‘randomized clinical trial’ OR RCT OR blinded OR ‘double blind’ OR ‘double blinded’ OR trial OR ‘clinical trial’ OR trials OR ‘Pragmatic Clinical Trial’ OR ‘Cross-Over Studies’ OR ‘Cross-Over’ OR ‘Cross-Over Study’ OR parallel OR ‘parallel study’ OR ‘parallel trial’. There were no date and language restrictions included in each of the database searches. Additional studies not captured by our database search were retrieved via a manual search of references from the originally identified reviews and research reports.
Study selection and eligibility criteria
Two authors (MZ and OA) reviewed the titles, abstracts, references and full texts of relevant articles to select eligible studies. The inclusion criteria were as follows: (1) adult participants who had supplemented with guar gum for ≥ 2 weeks; (2) the studies had a control group where the only difference between the treatment and control groups was the supplementation of guar gum; (3) the trial reported effects on TC, TAG, LDL or HDL; (4) the use of an RCT design; and (5) guar gum not being administered as part of a multicomponent supplement in either the experimental or control group. The exclusion criteria were as follows: (1) trials that enrolled children or pregnant women; (2) not focusing on our outcomes; and (3) animal, review, conference and case-report studies.
Risk of bias
The quality of eligible studies was assessed using the Cochrane risk of bias tool for RCT(Reference Higgins, Altman and Gotzsche36). Two independent investigators (TM and MDM) completed this checklist for each included paper. Methodological features applied for assessment were as follows: (1) adequate sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective outcome reporting (reporting bias) and (7) other potential sources of bias. Based on the mentioned items, studies were classified in terms of bias into three groups: low risk, moderate risk and high risk of bias(Reference Higgins, Altman and Gotzsche36).
Data extraction
The following data were extracted from included studies by two authors (MZ and OA) independently: study characteristics (the first author, country, year of publication, study design, sample size in each group, study duration, intervention type and dose), participant characteristics (mean age, sex and BMI), as well as the mean and standard deviation of TC, LDL, HDL and TAG concentrations in pre-intervention and post-intervention. In this study, all values were converted to a milligram per decilitres (mg/dl).
Statistical analysis
We performed this meta-analysis using STATA statistical software (version 14; STATA Corp LP). Treatment effects were defined as the weighted mean differences (WMD) and 95 % CI and were determined using the random-effects model, following the DerSimonian and Laird method(Reference DerSimonian and Laird37). We calculated changes in TC, LDL, HDL and TAG concentrations between the intervention and control groups from baseline to the end of the intervention period, and the following formula was used to calculate sd change from sd baseline and final: sd 2 baseline + sd 2 final – (2 × R × sd baseline × sd final)(Reference Borenstein, Hedges and Higgins38). Pre-specified subgroup analyses were performed according to baseline serum lipid profile (TAG, TC, LDL and HDL), guar dosage (≤ 15 g/d v. > 15 g/d), duration of the intervention (≥ 12 weeks v. < 12 weeks) and diabetes status of participants (diabetic v. non-diabetic participants). Sensitivity analyses were performed to assess the stability of the results by removing one study at a time to identify the impact of individual studies on the pooled effect size. Funnel plots and Egger’s regression test were used to assess the publication bias. A P-value of < 0·05 was considered statistically significant in this trial unless otherwise specified(Reference Egger, Smith and Schneider39). The potential non-linear effects of guar dose (g/d) and intervention duration (weeks) were investigated using fractional polynomial modelling. Also, we enforced the meta-regression to differentiate the confounders and linear relations among the effect size and sample size, duration and intervention dosage(Reference Mitchell40).
Certainty assessment
The overall certainty of evidence across the studies was graded according to the GRADE guidelines (Grading of Recommendations Assessment, Development and Evaluation) Working Group. According to the corresponding evaluation criteria, the quality of evidence was classified into four categories: high, moderate, low and very low(Reference Gordon, Oxman and Vist41).
Results
Study selection
In the primary search, we detected a total of 1940 records, where 823 duplicates were identified and removed. After screening based on title and abstract, twenty-six articles were retained for further evaluation. Three articles were excluded because they did not report lipid profiles. Also, four other studies were excluded from the study because they did not have a control group. Subsequently, nineteen eligible studies were included in qualitative and quantitative synthesis (meta-analysis)(Reference Dall’Alba, Silva and Antonio18,Reference Groop, Aro and Stenman19,Reference Khan, Khan and Mitchel23–Reference Makkonen, Simpanen and Saarikoski25,Reference Peterson, Ellis and Baylis28–Reference Ebeling, Yki-Järvinen and Aro30,Reference Aro, Uusitupa and Voutilainen42–Reference Vuorinen-Markkola, Sinisalo and Koivisto51) (Fig. 1).
