INTRODUCTION
Acute hepatitis A has a worldwide distribution with an estimated 1·5 million cases each year of symptomatic infection [Reference Yokosuka1–Reference Ling3]. It is primarily transmitted by person-to-person contact through faecal contamination, but common source epidemics from contaminated food and water also occur. In developed countries, the disease incidence is low, due to improvement of hygienic conditions [4]. In developing countries, the incidence remains high. Although the disease is usually self-limiting, hepatitis A can be severe, especially in adults and individuals with chronic liver disease [5, Reference Almasio and Amoroso6]. Mortality, usually due to fulminate hepatitis, is about 2% in adults aged >50 years [5]. The severity of illness increases with the age of patient. In children, hepatitis A is mainly asymptomatic or mild, while in adults usually leads to jaundice (>70% of cases). A patient with hepatitis A is infectious from 2 weeks before the appearance of jaundice to about 7 days thereafter. Hepatitis A causes considerable economic losses in many middle- or low-endemicity countries, in which the infection frequently affects adults [4, 5]. Even in a number of developed countries, groups living in poor sanitary conditions are susceptible to hepatitis A. Highly effective vaccines against hepatitis A are available, which generate long-term and possibly lifelong immunity [4, Reference Van Damme7]. Recommendations on vaccination vary from country to country, oscillating from selective vaccination of at-risk groups to universal vaccination [4].
Greece is included in the countries of low to intermediate endemicity, although in some existing ethnic minorities the disease may have greater impact. There has been a great reduction in the incidence of hepatitis A between 1980 and 1995; most cases now are sporadic. Public health surveillance of infectious diseases in Greece is based on a system of mandatory notification of identified cases. The physician making the diagnosis of hepatitis has to report the case to the regional public health authorities within a week. Over the past few years 150–200 cases of hepatitis A have been recorded annually, giving an annual incidence of 1·3–1·8 cases/100 000 population [Reference Kamaria8].
We investigated an outbreak in three distinct Roma populations. This outbreak was reported by the local public health authorities of three neighbouring prefectures, situated about 150–200 km apart. The objectives of the study were to investigate the characteristics of the outbreak, the environmental and socioeconomic conditions related to it, to examine the source of contamination, and the pathways of disease transmission.
METHODS
Outbreak investigation
The three prefectures of Xanthi, Rodopi and Evros are located in northeastern Greece bordering Turkey (Evros) and Bulgaria (all three). Their populations were 101 856 (Xanthi), 110 828 (Rodopi), and 149 354 (Evros) inhabitants. About 45% of the population of each prefecture is rural. In all three prefectures, there are important social minorities such as Roma and Islamic. All minorities can be considered as low-income minorities with poor sanitation.
A descriptive study was performed using notification data from local public health authorities. The study started in the first week of July 2007. For this investigation, cases were defined as having an onset date of symptoms between July and November 2007, and detection of positive immunoglobulin M (IgM) antibodies to hepatitis A virus (HAV). The cases were reported to the health departments of each prefecture.
A questionnaire was distributed to, and completed by the patients under the supervision of medical staff and public health authorities the day after the case was reported to the public health authorities. Data collected included age, sex, residence, date of onset of symptoms, hospitalization, and possible pathways of transmission, such as association with another case, living conditions, emigration, travelling, schooling, and common living.
Serum samples were also tested because they had already been collected by the public health authorities. Positive samples for HAV were confirmed by sequencing. We also analysed the phylogenetic relations between HAV isolates obtained from these outbreaks and recent epidemics in order to elucidate HAV genotypes.
Environmental samples
The EPA Information Collection Rule (ICR) has validated a standard method for collecting enteric viruses from large volumes of potable water [9]. It demands the use of one 1 MDS filter and an eluent of 1·5% beef extract with 0·05 m glycine (pH 9·5). According to this protocol, with some minor modifications [Reference Pina10], two water samples from Roma living areas were tested for HAV detection.
Serum samples
Sera samples from eight acute hospitalized HAV cases were collected and analysed for HAV. HAV RNA was extracted using the QIAamp Viral RNA kit (Qiagen, UK) according to the manufacturer's protocol. Purified viral RNA was subjected to reverse transcription and nested polymerase chain reaction (RT–PCR) [Reference Formiga-Cruza11]. A 290-bp fragment encompassing the 5′-NTR part was amplified with the following set of primers:
HAV1: 5′-TTGGAACGTCACCTTGCAGTG-3′,
HAV2: 5′-CTGAGTACCTCAGAGGCAAAC-3′
as well the nested primers:
neHAV1: 5′-ATCTCTTTGATCTTCCACAAG-3′,
neHAV2: 5′-GAACAGTCCAGCTGTCAATGG-3′.
