Metabolic or bariatric surgery supplemented with dietary, behavioural and lifestyle changes is the only effective method in facilitating permanent or long-term weight loss and improving medical co-morbidities in morbid obese patients(Reference Fried, Yumuk and Oppert1). Laparoscopic Roux-en-Y gastric bypass (RYGB) is considered the ‘gold standard’ for effective treatment of morbid obesity(Reference Neudecker, Sauerland and Neugebauer2).
The common channel of the RYGB is the part where most of the nutrients are absorbed and where enterohepatic circulation of bile salts and fat is preserved(Reference Gadiot, Grotenhuis and Biter3). It is hypothesised that the length of the common limb affects the amount of weight loss and preservation of weight loss on the long term, but a longer Roux limb may also influence the risk of malnutrition(Reference Lupoli, Lembo and Saldalamacchia4,Reference Obinwanne, Fredrickson and Mathiason5) . Until now, there is no consensus on the optimal length of the limbs of an RYGB.
Most common nutrient and vitamin deficiencies described after RYGB are Fe, vitamin B12, folic acid, vitamin D and Ca(Reference Dogan, Aarts and Koehestanie6,Reference Parrott, Frank and Rabena7) . The Interdisciplinary European Guidelines on Metabolic and Bariatric Surgery advises to prescribe to all bariatric patients lifelong daily vitamins (A, D, E and K, in water-soluble form) and micronutrient supplementation, a recommended minimally protein intake of approximately 90 g/d and extra supplements according to laboratory findings(Reference Fried, Yumuk and Oppert1,Reference Parrott, Frank and Rabena7) . Additionally, the National Institute for Health and Care Excellence advises a minimum follow-up period of 2 years in the bariatric surgical service and lifelong follow-up at the physician to monitor the patients’ nutrient and vitamin status(Reference O’Kane, Parretti and Hughes8). However, the efficacy of bariatric multivitamins in terms of prevention of nutrient deficiencies in RYGB with different lengths of the common limb is unclear.
The aim of the present study was to compare standard RYGB with a very long Roux limb RYGB (VLRL-RYGB) in terms of vitamin and nutrient deficiencies in patients undergoing a primary bariatric procedure.
Methods
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the institutional review board and the regional Medical Research Ethics Committee TWOR, Rotterdam, the Netherlands, with registration number NL43951.101.13.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Design and data collection
The present study was performed as a part of the Dutch Common Channel Trial (DUCATI), a multicentre randomised controlled trial comparing RYGB with VLRL-RYGB(Reference Gadiot, Grotenhuis and Biter3). In the DUCATI, 444 patients were included between 2014 and 2017 in two hospitals in the Netherlands. These patients were randomised with a 1:1 ratio between two procedure types: RYGB v. VLRL-RYGB. All patients found suitable for bariatric surgery according to the international IFSO guidelines and undergoing a primary laparoscopic RYGB were invited to participate in the DUCATI. DUCATI exclusion criteria were no informed consent, prior major abdominal surgery (such as colonic resection, septic abdomen, aorta surgery or other procedures with a high risk of intra-abdominal adhesions, which might jeopardise the possibility of performing a VLRL-RYGB), American Society for Anesthesiologists score ≥ IV and the inability or unwillingness to fill out follow-up questionnaires. For this separate analysis, patients were also excluded in case of conversion of the procedure type to a sleeve gastrectomy or a minigastric bypass.
Outcomes
Primary outcome measure of the current investigation was nutrient deficiency at 1 year postoperative. Secondary outcome measures were reoperations due to malabsorption. Data were collected on patient characteristics, vitamin usage, laboratory results at baseline and 1 year postoperative and reoperations due to malabsorption. Laboratory results of blood samples measuring albumin, Ca, ferritin, folic acid, Fe, K, Mg, Na, transferrin, vitamin B12 and vitamin D were collected preoperatively and 1 year postoperative. Nutrients were scored as deficient in case of a value below the lower bound of the reference value of the hospital’s laboratory (Table 2).
