Distressing mental imagery is common during anxiety. In phobic disorders imagery-evoked arousal predicted outcome to desensitisation (Reference Lang, Tuma and MoserLang, 1985) and fell after exposure therapy (Reference Marks, Marset and BoulougourisMarks et al, 1971). Similar imagery has been little studied in obsessive-compulsive disorder (OCD), although sufferers are commonly troubled by ritual-evoking images that they then try to cancel by generating neutralising images (also called mental rituals). This paper reports a new way of studying such imagery, which was designed for a pilot controlled study of functional magnetic resonance imaging (fMRI). The fMRI was done before and after exposure and ritual prevention (ERP) therapy. ERP was guided in one group by human therapists and in another by a computer-aided system called BTSTEPS (BT=behaviour therapy). BTSTEPS enables patients to do effective ERP at home by using a workbook and telephoning a computer interactive voice-response system for instruction, feedback and rating of progress (Reference Greist, Marks and BaerGreist et al, 1998; Reference Marks, Baer and GreistMarks et al, 1998; Reference Bachofen, Nakagawa and MarksBachofen et al, 1999). A third, control, group of patients had relaxation without ERP, by audio-tape and a manual.
METHOD
Each patient was studied twice during fMRI, 2 days before and 2 days after 10 weeks of treatment.
Mental imagery during fMRI scanning
Pre-scanning rehearsal outside the scanner
Before the first fMRI session, the researcher asked the patient to choose and mentally rehearse for a few minutes each of three tasks to be performed during scanning. Each task involved imagining (I), an image designed to induce one of three states: (i) urge to ritualise; (ii) non-OCD anxiety, (iii) neutral state; followed by (II), a second image designed to cancel the first one. The scenarios imagined were specific to each patient, and the researcher wrote on a card the detailed content of each of the six images ((I) and (II) for each of the three tasks) which patients then rehearsed for a few minutes until they could generate each image reliably.
For image (I) (300 s) of each task, patients had to imagine a scene they said would:
-
(a) in task (i), evoke an urge to ritualise, e.g. “I am walking in the street and think I left something at home in the wrong position, can't return there to change it, and worry that my family will therefore die”;
-
(b) in task (ii), evoke anxiety unconnected to the OCD, e.g. “wake on the morning of my exam having slept badly, worry about my poor preparation, feel awful, and can't find my books for final review”;
-
(c) in task (iii), evoke a neutral state, e.g. “I sit by a lake in the warm summer sun reading a book I enjoy and feel relaxed”.
For image (II) (300 s) of each task patients had to switch to another scene that would:
-
(a) in task (i), cancel their ritual-evoking image, e.g. “I've hurried home and moved the misplaced object into its correct position to avert danger”;
-
(b) in task (ii), stop their non-OCD anxiety-evoking image, e.g. “I've finished writing the exam and know I passed it and feel better”;
-
(c) in task (iii), switch from one neutral scene to another, e.g. “I lie dreamily on a lilo in a warm pool almost falling asleep.
Protocol inside the fMRI scanner
After preliminary adjustment and when patients felt comfortable (10-15 min) they had three 600 s fMRI scanning runs, one during each of the three tasks (ritual-evoking, anxiety-evoking, or pleasant). The order of the three tasks was allocated randomly across patients. Patients did not know which order of tasks they would be asked to do until the moment the researcher asked them to start each task.
During scanning patients were invited to lie still and to generate and hold the image for the given task when instructed. Immediately before the task the researcher read from the prepared card into the scanner microphone the image the patient was to imagine. When the subject said that he or she had generated the image clearly and vividly, fMRI scanning began and continued for 600 s. After 300 s (end of image (a)) the researcher read to the patient the cancelling image (b) to start and hold for 300 s.
During each 600 s period of imagery and scan, the researcher asked subjects every 30 s to rate their anxiety/discomfort rating on a 0-8 scale (0=no anxiety; 8=panic) by saying the relevant number out loud. This yielded a total of 20 self-ratings per 600 s task. Between the three tasks, patients rested in the scanner for 1-2 min.
