It has long been recognized that a cerebrospinal (CSF) pleocytosis may be observed after the occurrence of one or more epileptic seizures.Reference Patterson and Levi 1 - Reference Johnson, Michelson and Lyons 12 In many cases, it is readily apparent that the CSF pleocytosis is directly related to the etiology of the seizure(s). In other cases, and with support from a large number of publications in the literature, clinicians may conclude that a CSF pleocytosis was likely caused by the seizures themselves.Reference Patterson and Levi 1 - Reference Johnson, Michelson and Lyons 12 In light of important advances in diagnosing brain diseases with modern neuroimaging (e.g. magnetic resonance imaging), improved diagnosis of central nervous system infections using molecular techniques, and recognition of autoimmune encephalitis, we believed a reevaluation was needed. We sought to determine whether CSF pleocytosis associated with seizures is due to the underlying cause of the seizures or if there is strong evidence that seizures themselves can actually induce a CSF pleocytosis. We evaluated patients admitted to critical care units; many of the patients had status epilepticus (SE). Previous reports indicate that CSF pleocytosis tends to follow repeated or prolonged seizures.Reference Schmidley and Simon 5 , Reference Edwards, Schmidley and Simon 6 If seizures themselves can induce CSF pleocytosis, then this phenomenon is expected to be more readily observed in a patient group with SE.
Methods
Study Design and Patient Identification
We completed a retrospective chart review of adult patients (age 18 years or older) with seizures or SE in the medical and surgical intensive care units of the Winnipeg Health Sciences Centre, which is an urban tertiary care centre, over the 4-year period between January 2009 and December 2012. Patients were identified through the Critical Care Information Management Database at the Health Sciences Centre in Winnipeg, Canada. The database maintains a record of all patients admitted to critical care units with admission and acquired diagnoses (see http://www.wrha.mb.ca/prog/criticalcare/research.php for details). This database has previously been described in theliterature.Reference Bhaskaran, Johnson and Bolton 13 Each critical care patient has a diagnostic summary completed by the attending physician, which are reviewed by research nurses who input the information into a database. At discharge, data sheets are audited and any missing information is obtained through review of the chart. A database management committee supervises all aspects of the database, including software, data set, data verification, and collection procedures.Reference Bhaskaran, Johnson and Bolton 13
Laboratory Determination of CSF Parameters
CSF pleocytosis was defined as a CSF white blood cell (WBC) count >5×106/L, as per the laboratory range and consistent with conventional definitions. All CSF counts at our center were counted manually using a Neubauer chamber with a coefficient of variation (CV) of 45% (+90% for 2CV) with the same cell concentration.Reference Karcher and McPherson 14
Inclusion and Exclusion Criteria
Patients were included for analysis if they underwent lumbar puncture for CSF analysis within 5 days of their first seizure or at the beginning of the onset of SE. Only the first lumbar puncture results were included in the analysis. Patients were excluded if their CSF analysis did not include a cell count and differential, Gram stain, and culture. To ensure the uniformity of laboratory analysis, patients whose lumbar puncture was performed at a different site (i.e. before referral to our site) were excluded.
Results
A total of 426 patients admitted to the medical and surgical intensive care units were given the diagnosis of seizure during the study period. Of these, 51 met the study inclusion and exclusion criteria (Figure 1).

Figure 1 Consolidated Standards of Reporting Trials–style diagram showing how study patients were selected.
Patient Characteristics
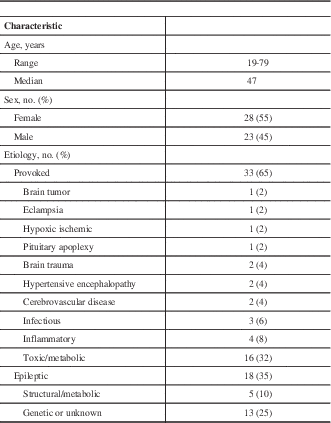
Information on the patients’ history including age, seizure semiology, seizure duration, time from seizure to lumbar puncture, and the treating team’s leading diagnosis were extracted from the medical charts. Laboratory investigations, electroencephalography, and computed tomography (CT) and/or MRI data were also collected. The clinical characteristics of all patients are shown in Table 1. The mean time interval between the noted seizure and lumbar puncture was 34.6 hours. Nine patients had previously diagnosed epilepsy.
Table 1 Patient characteristics of 51 patients with seizures and postictal CSF analyses