Table 1. Baseline characteristics (n 423)
(Mean values and standard deviations; numbers and percentages)
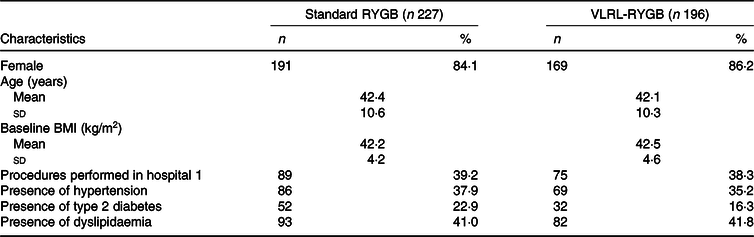
RYGB, Roux-en-Y gastric bypass; VLRL, very long Roux limb.
Table 2. Baseline laboratory results on nutrients and vitamins with lower and upper bound of normal values
(Median values and interquartile ranges (IQR))

RYGB, Roux-en-Y gastric bypass; VLRL-RYGB, very long Roux limb Roux-en-Y gastric bypass.
* Between parentheses the upper and lower bounds of the normal values.
Operation techniques
For both procedure types, a standard size of the pouch of 15–20 ml was used. For the RYGB, the biliopancreatic limb length was 60 cm and the alimentary limb length was 150 cm, resulting in a variable length of the common channel. For the VLRL-RYGB, the biliopancreatic limb length was also 60 cm, but the common limb was measured at 100 cm, resulting in a variable length of the alimentary limb. Total small bowel length was measured in all patients. The surgical techniques were described in more detail in the study protocol(Reference Gadiot, Grotenhuis and Biter3).
Diet and vitamin supplement usage
In the preoperative phase, patients received dietary advice for a healthy diet, low in refined sugars, rich in nutrients, a minimum intake of half a litre of dairy products and a distribution over six meals per d. Furthermore, a group lecture was held on the first postoperative day, in which patients were reminded of the content of the diet and importance of compliance to this diet. The dietary advice was the same for both procedure types. Patients were also counselled in the preoperative phase on the importance of using multivitamins that were specifically tailored for the bariatric patient. Commonly used multivitamin brands in the Netherlands are FitForMe© and Elan©. These multivitamins contain increased levels of multiple vitamins and minerals, in particular Fe, folic acid and vitamin B12(Reference Dogan, Aarts and Koehestanie6). As patients have to purchase these multivitamins themselves, they were free to choose the supplement of their preference. Patients were asked about the usage of (bariatric) multivitamins during the annual check-up at the outpatient clinic. Usage of vitamins was scored as ‘none’, ‘standard multivitamin’ or ‘bariatric multivitamin’.
Statistical analysis
Patients who were scheduled for VLRL-RYGB but underwent a regular RYGB because of technical difficulties or perceived unsafety were analysed as crossovers to the RYGB group. We chose to not exclude these patients, as their data remain valuable for the study aim: it is thought that the length of the common limb affects malabsorption and possibly nutrient and vitamin deficiencies. Mann–Whitney U tests were used to compare differences in the absolute values of each nutrient between the groups at baseline and at 1 year postoperative. The χ 2 test was applied to compare multivitamin usage between groups. Multiple linear regression analyses were used to compare changes in nutrient levels between the two types of surgery, adjusting for baseline patient characteristics (sex, age, BMI, presence of type 2 diabetes, hypertension and/or hypercholesterolaemia) and multivitamin usage. Unadjusted differences in deficiencies (yes/no) of each nutrient between groups were analysed using χ 2 tests. Unadjusted reoperation rates because of malabsorption were compared between the groups using χ 2 tests. No adjustment for multiple testing was made. All analyses were performed using SPSS (PASW) 25 software (SPSS Inc.). The results were evaluated at a significance threshold of P < 0·05 (two-sided).
Results
Fig. 1 illustrates the study profile. In total, 444 patients were included in the DUCATI study. Twenty-one patients were excluded because of conversion of the procedure type to a sleeve gastrectomy or a minigastric bypass. Sixteen patients crossed over from the VLRL-RYGB group to the standard RYGB group because of technical difficulties during the procedure. Table 1 shows the baseline characteristics of the 423 included patients by procedure type. Table 2 shows the absolute baseline laboratory results on nutrients divided by type of procedure. Between the groups, median levels of nutrients were comparable for all but K (4·0 mmol/l for standard RYGB v. 3·9 mmol/l for VLRL-RYGB, P = 0·009).