Positive samples for HAV identified by nested PCR were confirmed by sequencing of the PCR product. A full sequencing process of the positive DNA fragment was performed by Lark Technologies (Essex, UK). Multiple sequence alignment of sequences of HAV strains representing all the genotypes available in the GenBank database along with seven sequences of the Greek strains from the present study was performed using ClustalW2 software (www.ebi.ac.uk) (data not shown). To determine the relatedness between the different sequences, a phylogenetic tree was constructed by the neighbour-joining (NJ) method using MEGA 4.0.2 software [Reference Madhuri12]. The HAV genotype was determined by comparing the different sequences of the Greek strains included in the phylogenetic analysis with the reference sequences of different HAV genotypes. GenBank accession numbers were X75215, AB020567, AB020564 and AF357222 for IA; M14707 and M20273 for IB; AY644676 for IIA; AY644670 for IIB; AY644337 and AJ299464 for IIIA and D00924 for V genotypes.
RESULTS
A total of 124 cases were diagnosed with hepatitis A on the basis of their positivity for the IgM anti-HAV antibody between 2 July and 30 November 2007. All were negative for hepatitis B surface antigen (HBsAg), IgM anti-hepatitis B core antigen antibody (IgM-HBc), and anti-hepatitis C virus (HCV) antibody.
According to the historical regional notifications for the same period in the preceding 3 years, the number of HAV cases from January to July 2007 was as expected and increased steeply afterwards (Fig. 1). The epidemic curve by week of onset showed various peaks between 2 July and 30 November in the three prefectures (Fig. 2). The overall attack rate in these 5 months was 34·2/100 000 inhabitants for all three prefectures, ranging from 25·4/100 000 in Evros, and 38·8/100 000 in Rodopi to 42·2 cases/100 000 in Xanthi prefecture. Overall, 67 (54%) of the patients were male and 57 (46%) female, and their median age was 9 years (range 2–55). Patients aged 2–10 years accounted for 78·2% of the total (Fig. 3) and 82% were Roma. The other cases had a median age of 22·7 years (range 4–55 years) whereas Roma had a median age of 6 years (range 2–17 years). In total, 44·5% of the cases had a known association with another case and 39·5% shared common living conditions. Only 3·2% reported to have travelled abroad recently. All cases were of Greek nationality. Initially cases were reported in Xanthi prefecture (Fig. 2). All the cases reported in Evros prefecture were related to cases from Xanthi prefecture. In all, 91·2% of the cases were hospitalized. The geographical distribution of cases shows that there were cases in all three prefectures, mainly localized to areas where Roma people were living (Fig. 4). It is difficult to estimate with any reasonable degree of accuracy, the number of Roma living in the area, but the 2001 Comprehensive Plan of Action for the Social Integration of the Greek Roma estimated their number to be between 250 000 and 300 000 for the whole of Greece [13].