The treatments
The pilot randomised controlled trial used the protocol of a multi-centre randomised controlled test of BTSTEPS in North America done in parallel without fMRI scans (further details available from the author upon request), and was approved by the local Ethical Committee. Fifteen patients were randomly allocated (by computer-generated random numbers, with minimalisation regarding numbers per cell) to have one of three treatments: (a) ERP guided by BTSTEPS (group B, n=5); (b) ERP guided face-to-face by a therapist (group T, n=4); and (c) control relaxation treatment (group R, n=6) guided by an audio-tape plus manual given by a therapist. After treatment, 100 patients in group R who had not improved crossed over to have the group B treatment, and after that had a third fMRI scan.
The three treatment conditions
All three treatments were given over 10 weeks, and patients were asked to keep daily diaries of ERP, or of relaxation, homework done. Group T patients had 10 weekly 60-min sessions with a therapist. Patients in groups B and R, after being told after initial assessment what to do, had no further contact with a therapist; however, they saw the study coordinator 2 weeks before starting (for screening for suitability for the trial and to give written consent to participate), at baseline (randomisation to start of treatment), and at weeks 2, 6 and 10 (end of treatment) for rating of outcome and checking of mood, and they could contact the coordinator by telephone if there were problems.
Patients in group B were given a BTSTEPS workbook and an identification number and chose a personal four-digit password number for confidentiality. They could access the BTSTEPS computer tollfree 24 hours a day, 7 days a week. On the phone, patients performed repeated computer interviews for self-assessment and ERP self-treatment, driving their interviews mainly by key-presses on their telephone keypad. Users did each telephone interview after completing the relevant section in their workbook.
For group T, ERP was guided face-to-face by a live trained human therapist. Patients were taught prolonged daily self-exposure to situations that evoked rituals and obsessions and to resist ensuing urges to ritualise. One therapist left on maternity leave half-way through treatment, after which another continued the treatment.
Patients in group R were asked to do daily self-administered progressive relaxation exercises along the careful lines of Jacobsen (Reference Jacobsen1938), and were given a structured relaxation audio-tape and manual to help them.
All patients were asked to do ERP or relaxation homework exercises for an hour a day and to record these exercises in daily ERP or relaxation homework diaries.
Patient inclusion criteria
Minimum age 14; having had OCD (DSM-IV criteria; American Psychiatric Association, 1994) for over 2 years; total score on the Yale-Brown Obsessive Compulsive Scale (YBOCS; Reference Goodman, Price and RasmussenGoodman et al, 1989) over 15, including over 7 on ‘compulsions’ or over 9 on that sub-scale if the YBOCS total was 9-14; if on serotonergic antidepressants (clomipramine, paroxetine, sertraline, fluoxetine or fluvoxamine), they must have been on a stable dose for over 3 months before enrolment; must have given written informed consent.
Must have none of the following: other psychotropic medication in the past 3 months; electroconvulsive therapy in the past 6 months; suicidal plans; score greater than 15 on a depression scale based on the 17-item Hamilton Rating Scale for Depression (HRSD); bipolar disorder; history of major tics or Tourette's syndrome; schizophrenia; unstable medical condition; alcohol/substance abuse in the 6 months before enrolment; organic brain syndrome; developmental disorder or disability impairing participation or understanding of ERP or relaxation; schizotypal personality disorder; psychosurgery; need for in-patient care.
RESULTS
Two weeks before the trial started, a psychiatrist screened 35 patients face-to-face, of whom 20 were unsuitable (seven were unmotivated or did not attend, seven only wanted human-guided ERP, four had a disqualifying medical condition, one feared being scanned, one had just begun a course of selective serotonin-re-uptake inhibitor (SSRI)). The 15 patients enrolled were randomly allocated at week 0 (baseline) to have 10 weeks of treatment in one of the three conditions: ERP guided by the BTSTEPS workblook and computer (group B, n=5), ERP guided face-to-face by a therapist (group T, n=4) or relaxation guided by an audio-tape+manual (group R, n=6). After group R had finished the course, two patients who had not improved were then crossed over to have group B's treatment; they completed this and then had a further fMRI scan (the remaining patients in group R did not complete that final part of the protocol).