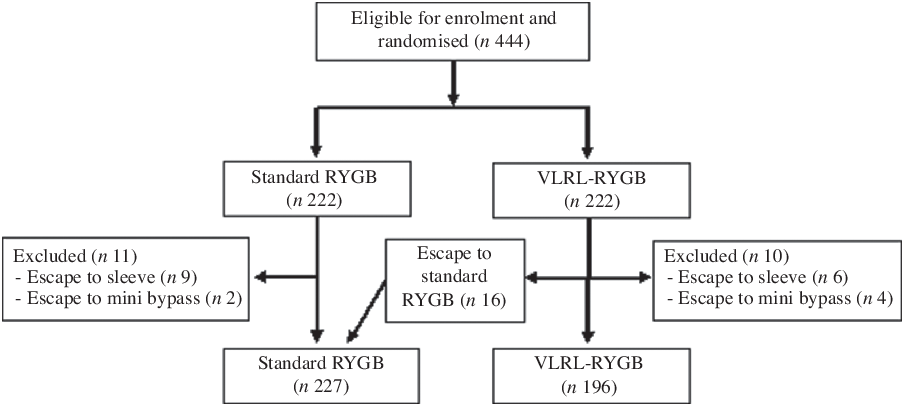
Fig. 1. Study profile: patient selection and randomisation. RYGB, Roux-en-Y gastric bypass; VLRL-RYGB, very long Roux limb Roux-en-Y gastric bypass.
Information on multivitamin usage at 1 year postoperative was missing for 27/227 (11·8 %) patients in the standard RYGB group and 28/168 (16·7 %) patients in the VLRL-RYGB group. At 1 year postoperative, 137/200 (68·5 %) patients in the standard RYGB group v. 120/168 (71·4 %) in the VLRL-RYGB group were documented to be using bariatric multivitamins. Sixty-one of 200 (30·5 %) in the standard RYGB group v. forty-seven of 168 (28·0 %) in the VLRL-RYGB group used standard multivitamins. Two of 200 (1·0 %) in the standard RYGB group v. one of 168 (0·6 %) in the VLRL-RYGB group used no vitamin supplements. There was no significant difference between the standard RYGB group and the VLRL-RYGB group at 1 year postoperative on usage of standard multivitamins (P = 0·718) or bariatric multivitamins (P = 0·657).
Fig. 2 shows the laboratory results on nutrients and vitamins at 1 year postoperative by type of surgery. Patients undergoing VLRL-RYGB had significantly lower levels of albumin, K, transferrin and vitamin D compared with those undergoing RYGB at 1 year postoperative.

Fig. 2. Laboratory results on nutrients and vitamins at 1 year postoperative in two groups. RYGB, Roux-en-Y gastric bypass; VLRL-RYGB, very long Roux limb Roux-en-Y gastric bypass. Data were missing for 10·4–44·0 %, depending on the specific laboratory value.
Fig. 3 shows the unadjusted percentages of patients with deficiencies of nutrients at 1 year postoperative by type of surgery. The differences in percentage of patients with nutrient deficiencies between the groups were significantly different for Fe (23·4 v. 35·6 %, P = 0·009), K (7·4 v. 15·2 %, P = 0·030) and vitamin D (22·7 v. 34·5 %, P = 0·011). Deficiencies that were equally present in both groups were in ferritin (17·2 v. 18·2 %, P = 0·811) and vitamin B12 (9·0 v. 9·9 %, P = 0·777).

Fig. 3. Deficiency rates in nutrients and vitamins at 1 year postoperative in two groups.  , Roux-en-Y gastric bypass;
, Roux-en-Y gastric bypass;  , very long Roux limb Roux-en-Y gastric bypass.
, very long Roux limb Roux-en-Y gastric bypass.