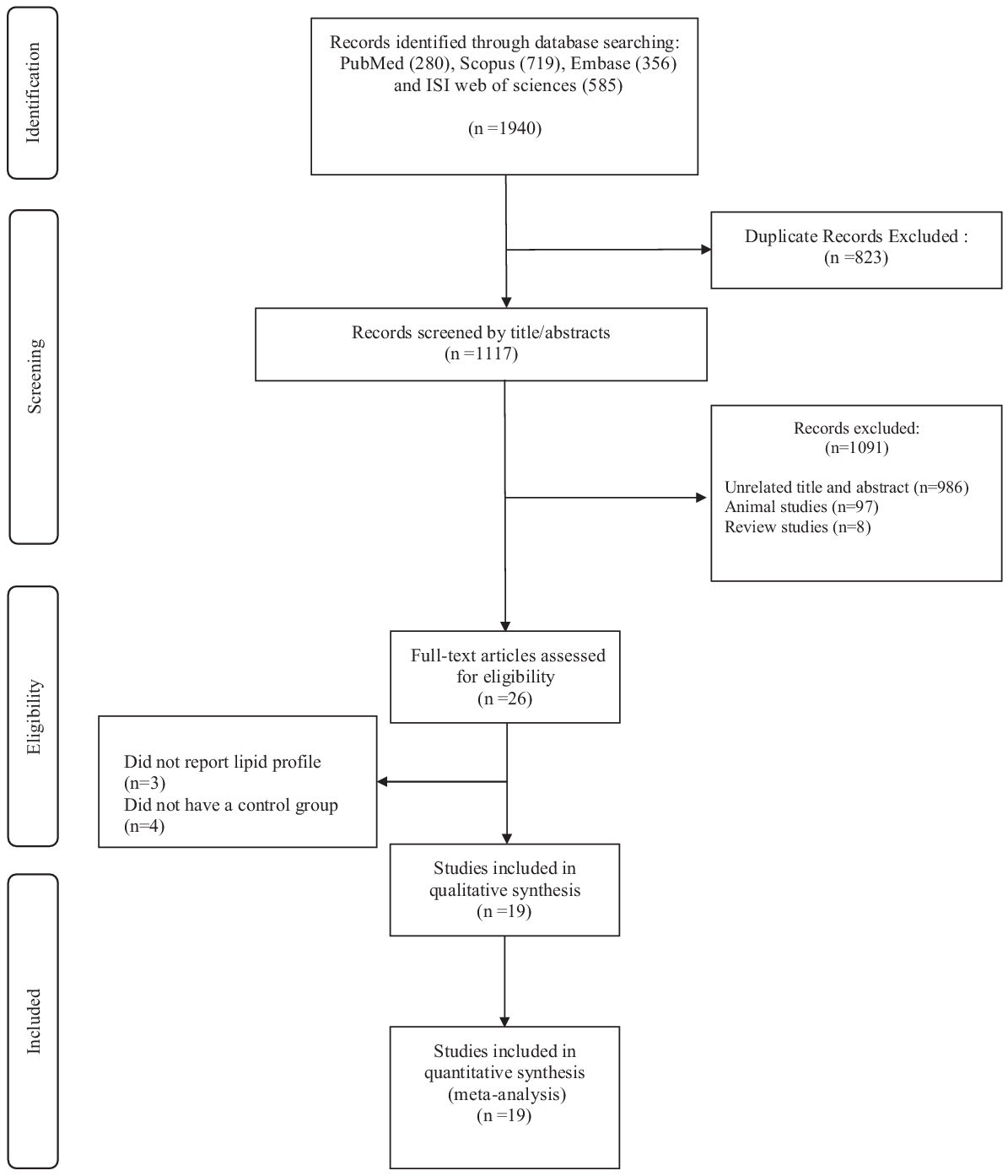
Fig. 1. Flow chart of study selection for inclusion trials in the systematic review.
Characteristics of the included studies
The general characteristics of the included studies are outlined in online Supplementary Table 1. The studies were published between 1980 and 2013 and were carried out in Finland(Reference Tuomilehto, Voutilainen and Huttunen24,Reference Makkonen, Simpanen and Saarikoski25,Reference Ebeling, Yki-Järvinen and Aro30,Reference Aro, Uusitupa and Voutilainen42,Reference Aro, Uusitupa and Voutilainen43,Reference Uusitupa, Siitonen and Savolainen47,Reference Tuomilehto, Silvasti and Aro49,Reference Vuorinen-Markkola, Sinisalo and Koivisto51,Reference Groop, Aro and Stenman52) , USA(Reference Khan, Khan and Mitchel23,Reference McIvor, Cummings and Van Duyn44,Reference Superko, Haskell and Sawrey-Kubicek48) , UK(Reference Peterson, Ellis and Baylis28,Reference Blake, Hamblett and Frost29,Reference Fuessl, Williams and Adrian45,Reference Lalor, Bhatnagar and Winocour50) , Ireland(Reference Farrell, Owens and Tomkin46), Brazil(Reference Dall’Alba, Silva and Antonio18) and Saudi Arabia(Reference Laajam, Jim and El-Bolbol53). The follow-up period ranged from 3 to 26 weeks, while the daily recommended dosage of guar gum varied between 8·3 and 39 g. All studies were conducted using both sexes, except for two trials performed exclusively on women(Reference Tuomilehto, Voutilainen and Huttunen24,Reference Makkonen, Simpanen and Saarikoski25) and two on men(Reference Aro, Uusitupa and Voutilainen43,Reference Superko, Haskell and Sawrey-Kubicek48) . The sample size in the included trials ranged from 14 to 58. Overall, 525 participants were amalgamated in these studies, of which 268 individuals were allocated to guar gum supplementation and 257 participants to the placebo group. The mean age of the participants ranged from 25 to 61·3 years old and included patients with hypercholesterolemia(Reference Tuomilehto, Voutilainen and Huttunen24,Reference Blake, Hamblett and Frost29,Reference Aro, Uusitupa and Voutilainen43,Reference Tuomilehto, Silvasti and Aro49,Reference Vuorinen-Markkola, Sinisalo and Koivisto51) , type 2 diabetes(Reference Dall’Alba, Silva and Antonio18,Reference Groop, Aro and Stenman19,Reference Peterson, Ellis and Baylis28,Reference Aro, Uusitupa and Voutilainen42,Reference McIvor, Cummings and Van Duyn44–Reference Uusitupa, Siitonen and Savolainen47,Reference Lalor, Bhatnagar and Winocour50) , type 1 diabetes(Reference Ebeling, Yki-Järvinen and Aro30), menopausal women(Reference Makkonen, Simpanen and Saarikoski25) and healthy participants(Reference Khan, Khan and Mitchel23,Reference Superko, Haskell and Sawrey-Kubicek48) (online Supplementary Table 1).