Description of patients
The 15 patients (nine men, six women) came from a waiting list for out-patient behaviour therapy. All were native English speakers living in greater London. Their mean age was 32 years (s.d.=7). Twelve were employed or were students. The mean of the OCD duration was 18 years (s.d.=10); their main problems were checking (six), washing (three), mental rituals (four), and a mixture of these in two patients. Seven were on a stable dose of clomipramine or an SSRI. The three treatment groups were similar in all these respects and as regards chronic moderate-to-severe OCD and work/social disability. At week 0, self-rated mean scores were: YBOCS 28.2 (s.d.=5.3; actual range 6-40, possible range 0-40); work/social adjustment (WSA; Reference MarksMarks, 1986) total 21 (s.d.=8.2; possible range 0-40), Hamilton depression (six-item Bech version; Reference Bech, Allerup and GramBech et al, 1981) 9.4 (s.d.=7; possible range 0-40) (higher values=more severe OCD). One subject did not complete the protocol because of claustrophobia in the scanner. Another persisted, but was overwhelmed by panic throughout. The analysis below therefore refers mostly to the 13 patients who completed the protocol.
Pre-treatment responses to task images
The three tasks were to imagine a ritual-evoking (OCD), anxiety-(non-OCD) evoking or neutral scene for 300 s (image (I)) and then to imagine a scene to cancel it for 300 s (image (II), see above). Patients rated discomfort every 30 s; their 10 discomfort ratings per 300 s image were pooled for that image.
Image (I) evoked substantial discomfort with both OCD and anxiety (non-OCD) scenes, but not with neutral scenes. On a scale of 0-8 (8=most discomfort possible), mean discomfort evoked by the OCD image was 5.25 (s.d.=1.6, range 2.3-7.9), by the anxious (non-OCD) image it was 4.85 (s.d.=1.68, range 2.5-7.5) and by the neutral scene was 1.55 (s.d.=1.3, range 0-4.5) (Fig. 1: OCD I, ANX I and NEU I). During both the OCD image and the anxiety (non-OCD) image, discomfort was significantly higher than during the neutral image (respectively t=6.07, t=5.12; P < 0.0001, P < 0.0001).
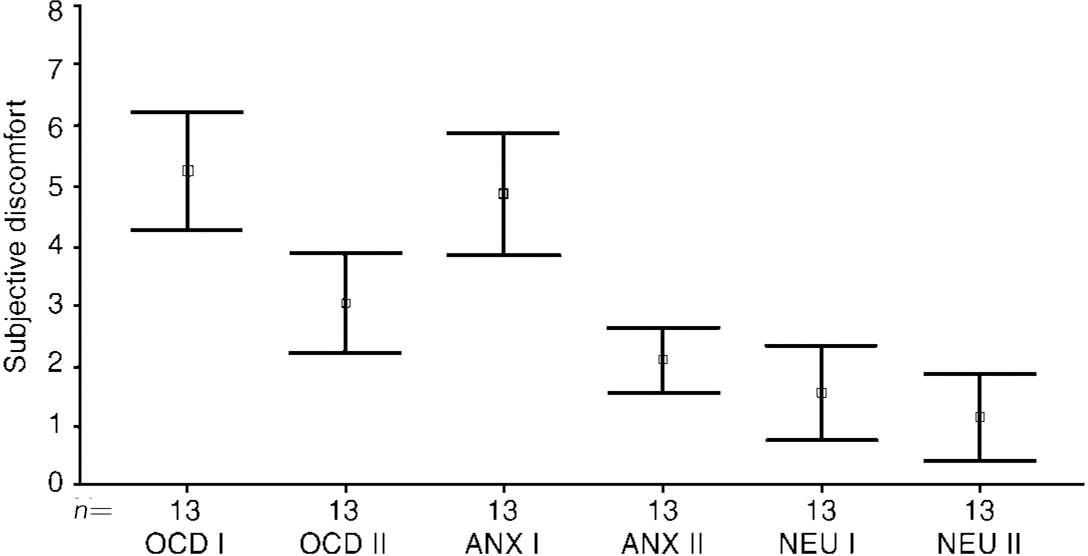
Fig. 1 Pre-treatment image (I) and cancelling image (II) (n=13). Type of image: OCD; anxiety (non-OCD); neutral. Higher score=more discomfort.