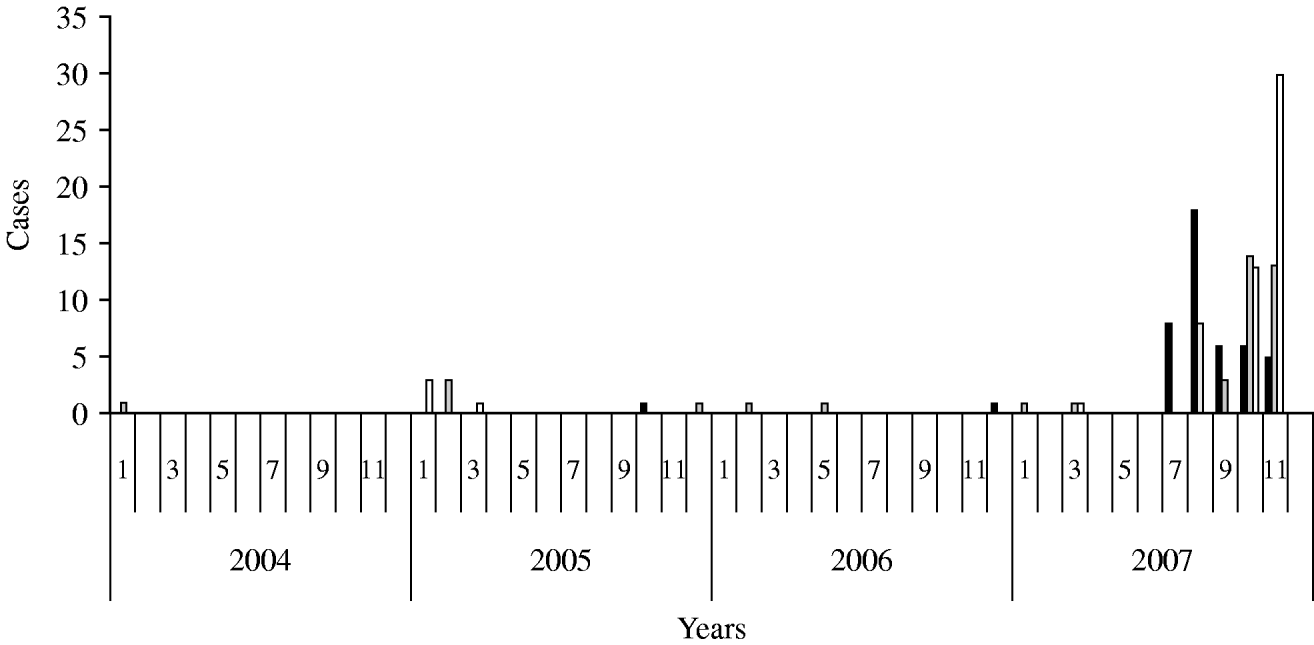
Fig. 1. Surveillance of cases in all three prefectures between 2004 and 2007. ▪, Xanthi;![]() , Evros; □, Rodopi.
, Evros; □, Rodopi.
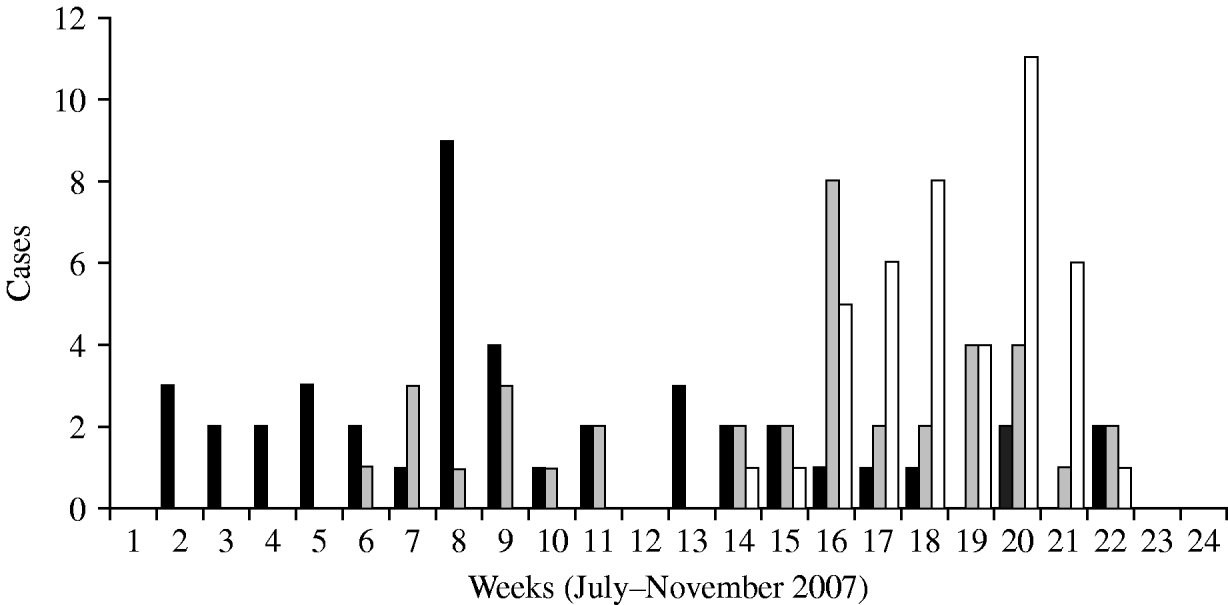
Fig. 2. HAV cases by week of onset, 1 July to 30 November 2007 in three prefectures. ▪, Xanthi;![]() , Evros; □, Rodopi.
, Evros; □, Rodopi.