Table 3 shows the results of the linear regression analysis of 227 patients undergoing RYGB and 196 patients undergoing VLRL-RYGB for nutrients pre- and 1 year postoperative, corrected for baseline patient characteristics (age, sex and BMI), presence of co-morbidities (hypertension, type 2 diabetes and dyslipidaemia), hospital and type of multivitamin use. Patients undergoing VLRL-RYGB had slightly but significantly lower levels of Ca (β −0·027, 95 % CI − 0·047, −0·007; P = 0·008), Fe (β −2·285, 95 % CI − 3·669, −0·901; P = 0·001) and vitamin D (β −8·935, 95 % CI − 13·422, −4·448; P < 0·001) at 1 year postoperative compared with those undergoing standard RYGB. Also, patients undergoing VLRL-RYGB had significantly higher levels of folic acid (β 3·698, 95 % CI 0·763, 6·632; P = 0·014) and Na (β 0·507, 95 % CI 0·045, 0·970; P = 0·032) compared with those undergoing standard RYGB.
Table 3. Linear regression analysis of nutrients and vitamins at 1 year postoperative on type of surgery, corrected for baseline value of nutrients and vitamins, patient characteristics, presence of co-morbidities and multivitamin use
(β-Coefficients and 95 % confidence intervals)

VLRL-RYGB, very long Roux limb Roux-en-Y gastric bypass; RYGB, Roux-en-Y gastric bypass.
* β-Coefficient VLRL-RYGB v. standard RYGB.
Reoperation rates due to malabsorption were not significantly different between the RYGB group (2/227, 0·9 %) and the VLRL-RYGB group (7/196, 3·6 %) (P = 0·088). All of these patients experienced severe diarrhoea, which was the main reason for reoperation.
Discussion
This study aimed to compare the presence of nutrient deficiencies in patients undergoing a standard RYGB v. a VLRL-RYGB. For both standard RYGB and VLRL-RYGB, most common deficiencies that occurred at 1 year postoperative were ferritin, Fe, K, vitamin B12 and vitamin D. Patients undergoing VLRL-RYGB had significantly lower levels of Ca, Fe and vitamin D compared with those undergoing standard RYGB at 1 year postoperative. However, levels of folic acid and Na were significantly lower in patients undergoing standard RYGB compared with VLRL-RYGB. Although not significantly different, there were more reoperations because of malabsorption/severe diarrhoea in the VLRL-RYGB group compared with the standard RYGB group.
Vitamin deficiencies were significantly more manifested in the VLRL-RYGB group. However, the absolute differences in mean nutrient and vitamin levels between the two procedure types were very small, and all mean postoperative results remained within the normal range. Furthermore, the clinical relevance of these slightly lower levels of nutrients and vitamins is debatable. Albumin is considered to be associated with malnutrition,(Reference Ziegler, Sirveaux and Brunaud9) and median levels of albumin were well above the lower reference limit for both procedure types.
Previous studies suggest that the preoperative levels of micronutrients also influence the postoperative nutrient status(Reference Parrott, Frank and Rabena7,Reference Mohapatra, Gangadharan and Pitchumoni10) . In our study, baseline laboratory values were comparable between the standard RYGB group and the VLRL-RYGB group on all laboratory values but K. However, the absolute difference in median K value between the groups was very small. After correcting for the baseline laboratory results and for patient and other characteristics, significant differences were found in levels of Ca, Fe and vitamin D. Once again, the clinical relevance of these statistically significant but very small absolute differences is questionable.
