Shigellosis is a major cause of bacillary dysentery worldwide [1]. In industrialized countries, Shigella sonnei predominates and tends to cause milder disease [Reference Keusch, Acheson and Schaechter2]. S. flexneri, S. dysenteriae and S. boydii are commonly linked to foodborne exposure during travel to less developed countries, the first two species causing the most severe disease (bloody diarrhoea and dysentery) [Reference Keusch, Acheson and Schaechter2]. In Montréal, S. sonnei has been responsible for person-to-person outbreaks within day-care facilities and in religious communities and men who have sex with men (MSM) [Reference Gupta3–Reference Daskalakis and Blaser5]. In MSM, S. sonnei is transmitted sexually through oral–anal contact. Due to the elevated prevalence of HIV/AIDS immunosuppression in MSM compared to other population groups, MSM experience increased carriage and transmission of Shigella [Reference Daskalakis and Blaser5]. This results in conditions favourable for outbreaks to occur. The surveillance of Shigella and other sexually transmitted infections (STI) in MSM, followed by prompt public health intervention, are important steps in preventing and controlling outbreaks in this community.
In February 2010, the Public Health Department of Toronto, another major Canadian city, reported a reversal in the predominant species from S. sonnei to S. flexneri (56% of cases notified in 2009) and increases in MSM and HIV co-infection in shigellosis cases [6]. The staff at the Montréal Public Health Department decided to conduct a timely epidemiological study to discover whether a shift to S. flexneri had recently taken place in MSM in Montréal and if so, to determine its impact on the severity of disease.
All Shigella infections reported to the Montréal Public Health Department from 1 January 2005 to 31 March 2010 were included. Information on demographics, hospitalization >24 h, risk factors (MSM status, travel to a less developed country 2 weeks before illness, food exposures and other local transmission) and self-reported HIV status was retrieved from routinely administered telephone questionnaires. If the case mentioned both MSM status and travel during the exposure period, the two risk factors were considered as overlapping in the analysis. Cases were also considered to be HIV positive if indicated by a laboratory report in the case file. As formal questions on MSM status during the exposure period were added to the questionnaire in 2007, we could not use MSM status for cases notified in 2005 and 2006 in the analysis. Cases in travellers returning from less developed countries were used as a reference group for all analyses of changes in notifications for MSM. Serotyping is not routinely conducted but the results were available for about half of the S. flexneri cases.
Case characteristics were examined by 2-year periods (2005–2006, 2007–2008, 2009–March 2010) that separated two known outbreaks of S. sonnei which occurred in a religious group from January to December 2005 and simultaneously in a religious group and in MSM in August 2007 to December 2008 [Reference Hannah and Allard4]. χ2 tests were used to examine whether increases in the proportions of risk groups (MSM, travel-related) and HIV-positive MSM in all shigellosis cases occurred between the time periods 2007–2008 and 2009–2010. This would indicate whether MSM became disproportionately affected by shigellosis in the recent period compared to the rest of the population.
To evaluate trends across time for all cases infected by S. sonnei and S. flexneri and then for risk groups infected by S. sonnei and S. flexneri, a Poisson regression analysis was applied [Reference Ely7]. The number of the month of notification counted onwards over the years starting in January 2005 was used as a predictor and the monthly number of cases as the outcome. Where the variance of the distribution of the monthly numbers of cases was greater than its mean, a negative binomial model was used instead. Results were expressed as incidence rate ratios (IRR) comparing the count for any given month to that of the previous month. To compare more finely the association between the risk groups and the three major species groupings [(1) S. sonnei, (2) S. flexneri, (3) combined S. dysenteriae and S. boydii] during two discrete time periods (2007–2008 and 2009–March 2010), a multinomial logistic regression was conducted [Reference Hosmer, Lemeshow, Hosmer and Lemeshow8]. Associations were expressed as the ratios of the odds of a given outcome (S. sonnei or combined S. dysenteriae and S. boydii) vs. the reference outcome (S. flexneri) for one risk group compared to another [odds ratios (OR)]. This analysis was performed using a main-effects model and a model controlling for potential confounders. Disease severity was evaluated by examining the changes in the proportion hospitalized annually by species in all shigellosis cases. Stata version 10.1 (Stata Corp., USA) was used for analysis. Significance was set at P<0·05.
Between 1 January 2005 and 31 March 2010, 502 shigellosis cases were reported. The epidemiological curve showed that S. flexneri infections remained below seven cases per month (Fig. 1). Multiple serotypes of S. flexneri were reported during the different time periods in cases that were serotyped: 2005–2006 (2A, 63%), 2007–2008 (1B, 31%; 2A, 31%; 3A, 28%) and 2009–2010 (3A, 30%; 1B, 21%). Serotype 3A appeared to have increased over time. The proportions of MSM cases did not change significantly between 2007 and 2008 (51/219, 23·2%) and 2009–2010 (20/82, 24·4%) (χ2=0·04, P=0·84) nor did the proportions of travel-related cases between 2007 and 2008 (66/219, 30·1%) and 2009–2010 (32/82, 39%) (χ2=2·15, P=0·14). The proportions of HIV-positive MSM cases did not change significantly between 2007 and 2008 (17/51, 33·3%) and 2009–2010 (9/20, 45%) (χ2=0·84, P=0·36).