Fig. 3. Cases of HAV infection: age distribution. Male cases (▪; n=67); female cases (□; n=57).
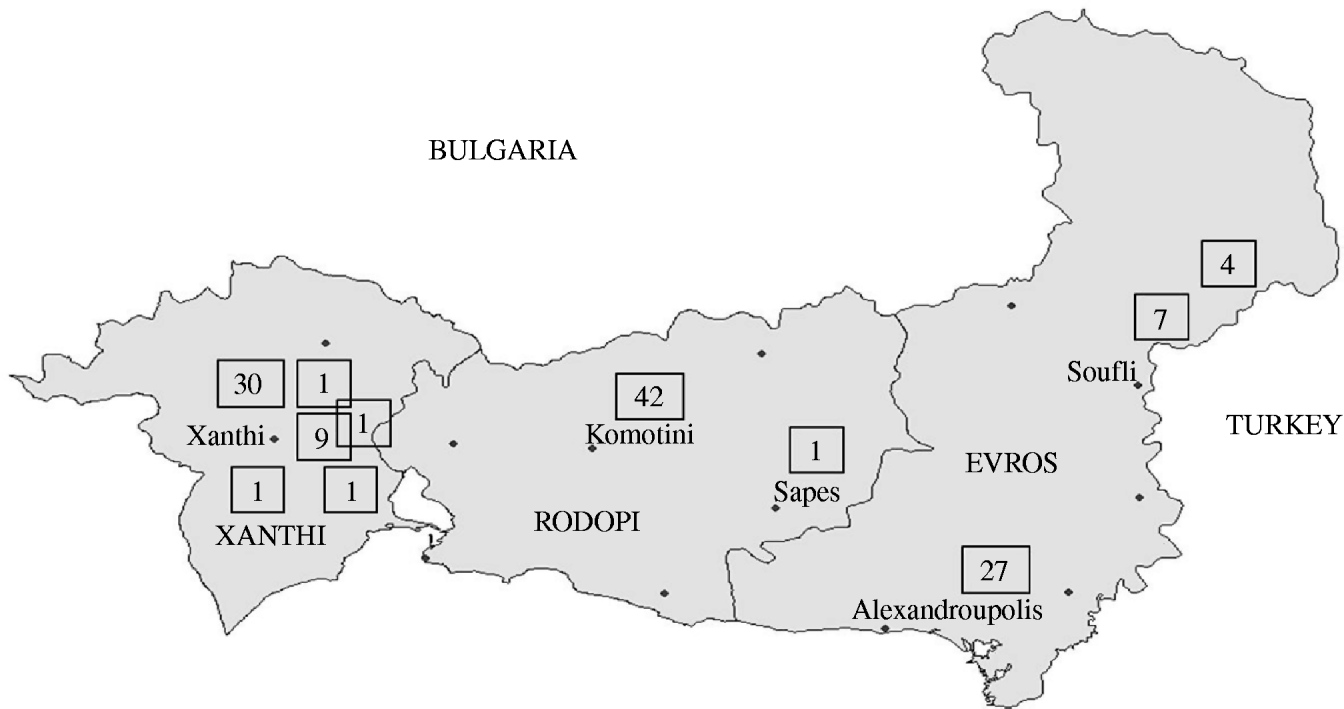
Fig. 4. Geographical distribution of the cases in three prefectures.
The acceptance rate for the serum survey for testing HAV genome was low. Sera from eight cases were obtained. The eight sera samples were collected between 16 August and 23 October from patients residing in all three prefectures. Samples were collected on average 3 days after the onset of symptoms. All eight cases had been confirmed by HAV IgM antibodies, tested in hospital laboratories. All these patients were positive for the HAV genome and had genotype IA. A phylogenetic tree was also constructed. It showed that all the positive RNA samples had >99·8% identity (Fig. 5). The nucleotide sequences of a total of 27 human and one simian HAV isolates comprising all HAV genotypes were retrieved from the GenBank database. These comprise most of the strains circulating globally over the last three decades. The nucleotide sequences of the HAV strains and seven Greek strains were included for comparative analysis. The eighth sample was not used due to poor quality of sequencing of the PCR product supplied. The Greek strains grouped together with the X75215 HAV strain of genotype IA, isolated in Germany. As expected, the simian strain D00924 was in a distinct clade to all human strains.