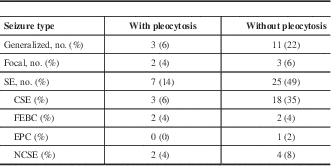
Seizures were classified according to the 2010 International League Against Epilepsy (ILAE) definition.Reference Berg, Berkovic and Brodie 15 Although the ILAE definitions from 1989 continue to be in common use in clinical practice, the 2010 definitions use more accurate and evidence based terminology. Seizures were broadly classified by mode of onset into generalized, focal, or SE (Table 2). Isolated seizures were then classified into provoked seizures and epileptic seizures, with epilepsy further subdivided into structural/metabolic or genetic/unknown subtypes. SE was defined using the 2015 definition of the ILAE Task Force on Classification of Status Epilepticus.Reference Trinka, Cock and Hesdorffer 16 SE was further classified into SE with and without prominent motor symptoms.
Table 2 Seizure characteristics of 51 patients with seizures and postictal CSF analyses

CSE=convulsive status epilepticus; EPC=epilepsia partialis continua; FEBC=focal evolving to bilateral convulsive; NCSE=nonconvulsive status epilepticus.
Of the 51 patients who met the inclusion/exclusion criteria, 12 patients had a CSF pleocytosis and all had a probable etiology for the CSF pleocytosis related to an underlying acute or chronic brain disorder (Table 3).
Table 3 Probable cause of CSF pleocytosis in 12 patients

FLAIR=fluid-attenuated inversion recovery; ND=not done.
* The significance of these findings is uncertain and could be attributable to the paramagnetic effect of supplemental oxygen.Reference Anzai, Ishikawa, Shaw, Artru, Yarnykh and Maravilla 33
Discussion
A CSF pleocytosis was noted after the occurrence of seizures in a report from 1926 by Patterson and LeviReference Patterson and Levi 1 and other reports soon followed.Reference Neel 2 , Reference Lennox and Merritt 3 A systematic literature review (Table 4) reveals a variety of studies (Table 5), many performed decades ago, that have led to a widely held belief that seizures themselves may be the cause of CSF pleocytosis in a minority of cases.
Table 4 CSF pleocytosis search strategy