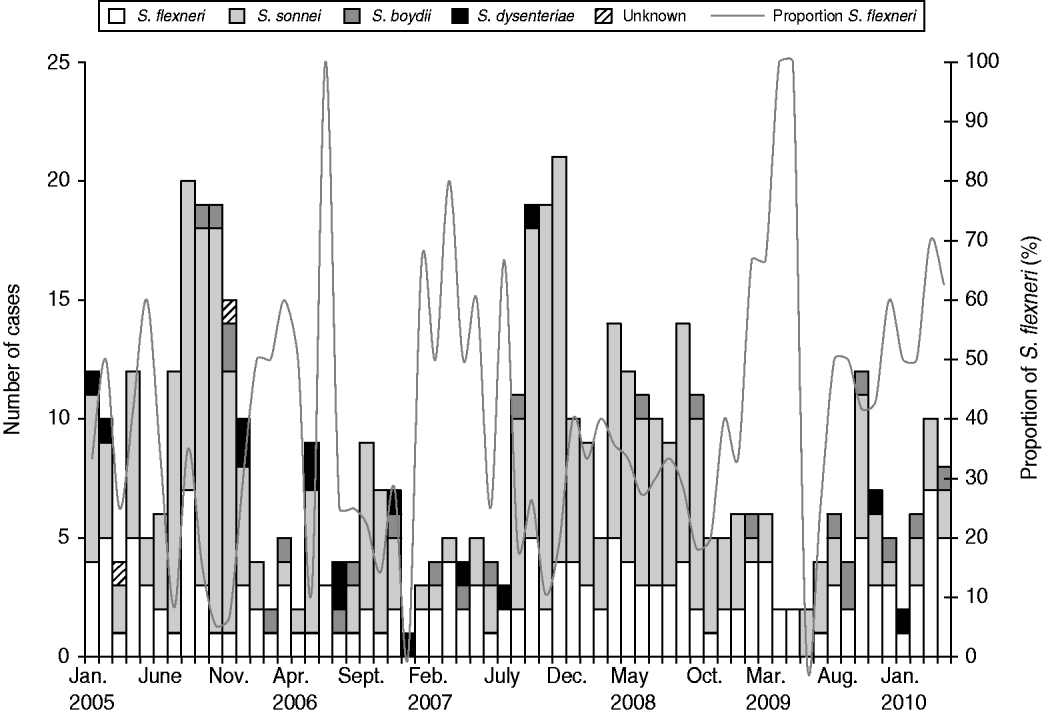
Fig. 1. Shigella cases by species and month, Montreal, 1 January 2005 to 31 March 2010.
The analysis of time trends in all cases from 2005 to 2010 showed an average reduction in incidence of S. sonnei of 1·4% per month [IRR 0·99, 95% confidence interval (CI) 0·97–1·0, P=0·06] and no significant change in S. flexneri (Table 1). During 2007–2010 for MSM an average reduction of S. sonnei of 10·8% per month (IRR 0·9, 95% CI 0·82–0·98, P=0·02) and an average increase of 6·7% per month of S. flexneri (IRR 1·07, 95% CI 1·02–1·12, P=0·004) were observed. No changes in the time trends of S. sonnei or S. flexneri in travel-related cases were observed. To assess the associations between MSM status and travel status with species over discrete time periods, the multinomial logistic model was adjusted for age, sex, local transmission and any interactions. In 2007–2008, MSM were more likely to be infected by S. sonnei compared to S. flexneri (OR 4·3, 95% CI 1·33–13·95) and no significant association was found between travel and S. sonnei. Between 2009 and 2010, MSM were less likely to be infected by S. sonnei compared to S. flexneri (OR 0·03, 95% CI 0·002–0·31) and travellers were more likely to be infected by S. sonnei compared to S. flexneri (OR 4·7, 95% CI 1·25–17·92). The time periods (2007–2008 and 2009–2010) were strong effect modifiers for the associations between risk factors and species, sometimes resulting in a reversal of the direction of the association (such as between species and sexual orientation). The proportions of cases hospitalized by species was too low to provide an insightful comparison of results [S. flexneri (13/166, 7·8%), and S. sonnei (20/296, 6·8% over the entire time period)].
Table 1. Time trends of S. sonnei and S. flexneri (1) by species and (2) by species and risk factor, Montréal, 2005 to 31 March 2010
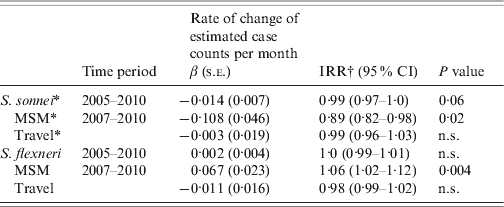
β (s.e.), Regression coefficient and standard error; IRR, incidence rate ratio; CI, confidence interval; MSM, men who have sex with men; n.s., not significant.