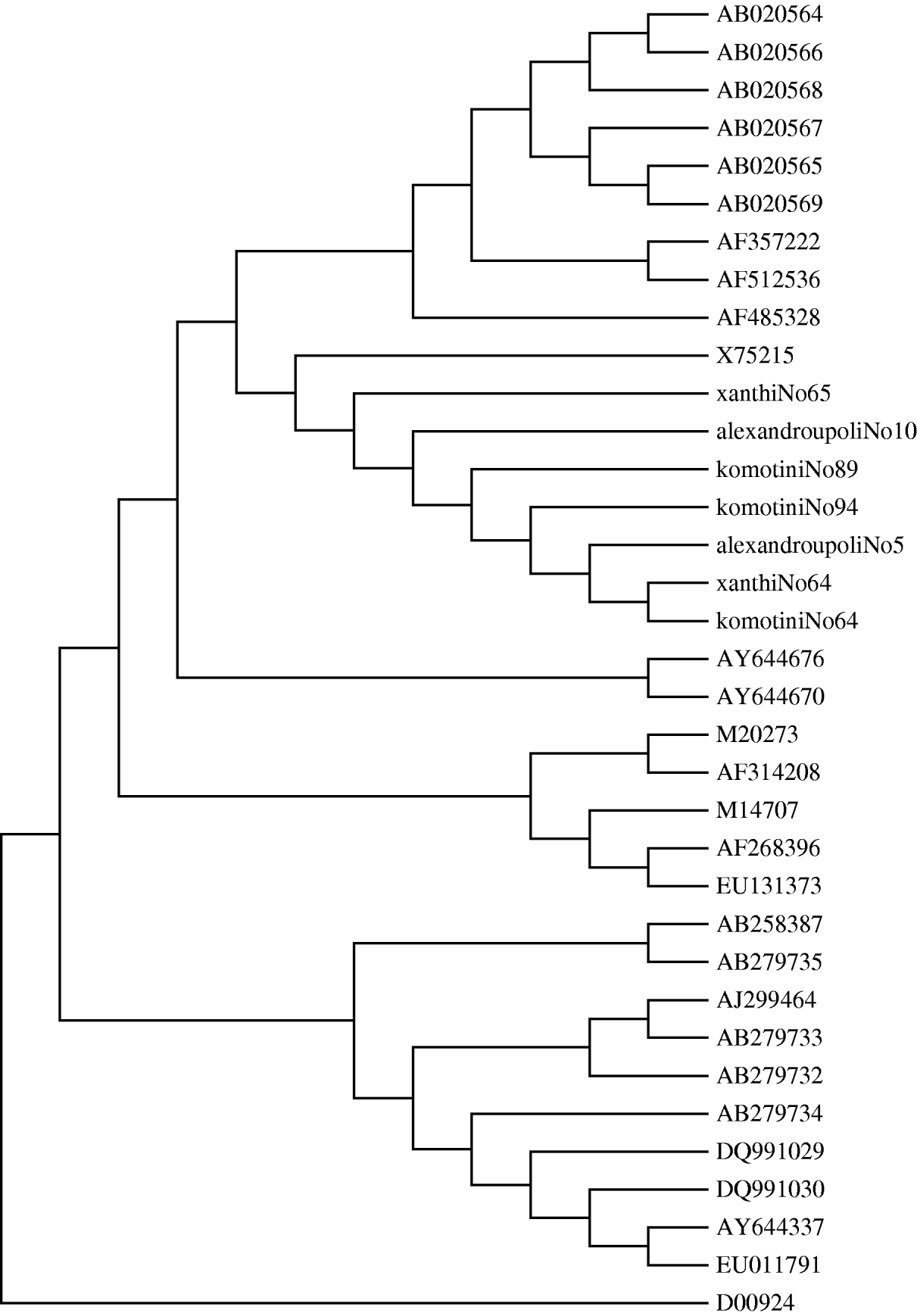
Fig. 5. Phylogenetic tree analysis of HAV. The phylogenetic tree was obtained by the neighbour-joining method. Strains in the tree are shown by their accession number (GenBank database).
The two water samples analysed were found negative for HAV.
Discussion
To our knowledge, this is the first study describing a HAV outbreak in Roma in northeastern Greece. This outbreak is remarkable because of the significant number of affected subjects, the wide geographical area involved, and the investigation performed, which is not common in Greece. This was a descriptive study with a virological characterization of the agent identified from cases as well as phylogenetic analysis.