Effect of guar gum supplementation on TAG concentrations
Sixteen studies, including a total of 477 participants (case = 283 and control = 272), reported TAG as an outcome measure. Overall results from the random-effects model suggested that guar gum supplementation resulted in a non-significant decrease in TAG concentrations (WMD: −2·80 mg/dl, 95 % CI −10·05, 2·45, P = 0·233) (Fig. 2(a)). Heterogeneity was not observed between studies. Also, subgroup analysis demonstrated the same results (online Supplementary Table 3).
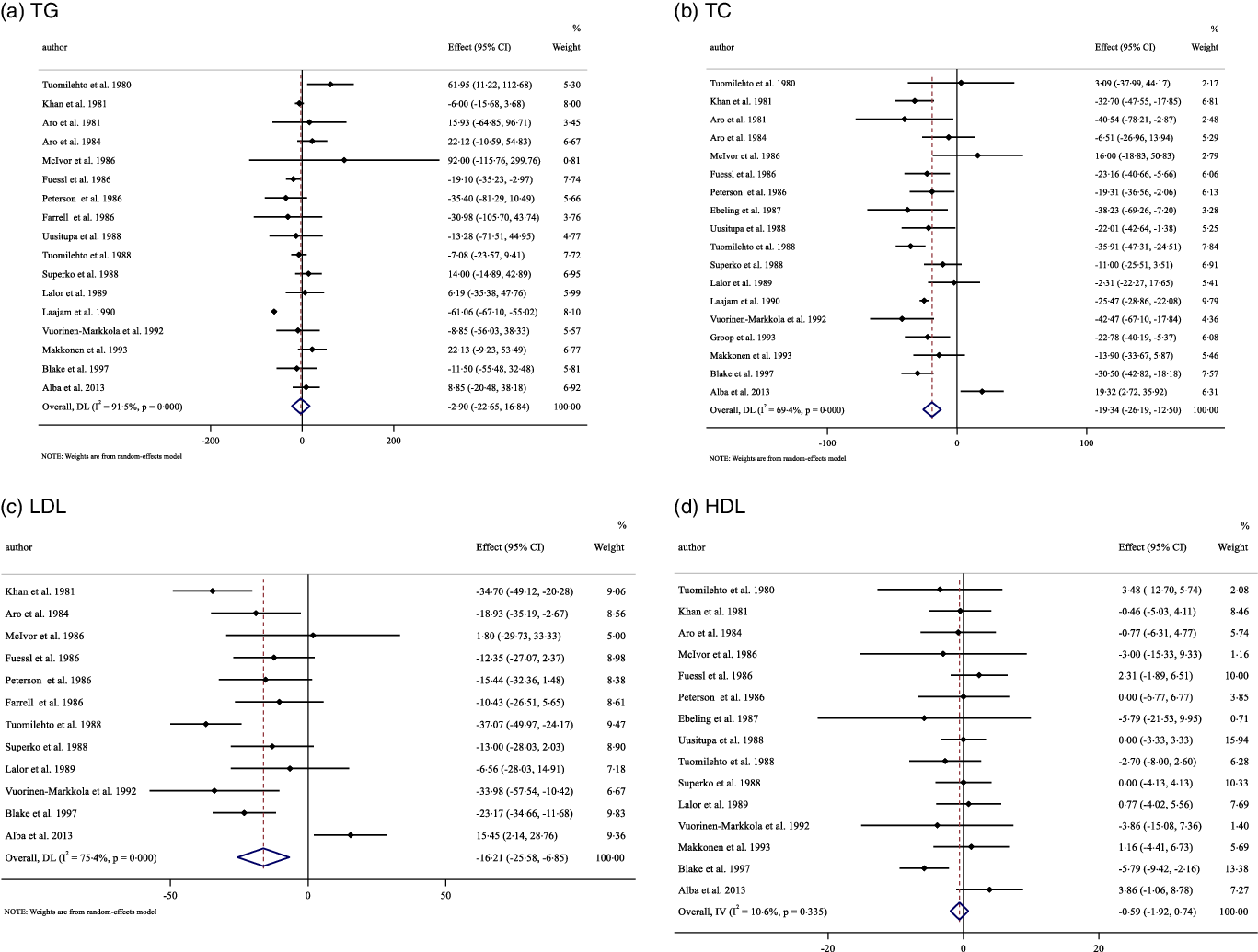
Fig. 2. Forest plot detailing weighted mean difference and 95 % CI for the effect of guar gum supplementation on: (a) TAG; (b) TC; (c) LDL-cholesterol and (d) HDL-cholesterol.
Effect of guar gum supplementation on total cholesterol concentrations
Pooling effect sizes from seventeen publications, including 499 participants (case = 289 and control = 288), we found that guar gum supplementation had a significant effect on TC concentrations compared with placebo (WMD: −19·34 mg/dl, 95 % CI −26·18, −12·49, P < 0·001), with considerable between-study heterogeneity (I2 = 69·4 %, P < 0·001) (Fig. 2(b)). Subgroup analysis based on guar gum dosage, baseline TC concentrations, trial duration and diabetes status revealed that these factors explained the heterogeneity. In addition, diabetes status is known as a source of heterogeneity (online Supplementary Table 3).