The cancelling image (II) successfully reduced discomfort to both OCD and anxiety (non-OCD) scenes and there was little discomfort during the neutral scene (Fig. 1: OCD II, Anx II and NEU II). Mean discomfort scores during the OCD task changed from 5.25 (s.d.=1.61) for image (I) to 3.05 (s.d.=1.37) for its cancelling image (II) (t=5.85; P=0.001) (Fig. 2). Mean discomfort scores during the anxiety (non-OCD) task changed from 4.85 (s.d.=1.68) for image (I) to 2.09 (s.d.=0.89) for its cancelling image (II) (t=6.12; P=0.001). Though the cancelling image (II) significantly reduced discomfort from image (I), that reduction was not right down to the level found during neutral image (II).
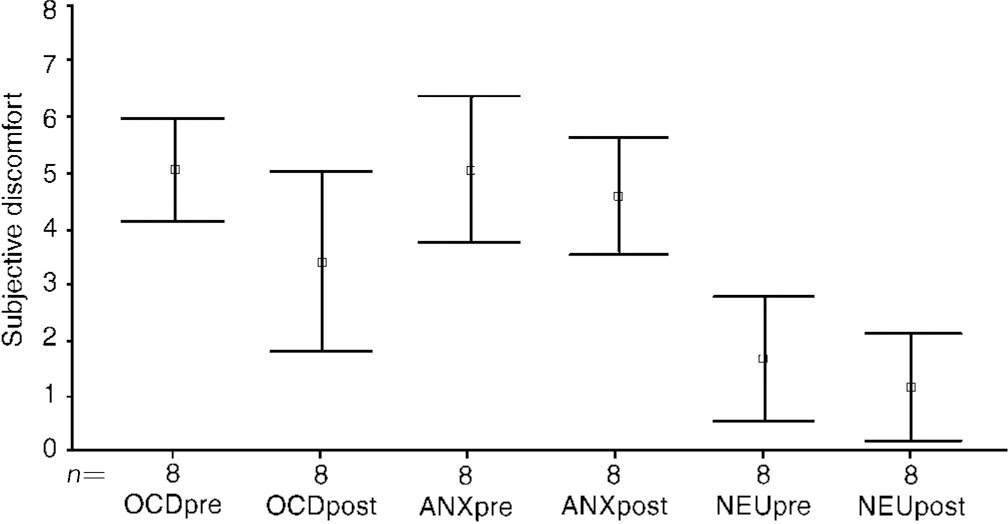
Fig. 2 Image (I): pre- and post-exposure treatment (n=8). Type of image (I): OCD, anxiety (non-OCD); neutral
Change from before to after treatment in response to image (I)
During OCD image (I), discomfort fell significantly from before to after the treatment in the two ERP groups B and T pooled, from 5.1 (s.d.=1.08) to 3.4 (s.d.=1.9) (t=2.68, P=0.03) (Fig. 2: OCDpre and OCDpost). For the two ERP groups separated, the fall in group B was from a mean of 4.9 (s.d.=1) to 2.8 (s.d.=1.4) (t=2.5, P=0.07), and less in group T, from 5.5 (s.d.=0.7) to 4.4 (s.d.=2.6) (t=9, P=0.5). Discomfort during OCD image (I) remained unchanged before and after treatment in group R: 5.7 (s.d.=3.0) to 5.8 (s.d.=2.7).
During anxiety (non-OCD) image (I), discomfort showed almost no change from before to after treatment in groups B (4.0→4.2), T (5.0→5.3) or R (5.2→5.4).
During the neutral image (I) too, there was almost no change from before to after treatment: group B 1.2→0.8, group T 2.1→2.3, group R (0.9→0.6).
Change from before to after treatment in response to image (II)
Before treatment, the cancelling image (II) had reduced discomfort during OCD and the non-OCD anxiety image (I), but some discomfort had remained during image (II). From before to after treatment, during OCD image (II) discomfort fell significantly in the two ERP groups B and T pooled, from a mean of 3.5 (s.d.=1.0) to 1.8 (s.d.=1.8) (t=1.9, P=0.02) (Fig. 3: OCDpre and OCDpost). For the two groups separated, the fall in group B was from a mean of 3.5 (s.d.=1.2) to 1.6 (s.d.=1.3) (t=2.19, P=0.09), and less in group T from 3.5 (s.d.=1.0) to 2.0 (s.d.=1.9) (t=0.17, P=0.23). Discomfort during OCD image (II) rose marginally though significantly from before to after treatment in group R, from 2.7 (s.d.=2.1) to 3.1 (s.d.=2.0) (P=0.02).