The most common nutrient deficiencies in our cohort are in line with the results from previous studies(Reference Lupoli, Lembo and Saldalamacchia4,Reference Dogan, Aarts and Koehestanie6,Reference Parrott, Frank and Rabena7,Reference Fieber, Sharoky and Wirtalla11–Reference Dogan, Homan and Aarts15) . Nutrient deficiencies after metabolic surgery can exist due to several reasons. First, pre-existing deficiencies can persist or worsen after surgery, such as Fe deficiency caused by impaired expression of transporter proteins due to chronic inflammation. Preoperatively, patients often have inappropriate eating behaviour, their diet containing high energy density and few micronutrients(Reference Frame-Peterson, Megill and Carobrese16). Also, different procedures can cause specific alterations to digestion and absorption and result in small intestinal bacterial overgrowth, which can cause pain, watery diarrhoea, dyspepsia and weight loss. This causes malabsorption of thiamine, vitamin B12 and fat-soluble vitamins(Reference Stein, Stier and Raab17). After surgery, prolonged nausea and vomiting, food intolerance, dietary and non-adherence to eating behaviour, meal pattern and supplement recommendations can play a crucial role in the cause of nutrient deficiencies(Reference Stein, Stier and Raab17). Patients in our study requiring reoperations all experienced severe diarrhoea, most likely caused by the alterations to digestion and absorption after a standard RYGB or VLRL-RYGB.
It is thought that the length of the common limb affects the malabsorption and herewith the amount of weight loss(Reference Brolin, LaMarca and Kenler18–Reference Sugerman, Kellum and DeMaria21), but possibly also leading to nutrient and vitamin deficiencies(Reference Fox, Fox and Oh22–Reference Thurnheer, Bisang and Ernst25). A systematic review by Orci et al. in 2011 identified a trend supporting that the construction of a longer Roux limb was more effective in super obese patients in terms of weight loss, and no differences were found regarding nutritional outcomes between groups(Reference Orci, Chilcott and Huber26). Mahawar et al. published a systematic review in 2016, stating that a range of 100–200 cm for a combined length of biliopancreatic or alimentary limb gives optimum results with RYGB in most patients, with a low degree of macronutrient malabsorption(Reference Mahawar, Kumar and Parmar27). The most recent systematic review on this topic was published by Gan et al. in 2018, which concluded that a standard alimentary limb length (130–150 cm) could be preferred since long alimentary limb length (170–250 cm) may result in greater nutritional deficiencies(Reference Gan, Wang and Zhou28). In our study, patients with a long limb length had slightly greater nutritional deficiencies as well. However, the differences in deficiencies between the two procedure types were very small, and therefore, the clinical relevance of these slightly lower levels of nutrients and vitamins is debatable.
Partly in line with our findings, recent randomised controlled trials comparing different lengths of the biliopancreatic limb showed significant differences in deficiencies of vitamin B12, folic acid and vitamin A at 1 year postoperative(Reference Ruiz-Tovar, Vorwald and Gonzalez-Ramirez29,Reference Shah, Nergard and Fagerland30) . However, one of these studies describes the usage of multivitamins that are not specific for bariatric patients, and the other study does not report on multivitamin usage at all. The usage of multivitamin supplements specifically manufactured for bariatric patients has been proven to result in less deficiencies of vitamin B12, vitamin D, folic acid and ferritin(Reference Schijns, Schuurman and Melse-Boonstra31). Additional micronutrient supplementation in addition to two daily multivitamins is recommended in the American Society for Metabolic and Bariatric Surgery Guidelines(Reference Mechanick, Youdim and Jones32). The results from our study were corrected for the different types of multivitamins and therefore have additional value to the previous publications on this topic.
Although a limitation of this study is the missing data on multivitamin usage (13·9 %) and laboratory results (10·4–44·0 %), the numbers were sufficient to form reliable conclusions, as other randomised controlled trials on this matter analyse similar or even smaller patient numbers. Future research should focus on a longer follow-up period.
Conclusion
Patients undergoing VLRL-RYGB had significantly lower levels of Ca, Fe and vitamin D compared with those undergoing RYGB at 1 year postoperative, but higher levels of folic acid and Na. Also, there was no difference in the reoperation rates because of malabsorption between the groups. Close monitoring on nutrient deficiencies should be performed in patients undergoing VLRL-RYGB, and the usage of multivitamins specific for bariatric patients should be encouraged.
Acknowledgements
There was no financial support for this study.
M. L. and R. G. wrote the manuscript. R. G. and U. B. were responsible for the study protocol. J. W. and J. A. collected data. E. B. was responsible for the statistical analysis. M. D. supervised the project.
The authors declare that there are no conflicts of interest.