Effect of guar gum supplementation on LDL concentrations
The influence of guar gum supplementation on LDL concentrations was evaluated in twelve trials, including 369 participants (case = 190 and control = 179). The pooled estimates highlighted that in participants who supplemented with guar gum, LDL concentrations were significantly decreased compared with placebo (WMD: −16·19 mg/dl, 95 % CI −25·54, −6·83, P = 0·001). There was a significant between-study heterogeneity (I2 = 76·2 %, P < 0·001) (Fig. 2(c)). Subgroup analysis revealed that baseline LDL concentrations and diabetes status were sources of heterogeneity. In addition, the results remained significant when baseline LDL ≥ 130 mg/dl, trial duration < 12 or ≥ 12 weeks, and intervention dose < 15 g/d and in non-diabetic participants (online Supplementary Table 3).
Effect of guar gum supplementation on HDL concentrations
Overall, fifteen eligible studies, including a total of 441 participants (case = 226 and control = 215), examined the effect of guar gum supplementation on HDL concentrations. Combining their findings based on a random-effects model, we found that HDL concentrations did not significantly change after intervention (WMD: −0·59 mg/dl, 95 % CI −1·92, 0·73, P = 0·382) compared with the control group, with no significant between-study heterogeneity (Fig. 2(d)). Subgroup analysis showed that guar gum supplementation significantly increased HDL concentrations only when baseline HDL concentrations were ≥ 50 mg/dl (online Supplementary Table 3).
Publication bias
Evaluation of publication bias by visual inspection of funnel plot revealed no evidence of publication bias in the meta-analysis of guar gum supplementation on TAG, TC, LDL and HDL concentrations, respectively (Fig. 3(a)–(d)).
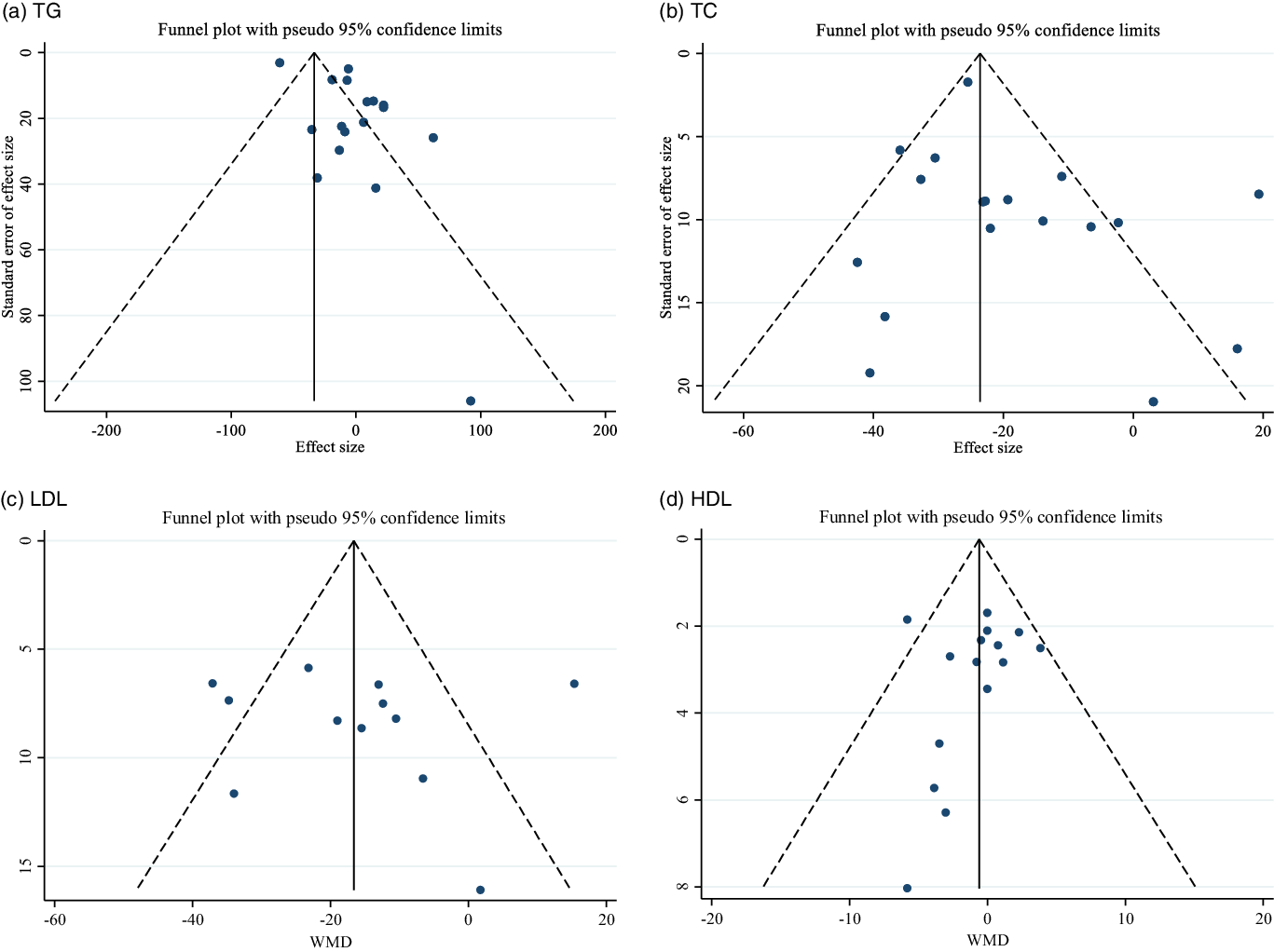
Fig. 3. Funnel plot for the effect of guar gum supplementation on; (a) TAG; (b) TC; (c) LDL-cholesterol and (d) HDL-cholesterol.