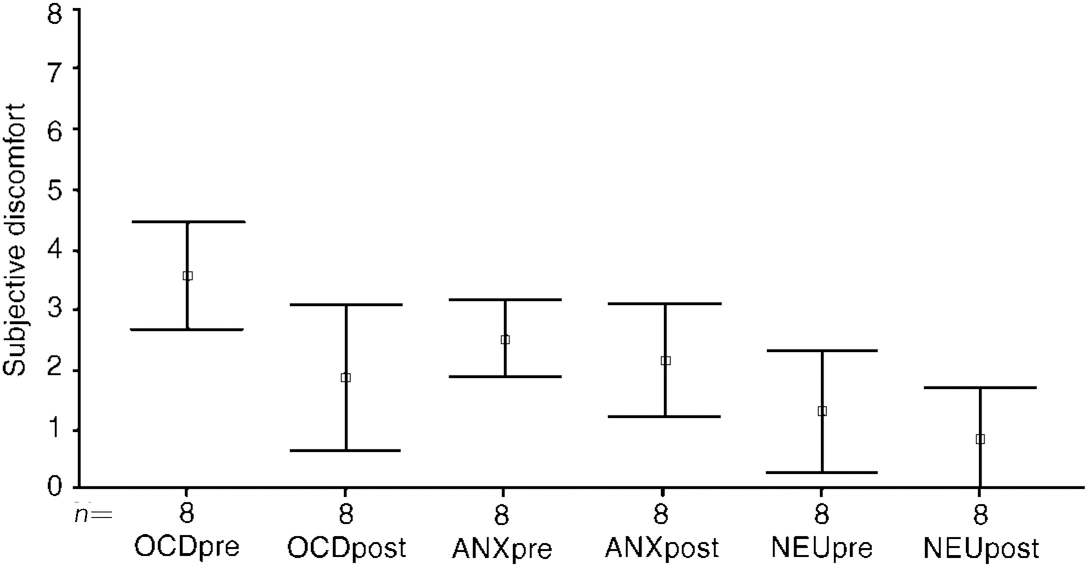
Fig. 3 Cancelling image (II) pre- and post-exposure treatment (n=8). Type of image (II): OCD; anxiety (non-OCD); neutral.
During the anxiety (non-OCD) image (II), discomfort did not change significantly from before to after treatment in the two ERP groups B and T pooled (2.4→2.08, t=1.44, P=0.19) or separated (B: 2.14→1.5, t=1.8, P=0.14, T: 2.9→3.0, t=1.0, P=0.4) or in group R (1.9→2.2, t=0.87, P=0.5).
During the neutral image (II), discomfort remained low in all groups from before to after treatment: groups B and T pooled, 1.2 (s.d.=1.2) to 0.79 (s.d.=0.8), t=2.4, P0.05; group B 0.92→0.44, t=2.2, P=0.1; group T 1.7→1.4, t=0.99, P=0.43; group R 1.4→0.7, t=1.5, P=0.28. For the two ERP groups pooled, the low discomfort during neutral image (II) fell significantly further after treatment.
The fall in discomfort during the cancelling OCD image (II) from before to after treatment was thus significant only after exposure, not relaxation. After treatment, although the cancelling image still reduced discomfort from both OCD and anxiety (non-OCD) image (I), this reduction was not right down to the low level rated during the neutral image (II).
Changes in imagery v. changes in scores on clinical scales
Before treatment, during the OCD imagery, neither the amount of discomfort it evoked nor the discomfort remaining after its cancellation correlated significantly with clinical scores, respective correlations being: YBOCS (r=0.42 and 0.00), WSA (r=0.33 and 0.34) or HRSD (r=-0.02 and 0.15). Discomfort during the OCD image at baseline and after its cancellation did not correlate significantly with post-treatment YBOCS or WSA scores.