* Negative binomial regression was used where distribution was overdispersed (variance >mean).
† IRR=exp{case count for month (x+1)/case count for month x}.
Our results suggest that for MSM, a shift towards dominance by S. flexneri occurred from 2009 onwards. It appeared to be the combined effect of a slight decrease in the proportion of S. sonnei infections probably owing to the end of the outbreak in MSM in 2007–2008 and a greater simultaneous rise in the proportion of S. flexneri infections in MSM. The IRRs for MSM populations show that the decrease in S. sonnei and increase in S. flexneri in MSM are statistically significant in absolute numbers and not only relational. Changes in species dominance were confined to MSM cases.
There are several limitations to this analysis. Passively reported surveillance data from a medium-sized city was used and therefore the dataset of 502 cases is small for statistical analysis. Accordingly, we reported confidence intervals as measures of uncertainty. The reader may also choose to base his/her interpretation of the ORs on the limit of the confidence interval that is closer to 1 and not on the point estimate of the OR. Trend analyses of S. sonnei are affected by the presence of outbreaks during the time period. Therefore, the magnitude of the results should be interpreted with caution. Finally, the self-reporting of MSM status and HIV status may have led to underestimations and precluded the detection of significant changes in these factors.
The presence of a large increase and the absence of a sharp spike in S. flexneri infections in MSM during the recent period may be explained by the increased opportunities for S. sonnei to cause an outbreak. Given its lower virulence and pathogenicity, infection by S. sonnei may cause milder disease so that persons infected are less incapacitated and may continue the risky sexual behaviour that contributes to transmission [Reference Aragón9]. The determinants of the more stable shift towards S. flexneri are unclear. In the USA, a large increase in the proportion of S. flexneri in males aged 20–49 years between 1975 and 1985 was documented nationally, suggesting a long period of dominance in MSM [Reference Tauxe10]. A shift back towards S. sonnei dominance in males occurred by 2000–2002 although some states continued to report a predominance of S. flexneri in the general population [Reference Gupta3]. From 2009 to 2011, London, UK and Vancouver, Canada reported small S. flexneri serotype 3A outbreaks in MSM [11, 12]. Of note, serotype 3A was the most frequently reported serotype in isolates serotyped in Montréal since 2009.
Although no significant increase in the proportion of HIV/shigellosis co-infection in MSM was found, this remains a sub-population vulnerable to increased transmission of shigellosis. Several factors contribute to this vulnerability including high-risk sexual practices within networks of HIV-positive MSM (serosorting) and increased carriage and shedding in HIV-positive persons [Reference Daskalakis and Blaser5]. Of note, clusters of STIs, including Entamoeba histolytica, ciprofloxacin-resistant Campylobacter coli (R. Ratnayake, unpublished observation) and ciprofloxacin-resistant S. sonnei [Reference Gaudreau13], have also affected HIV-positive MSM in Montréal since 2008, providing additional signals of a rise in high-risk sexual behaviours.
Like many surveillance systems, the current system in Montréal automatically analyses all shigellosis cases irrespective of species. Hence, it could not easily detect shifts in dominance of S. flexneri in MSM. Although a complex analysis was applied here in a retrospective fashion, we recommend testing for Shigella species shifts be conducted on a routine, timely basis as a first step to monitor shifts in their early stages. Upon detection of a shift, further geographical investigation of aggregated patient addresses or clinics reporting cases can help to more precisely identify high concentrations of species in short time periods in the neighbourhoods where many MSM may live or frequent. If a particular serotype of S. flexneri is detected with increased frequency, serotyping can be used for each isolate detected to prioritize the investigation of certain clusters, in particular those whose serotype links them with other, possibly out-of-town, clusters. Further, molecular subtyping using pulsed-field gel electrophoresis can enable linkage of cases and clusters elsewhere in Canada and the USA using patterns of isolates characterized in the PulseNet database (http://www.cdc.gov/pulsenet/). Gathering additional epidemiological information, such as exposure to oral–anal sex during the exposure period and serosorting behaviour in HIV-infected individuals, is useful in identifying high-risk sexual practices that can be addressed by prevention measures.
Given the potential for future rises in S. flexneri and more severe disease in HIV-positive MSM cases, increased attention to shifts in species can provide an early warning for intervention measures. In summary, our results provide the rationale for the routine analysis of species-level surveillance data to detect shifts in risk groups in a timely manner in Montréal and other urban public health units worldwide.
ACKNOWLEDGEMENTS
We thank our colleagues from the Montréal Public Health Department including Maryse Lapierre for assistance with medical records and Lucie Bédard and Jérôme Latreille for reviewing an earlier report. We thank Lisa Hansen and Nashira Khalil from the Canadian Field Epidemiology Program and the two reviewers for their comments on the draft.
DECLARATION OF INTEREST
None.