Quality assessment
All studies were low risk for random sequence generation, except for one study that was unclear(Reference Groop, Aro and Stenman19). None of the studies reported allocation concealment, and three of the studies had high risk(Reference Dall’Alba, Silva and Antonio18,Reference Groop, Aro and Stenman19,Reference Groop, Groop and Tötterman54) . All studies had a high risk of bias for other sources of bias. All studies had a low risk of bias regarding selective reporting except for seven studies(Reference Groop, Aro and Stenman19,Reference Tuomilehto, Voutilainen and Huttunen24,Reference Makkonen, Simpanen and Saarikoski25,Reference Ebeling, Yki-Järvinen and Aro30,Reference Aro, Uusitupa and Voutilainen42,Reference Farrell, Owens and Tomkin46,Reference Uusitupa, Siitonen and Savolainen47) . Moreover, all studies had a low risk of bias regarding blinding participants and personnel, except for three studies(Reference Groop, Aro and Stenman19,Reference Peterson, Ellis and Baylis28,Reference Superko, Haskell and Sawrey-Kubicek48) . Four studies had a low risk of bias regarding blinding outcome assessors(Reference Dall’Alba, Silva and Antonio18,Reference Groop, Aro and Stenman19,Reference Blake, Hamblett and Frost29,Reference Superko, Haskell and Sawrey-Kubicek48) (online Supplementary Table 2).
Grading of evidence
The GRADE profile for the certainty of the evidence is included in online Supplementary Table 4. Both TAG and HDL were regarded as moderate quality due to serious limitations in indirectness and imprecision. The evidence relating to TC was moderate quality because of serious limitations in indirectness and inconsistency. Evidence regarding LDL was identified as low quality, owing to very serious limitations in inconsistency and serious limitations in indirectness.
Linear meta-regression and non-linear relationship between dose and duration of intervention and changes in lipid profile
There was no linear (Figs. 4 and 5) and non-linear (Figs. 6 and 7) relationship between changes in dose and duration of intervention and changes in lipid profile. However, there was a significant non-linear relationship between changes in dose of guar supplementation and alteration in HDL (coefficiency = 1·496 724, P = 0·044).
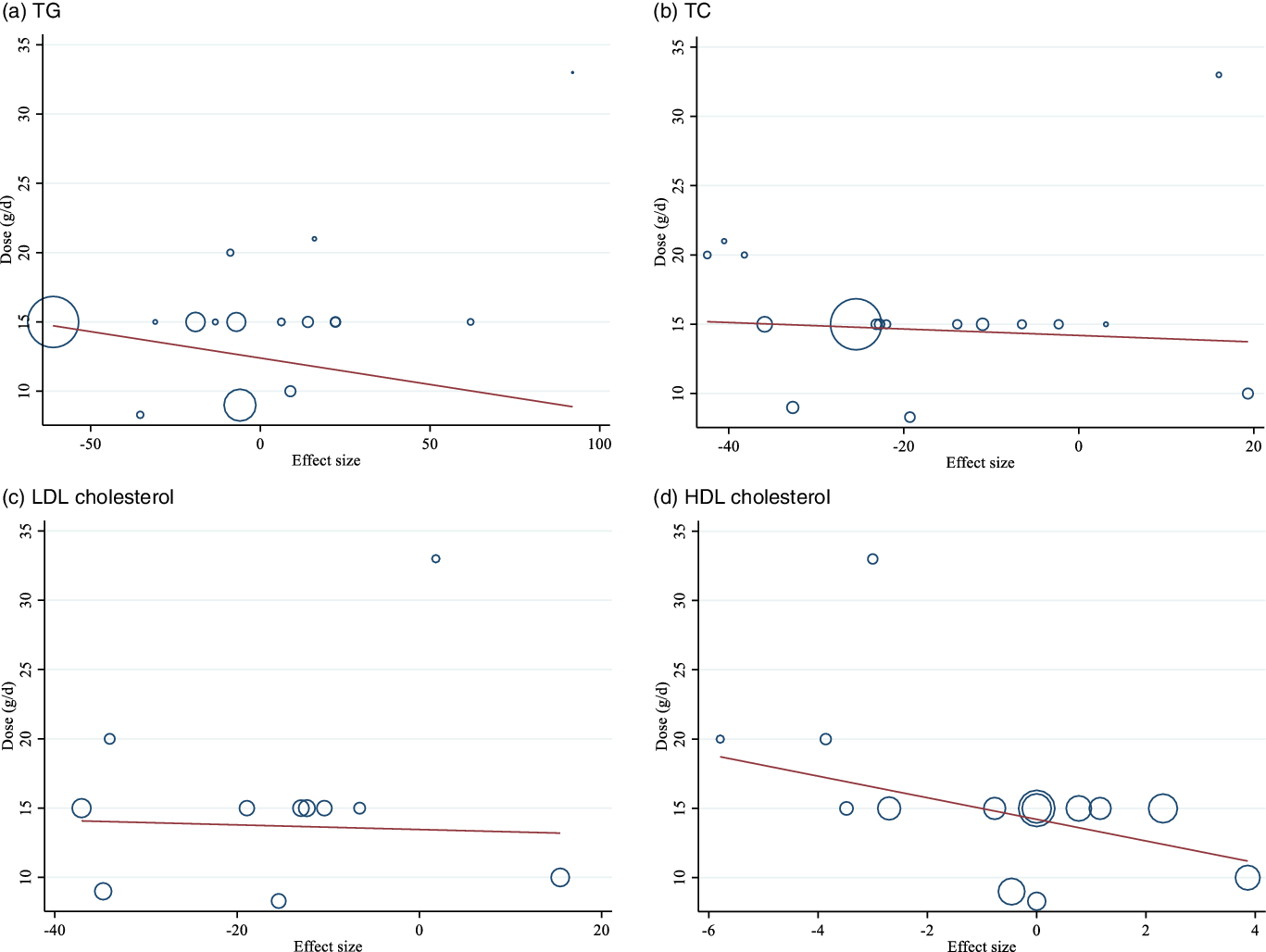
Fig. 4. Random-effects meta-regression plots of the association between dose of intervention (week) and weighted mean difference of: a) TAG; b) TC; c) LDL-cholesterol and d) HDL-cholesterol.