Table 5 Key reports in adult patients with seizures and CSF pleocytosis

GTC=general tonic-clonic.
* We have excluded all cases in which the authors defined CSF pleocytosis as a WBC >1×106 polymorphonuclear leukocyte/l with <5×106 WBC/l.
† This study excluded patients who had known diseases associated with CSF pleocytosis.
‡ We excluded a patient with subarachnoid hemorrhage with bloody CSF.
§ CSF pleocytosis was defined as >3×106 WBC/l. Patients with seizures resulting from electrolyte disturbances, metabolic causes, acute brain disease, or trauma were excluded. Five patients were diagnosed with cerebral tumors.
|| CSF pleocytosis was defined as >4×106 WBC/l.
In a critical care population, we identified 51 patients over a 4-year period with seizures and postictal CSF analyses that met the study inclusion criteria. Twelve of these 51 patients (23.5%) had a CSF pleocytosis, and we identified a probable cause for the pleocytosis in all of these cases (Table 3). Some of the established etiologies had very strong associations with the presence of CSF pleocytosis (e.g. cryptococcal meningitis), whereas for others such as cocaine intoxication there was evidence with less strong support in the literature.Reference Alexopoulou, Deutsch and Dourakis 27 , Reference Gradon and Wityk 28 A nonprogressive vasculitis may explain CSF pleocytosis in cocaine intoxication.Reference Alexopoulou, Deutsch and Dourakis 27 , Reference Gradon and Wityk 28 Structural lesions were demonstrated with MRI in eight of the 10 cases (80%) in which this imaging was performed (Table 3); hence, we did not find a single case of CSF pleocytosis in which the seizures themselves were the only identified probable cause of the pleocytosis.
Many classical studies, mostly performed decades ago, indicated that 2% to 20% of patients had a CSF pleocytosis in association with seizures in the absence of an identified underlying brain process. We reevaluated these heterogeneous studies that had variable definitions of CSF pleocytosis (Table 5). Many of these reports excluded patients with known underlying causes for CSF pleocytosis such as infection, inflammation, trauma, or neoplastic processes, and the exclusion criteria were not always clearly laid out. It is interesting to note that alcohol withdrawal was not infrequently associated with seizures and CSF pleocytosis (14 patients in two reports) (Table 5). We suspect that this may, at least in part, be related to unrecognized head injuries resulting in seizures and CSF pleocytosis as previously suggested.Reference Barry and Hauser 11 The relationship of CSF pleocytosis with seizures related to isoniazid intoxication in two of the reports (involving five patients)Reference Aminoff and Simon 4 , Reference Schmidley and Simon 5 is interesting. This has been subsequently reported in an additional case report.Reference Ehsan and Malkoff 34 Isoniazid intoxication may induce a CSF pleocytosis through a mechanism that has not yet been defined. Observations from these studies gave rise to the widely held and accepted concept that in a minority of cases seizures themselves are the probable cause of CSF pleocytosis through unknown mechanisms. It is likely that potential causes of the CSF pleocytosis were underrecognized, in part, because of utilization of insensitive imaging investigations (e.g. CT scans) from a previous era.
Limitations of this study include the retrospective design, heterogeneous seizure population, and variable time from seizure to lumbar puncture (mean, 34.6 hours). Strengths of the study include the critical care setting because these patients are typically more thoroughly investigated and have more detailed charts than non–critical care patients. In addition, studying patients with the most severe seizure types such as SE, ensures CSF pleocytosis was not overlooked by only assessing self-limited or milder seizure types. Although our patient selection represents a referral bias, this bias aims to identify the patients most likely to have CSF pleocytosis given the type and severity of illnesses seen in a critical care setting.
Previous studies have suggested, but do not provide supportive evidence, that seizures may cause structural breakdown of the blood-brain barrier (BBB), leading to CSF pleocytosis.Reference Barry and Hauser 11 , Reference Johnson, Michelson and Lyons 12 Although BBB dysfunction with seizures is very well-documented, the available evidence does not provide an explanation for the presence of cells in the CSF.Reference Gorter, van Vliet and Aronica 35 - Reference van Vliet, Aronica and Gorter 37 Numerous experimental models have established BBB dysfunction in the setting of seizures by showing extravasation of albumin, but not of cells, out of the blood vessels. In fact, one study showed that “neither acute-induced nor chronic seizures correlate with WBC brain parenchymal migration while albumin and [immunoglobulin]G brain leakage is a hallmark of acute and chronic seizures.”Reference Marchi, Teng and Ghosh 38 This indicates that BBB dysfunction with seizures is sufficient to lead to extravasation of smaller molecules, but not of larger blood components such as cells. This is an area requiring further investigation, but currently a clear BBB mechanism for induction of CSF pleocytosis following seizures cannot be confirmed.
There is an accumulating body of research evaluating whether seizures in the absence of an underlying brain process can induce inflammation in the central nervous system and CSF. A recent meta-analysis concludes that “inflammatory pathways are involved in epilepsy” and looks at the role of cytokines in the blood, brain, and CSF.Reference de Vries, van den Munckhof, Braun, van Royen-Kerkhof, de and Jansen 39 Certain cytokines (interleukin-17 and interleukin-22) that were found to be elevated in brain tissue can also cause BBB dysfunction and potentially attract inflammatory cells, but their role in producing a CSF pleocytosis has not been addressed. Current opinion on the mechanism of seizures indicates that epileptic seizures arise as an imbalance between excitatory and inhibitory forces in the brain.Reference Staley 40 The role of inflammation in this process and the mechanism by which seizures are initiated, sustained, and terminated are unknown.Reference Trevelyan, Muldoon, Merricks, Racca and Staley 41 , Reference de Curtis and Avoli 42 The lack of a clear understanding of these mechanisms complicates our understanding of the inflammatory effect of seizures on the brain at a cellular and tissue level.
A patient’s underlying acute brain pathology or, less commonly, a chronic brain process, provides the best explanation for both the occurrence of seizures and CSF pleocytosis. Many acute pathologies such as meningitis, trauma, and stroke are well-known to cause both seizures and CSF pleocytosis. We conclude that seizures, in the absence of an underlying acute or chronic brain process, are very unlikely to be the cause of postictal CSF pleocytosis and that with appropriate investigations an underlying cause for CSF pleocytosis can be found for most patients. It is unclear if seizures themselves are at all capable of directly inducing a CSF pleocytosis. However, if this actually occurs, then it must occur very rarely and seizures should never be assumed to be the cause of a CSF pleocytosis. Appropriate investigations, including neuroimaging and other laboratory investigations, should always be performed to exclude treatable causes.
Acknowledgements and Funding
The Critical Care Information Management Database at the Winnipeg Health Sciences Centre is supported by the Department of Internal Medicine at the University of Manitoba.
Disclosures
CS and ACJ do not have anything to disclose.