The epidemic curve included 124 cases of HAV occurring in three prefectures between 2 July and 30 November 2007 and showed several peaks of different sizes, spaced a few weeks apart. As observed in outbreaks located in southern Italy [Reference Malfait14, Reference Lopalco15] and Slovakia [Reference Hrivniaková16], this episode mostly affected younger age groups with an attack rate in the <10 years age group more than double that observed in the general population. The higher incidence of HAV infection in children could be explained by a lower degree of natural immunity to HAV as well as to more frequent contacts between them. Moreover, as children are more likely to have asymptomatic infection with HAV, the infection rate is likely to have been considerably higher than that observed in this study of clinical cases. A relative improvement in sanitary conditions and a switch from high to intermediate level of endemicity is probably taking place now in Roma populations. This could be the reason why the outbreak involved mainly children and adolescents, who may previously have had restricted exposure to the virus.
The epidemic curve showed a first peak in July in Xanthi, compatible with the start of the circulation of HAV infection. It is possible that there was a common source of infection at this time. The other peaks observed during the outbreak may have been caused by other common sources (food or water) in other Roma communities and/or case-to-case dissemination of HAV. It was not possible to verify any hypothesis about the source of the outbreak based only on descriptive epidemiological data. The role of possible risk factors could have been investigated by a case-control study. However, such a study was not conducted, as many cases were recorded retrospectively by our study group, using notifications obtained by local public health authorities; the contacts between these patients or their families presented many difficulties, because of their culture and inability to provide a reliable history. The long duration of the epidemic suggests an important role of person-to-person transmission, although it was difficult to identify primary and secondary cases.
Indeed, nearly all cases tested for the presence of HAV were found to be infected by similar viral strains belonging to the same IA genotype. This genotype, the most common type in Greece, was also described recently in a cluster of 26 cases observed in a region in southern Italy [Reference Malfait14]. Molecular characterization of HAV strains allows the elucidation of their geographical origin and their transmission patterns. Despite the limited amino-acid heterogeneity of HAV, a significant degree of nucleic acid variability has been observed in different isolates from different regions of the world [Reference Lopalco15]. Nucleotide sequences of seven Greek strains along with representative GenBank HAV strains of all genotypes were used in our study. A 234 nucleotide fragment was used. All the seven strains collected from this outbreak in northeastern Greece were of a single subgenotype IA, suggesting a single source of infection.
Public health surveillance of infectious diseases in Greece is based on a system of mandatory declaration of identified cases. The physician making the diagnosis of hepatitis A has to report the case to the regional public health authorities within a week. It is estimated that a large number of hepatitis A cases are not recorded through this surveillance system. Only a small number of cases are reported annually in the study area. As a consequence, many cases of hepatitis A that occurred during the outbreak may not be declared. The high proportion of hospitalized cases may be related to an underestimation of cases that did not attend hospital. However, in an outbreak escalating within a specific population it is expected that local physicians would be much more prepared to notify cases.
Our investigation could not clearly differentiate between the role of secondary and household transmission from asymptomatic children. A specific source of HAV could not be identified. Previous HAV outbreaks have not been reported in Greece, so there are no previous data to compare with. However, in previous studies in Slovakia [Reference Hrivniaková16] and Portugal [Reference Rodrigues17], an increased occurrence of hepatitis A in Roma children was suggested.
Vaccinations of the population by the health departments of the prefectures as well as sanitation measures were performed and the epidemic was controlled.
In conclusion, a large outbreak of hepatitis A occurred in northeastern Greece, affecting mainly the Roma population living in this area. The identification of a single subgenotype suggested a common source of infection. The geographical variation and the epidemic curve indicated that person-to-person transmission was an important route of disease dissemination, although several peaks of the epidemic curve might also be related to common sources of infection. Sanitary conditions and continual movement of the Roma population were likely to have had an important role in the emergence and development of the outbreak. The restricted number of water samples analysed does not allow us to make any conclusion about waterborne transmission.
It is probable that Roma communities are experiencing a relative improvement in sanitary conditions and a switch from high to intermediate level of endemicity. This could be an explanation for the occurrence of the outbreak that involved mainly children and adolescents. As a consequence, vaccination might offer an effective preventive measure for the Roma populations and other populations with similar living conditions in Greece.
ACKNOWLEDGEMENTS
The authors thank all the local public health and hospital staff of three prefectures for their assistance with interviewing study participants.
DECLARATION OF INTEREST
None.