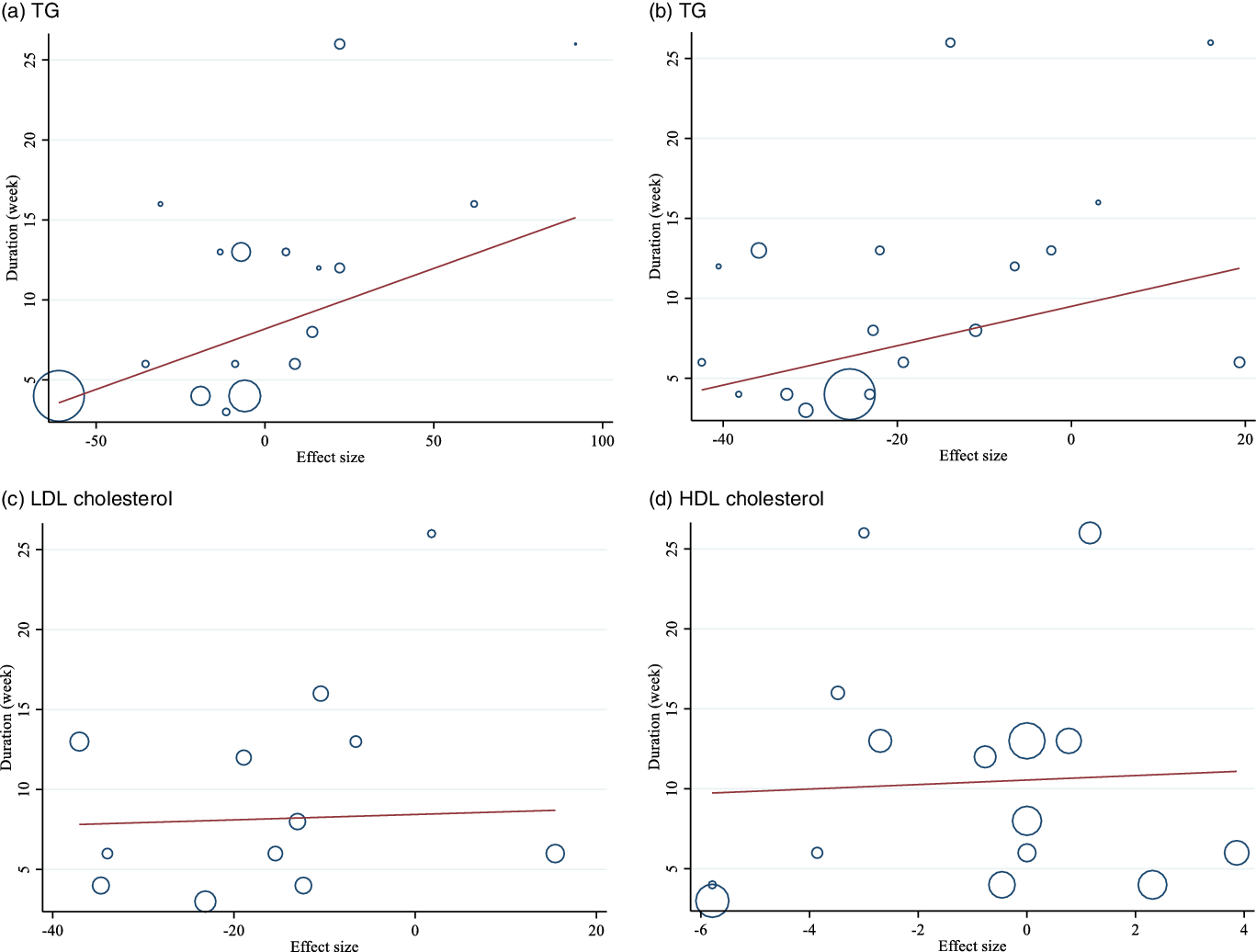
Fig. 5. Random-effects meta-regression plots of the association between duration of intervention (week) and weighted mean difference of: a) TAG; b) TC; c) LDL-cholesterol and d) HDL-cholesterol.

Fig. 6. Random-effects meta-regression plots of the association between dose of intervention (week) and weighted mean difference of: a) TAG; b) TC; c) LDL-cholesterol and d) HDL-cholesterol.