Change in clinical scale scores from before to after treatment
Available results for group B are for six patients (four who were in the original group B and two who had group B's treatment after they had been in group R without improving), for group T for three patients, and for group R for four patients. Improvement from before to after treatment on the YBOCS total score was, for groups B, T and R respectively, a mean of 9.1 (29.7→18.1=36%, t=9.1, P < 0.001), 2.7 (27.3→24.6=10%, P=0.057) and ‒4.2 (28.5→32.7=-15%). For the two ERP groups B and T pooled, the fall in YBOCS score from before to after treatment remained significant, from 28.3 (s.d.=5.1) to 20.3 (s.d.=5.8) (t-4.5, P=0.002). The improvement on the WSA total score after treatment was, for groups B, T and R respectively, a mean of 9.0 (19→10=47%, P=0.03), 2.0 (25→23=8%) and ‒1.2 (24.5→25.7). For the two ERP groups B and T pooled, the WSA score improved significantly from a mean of 21 (s.d.=8.6) before treatment to 14.1 (s.d.=9.6) after treatment (t=-4.22, P0.003). The HRSD score did not change for groups B and T pooled, the mean values being: before treatment 12 (s.d.=8.5), after treatment 11.7 (s.d.=8).
Group R patients did not improve from before to after treatment (YBOCS 28.5→32.7; WSA 24.5→25.7; HRSD 8.3→20.3).
DISCUSSION
Intended effects of the design
The experimental method had the intended effects of facilitating the study of mental imagery in OCD and the disentangling of OCD-related from non-OCD-related anxiety. Before treatment, 13 of the 15 subjects with OCD could imagine the individually tailored scenes, and developed substantial discomfort during OCD and non-OCD anxiety imagery but not during neutral imagery. Patients rapidly reduced their discomfort by imagining individually tailored cancelling scenes (imagining that they had satisfactorily completed rituals in the case of OCD discomfort; imagining the end of the anxiety-evoking scene in the case of non-OCD anxiety). This result confirms what has long been suspected clinically but has not been demonstrated experimentally until now, that mental cancelling (neutralising) rituals of OCD can indeed lessen discomfort. The discomfort, however, was not completely reduced to the low level rated during a neutral image.
The experimental method also had its intended specific effect after treatment. After exposure therapy, discomfort fell significantly during the OCD image but not during the anxiety (non-OCD) image (after relaxation therapy there was no change in discomfort during either image). This specificity of the drop in discomfort during particular imagery fits the specific decrement in treated symptoms that usually occurs with exposure therapy. Interestingly, initial discomfort evoked by OCD scenes did not predict clinical response to exposure therapy.
It is noteworthy that patients were able to evoke different types of imagery despite having to give brief subjective discomfort ratings at 30-second intervals during the imagery tasks. Both type of image and type of treatment had been randomised.
Caveats about the small numbers
Only tentative conclusions can be drawn from the clinical measures, because of the small numbers of patients per cell. Exposure guided by BTSTEPS led to significant improvement which was clinically meaningful, with patients resuming more normal work and social lives. Gains from exposure guided by a therapist were unexpectedly small, and might perhaps be due to some patients having to change therapist half-way when their original therapist had to go on maternity leave. The facts that relaxation (without exposure) guided by an audio-tape and manual did not help patients, and that two patients who did not improve with relaxation went on to improve with subsequent exposure guided by BTSTEPS, fitted past results with OCD, in which relaxation was guided by a therapist (Reference MarksMarks, 1987).
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Ritual-evoking mental imagery in obsessive-compulsive disorder (OCD) can be studied systematically.
-
▪ Ritual-evoking imagery evokes more discomfort than neutral imagery; that discomfort can be reduced by cancelling imagery.
-
▪ Discomfort during ritual-evoking imagery falls significantly after successful exposure therapy for OCD, unlike discomfort associated with imagery that evokes non-OCD anxiety.
LIMITATIONS
-
▪ Numbers of patients were small so conclusions can only be tentative.
-
▪ Improvement in the therapist-guided exposure group was atypically small.
-
▪ The relaxation treatment was delivered by audio-tape.
ACKNOWLEDGEMENTS
Part of the work was funded by a grant from Pfizer-US. Professor Steve Williams facilitated carrying out of the testing during fMRI scanning.
I.M.M., J.G. and L.B. have intellectual property rights in the computer system that guided exposure therapy.






eLetters
No eLetters have been published for this article.