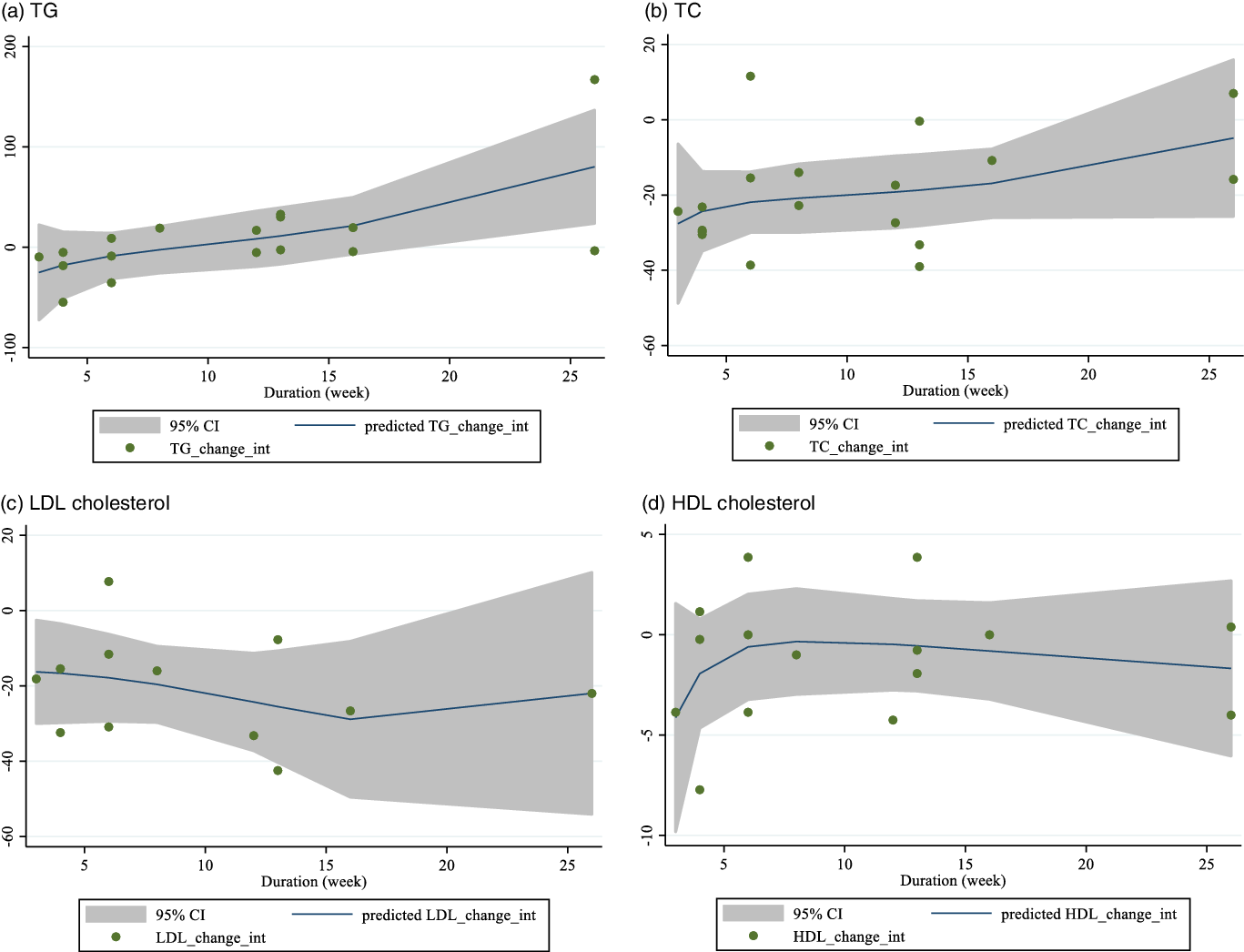
Fig. 7. Random-effects meta-regression plots of the association between duration of intervention (week) and weighted mean difference of: a) TAG; b) TC; c) LDL-cholesterol and d) HDL-cholesterol.
Discussion
The present systematic review and meta-analysis of RCT examined the effects of guar gum supplementation on lipid profile in adult populations. Our results showed that guar gum supplementation significantly reduces TC and LDL; however, there was no significant effect on TAG and HDL concentrations. Moreover, there was a significant non-linear relationship between changes in dose of guar supplementation and alteration in HDL concentrations.
In addition to pharmacological therapy for the treatment of dyslipidaemia, a primary focus of many researchers has been to establish efficacious and alternative treatments with lesser side effects. Indeed, many experimental and human studies have shown that guar gum supplementation exhibits hypocholesterolemic properties(Reference Trautwein, Kunath-Rau and Erbersdobler55). Accordingly, the present study indicated that guar gum supplementation significantly decreased TC and LDL concentrations. Concordant with our findings, a non-randomised clinical study demonstrated that daily guar gum supplementation for 3 months significantly decreased TC, with no effect on TAG concentrations(Reference Figueiredo, Alfenas and Franceschini56). In addition, in a double-blind crossover trial conducted by Niemi et al., 12 weeks of guar gum supplementation resulted in 10 % lower cholesterol, without any significant change in HDL or TAG concentrations(Reference Niemi, Keinänen-Kiukaanniemi and Salmela32). Further, Wirth et al. showed that, in patients with familial hypercholesterolemia, guar gum supplementation significantly reduced plasma TC and LDL concentrations after 3 months when administered concurrently with bezafibrate(Reference Wirth, Middelhoff and Braeuning57). Similarly, Tuomilehto et al. reported that a combination of gemfibrozil and guar gum supplementation significantly lowered TC and LDL concentrations and improved HDL:LDL ratio(Reference Tuomilehto, Silvasti and Manninen58). Clinically, declines of 39 mg/dl in TC and LDL concentrations can lessen all-cause mortality by 25 % and 16 % and CHD mortality by 25 % and 28 %, respectively(Reference Gould, Davies and Alemao59). Therefore, the results from our pooled analysis showing significant reductions in TC (–19·34 mg/dl) and LDL (–16·19 mg/dl) concentrations support the clinical significance of guar gum supplementation as a non-pharmacological strategy for lipid control.
Our study did not show any significant influence of guar gum supplementation on TAG and HDL concentrations. Indeed, findings from previous studies in this area align with our outcomes(Reference Aro, Uusitupa and Voutilainen16,Reference Knopp, Superko and Davidson60,Reference Krotkiewski61) . Bosello et al. showed that a 60-d intervention with guar gum supplementation decreased TC and led to plasma TAG concentrations reduction in patients suffering from familial combined hyperlipoproteinemia(Reference Bosello, Cominacini and Zocca62). A murine experimental study indicated that when rats were fed with a guar gum diet, a significant decrease in cholesterol and TAG concentrations along with higher HDL and HDL:LDL ratio was noted(Reference Frias and Sgarbieri22). In another study conducted by Gatti et al., guar gum supplementation yielded a reduction of TAG and TC concentrations when administered as guar-enriched pasta in diabetic and hyperlipidaemic patients(Reference Gatti, Catenazzo and Camisasca63). Dietary treatment in this study was so strict that the intake of ethanol and sucrose, with their known hypertriacylglycerolaemic effects, was excluded(Reference Gatti, Catenazzo and Camisasca63,Reference Burgeiro, Cerqueira and Varela-Rodríguez64) . Yamamoto et al. observed that a mixture of xanthan and guar gum supplementation had a hypertriacylglycerolaemic effect in diabetic rats, which might be due to high viscosity of intestinal content that resulted in delayed absorption of triacylglycerol(Reference Yamamoto, Sogawa and Nishina65). Moreover, Pasquier et al. hypothesised that high- and medium-viscous fibres could change the emulsification of dietary lipids and a subsequent reduction of TAG lipolysis in the duodenum(Reference Pasquier, Armand and Castelain66) as our subgroup analyses showed that guar gum supplementation, specifically in diabetic patients, decreased TAG concentrations. One possible explanation for this result is the use of antidiabetic drugs such as metformin, which has been reported to be accompanied by decreases in TAG(Reference Zhou, Massey and Story67). Another explanation for a tendency towards reducing TAG concentrations might be due to small but significant weight loss, as reported by Jenkins et al. (Reference Jenkins, Reynolds and Slavin68). Thus, further investigations are necessary to clarify the effect of guar gum supplementation on TAG concentrations.
The mechanisms through which soluble fibres, such as guar, might affect lipid profile are unclear. Studies suggest that when micelles are forming in the lumen, soluble fibres bind to bile acids or cholesterol, leading to decreased enterohepatic circulation(Reference Turner, Tuomilehto and Happonen69,Reference Brown, Rosner and Willett70) . Viscosity, a physicochemical property associated with dietary fibres (particularly soluble dietary fibres), also contributes to the physiological effects of dietary fibre via decreases in the diffusion of nutrients(Reference Dikeman and Fahey71). This is accomplished by reduced contact between food and digestive enzymes, altered contractile movements, slowed gastric emptying, and a thickening of the unstirred water layer through which glucose and cholesterol diffuse in the lumen(Reference Dikeman and Fahey71). Soluble fibres inhibit the reabsorption of bile acids from the intestine, and they might also interfere with cholesterol absorption(Reference Lalor, Bhatnagar and Winocour50). As the faecal loss of bile acids increases and the bile acid pool decreases, the liver produces more bile acids from cholesterol, lessening hepatic free cholesterol concentrations(Reference Rideout, Harding and Jones72). When cholesterol reduces, an up-regulation of LDL receptors occurs, leading to higher clearance of LDL(Reference Brown, Rosner and Willett70). Another suggested mechanism is the mediating effect of SCFA produced due to bacterial fermentation that contributes to the prevention of hepatic fatty acid synthesis(Reference Chen, Anderson and Jennings73).
Some limitations need to be considered when interpreting our results. Although guar gum was used in different dosages and forms, the impact of the various forms of guar gum was not examined. Further investigations are required to address questions specific to efficacy, compliance and side effects. In addition, the intervention period varied among the studies. Finally, except for one study, all the included studies were conducted among Western countries; therefore, examining the influences of guar gum supplementation in non-Western countries and other races is necessitated.
Conclusion
In conclusion, guar gum supplementation seems to favourably affect TC and LDL but without significant alterations in TAG and HDL concentrations. Given the limitations of the included studies, further investigations with various guar forms in different countries are needed to shed light on this issue.
Acknowledgements
Financial support: None.
Authorship: Conceptualization: [LS and SP]; Methodology: [OA, MRK, RB, and DAL]; Formal analysis and investigation: [OA]; Writing - original draft preparation: [LS, MZK, RB, SP, and OA]; Writing - review and editing: [AW, CCTC, DAL, RB, KS, and MG]; Supervision: [RB and MG].
The authors declare no conflict of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522002136










