Primary liver cancer is the third leading cause of cancer-related death worldwide(Reference Bray, Ferlay and Soerjomataram1). Hepatocellular carcinoma (HCC) is the most common (>80 %) histological type of liver cancer. There are large variations in geographical distribution of liver cancer worldwide. The disease burden is highest in areas with endemic hepatitis B virus (HBV) infection, such as in Asian countries, specifically in the East and South-East Asia, while North and South America have a relatively low incidence(Reference Petrick, Florio and Znaor2). About 72 % of all liver cancer occurs in Asia, with China accounting for 47 % of the global burden(Reference Petrick, Florio and Znaor2). Established risk factors of liver cancer include chronic infection with HBV or hepatitis C virus (HCV), excessive alcohol consumption, non-alcoholic fatty liver disease (NAFLD) and aflatoxin exposure. Beyond chronic hepatitis infections(Reference Mittal and El-Serag3–Reference Yang, Gao and Li5), as shown in previous and our own studies, liver cancer risk consistently increased with obesity(Reference Yang, Petrick and Kelly6–Reference Liu, Warren Andersen and Wen11) and type 2 diabetes (T2D)(Reference Campbell, Newton and Freedman10,Reference Petrick, Freedman and Demuth12,Reference Chen, Wu and Saito13) , suggesting an important role of insulinaemic and inflammatory pathways in hepatocarcinogenesis.
Currently, the population seroprevalence of HBV or HCV continues to decline, which was possibly due to large-scale HBV vaccination in newborns and common anti-HCV treatment worldwide(Reference Zhu, Shao and Chen14,Reference Thrift, El-Serag and Kanwal15) , and may contribute to the future declining rates of liver cancer in traditionally high-incident areas including East Asia(Reference Petrick, Florio and Znaor2). However, such gains in the control of liver cancer are at risk of being reversed by the rising rates of T2D, obesity and NAFLD, which were largely related to unhealthy diet(Reference Ng, Fleming and Robinson16,Reference Wang, Gao and Zhang17) . For example, in historically low-incident areas such as the USA, the liver cancer rates have tripled since the 1980s(Reference Petrick, Florio and Znaor2), and metabolic diseases are more prevalent than HBV or HCV infections. Therefore, HCC cases that are attributed to metabolic disorders (32 %) are higher than HBV (4 %) and HCV (21 %)(Reference Makarova-Rusher, Altekruse and McNeel18). Diet is a modifiable component of lifestyle that was suspected to influence liver cancer development, but dietary aetiology of liver cancer remains poorly understood. The World Cancer Research Foundation International/American Institute for Cancer Research (WCRF/AICR) concluded in their most recent Continuous Update Project that the established dietary factors for liver cancer only include aflatoxin-contaminated foods and heavy alcohol drinking, and possibly coffee consumption(19).
The liver plays a key role not only in the metabolism of carbohydrates, fats and proteins, but also in detoxification and hormone production. Thus, diet has measurable biological impacts on key pathways hypothesised to be involved in liver cancer risk. The proposed mechanisms linking diet and liver cancer development are shown in Fig. 1. For example, dietary carbohydrates are the primary determinants of the post-prandial glucose and insulin response(Reference Ludwig20), impacting circulating blood glucose, insulin demand and the bioavailability of insulin-like growth factor-1 (IGF-1)(Reference Malaguarnera and Belfiore21,Reference Shan, Shen and Liu22) . The essential branched-chain amino acids (BCAA), namely, leucine, isoleucine and valine, are essential amino acids that have known chemopreventive properties in patients with cirrhosis(Reference Muto, Sato and Watanabe23), via inhibition of hepatic IGF signalling and blockade of the phosphatidylinositol-3-kinase/protein kinase B (PI3k/Akt)/mammalian target of rapamycin (mTOR) pathway(Reference Iwasa, Shimizu and Shiraki24,Reference Alexia, Fallot and Lasfer25) . Recently, emerging evidence also showed that poor diet may cause prominent changes in gut microbiota which induce intestinal bacterial overgrowth, dysbiosis, intestinal permeability, bacterial translocation and endotoxaemia, leading to liver inflammation, chronic fibrosis, liver cirrhosis and hepatocarcinogenesis through the gut–liver axis(Reference Yu and Schwabe26,Reference Gupta, Youn and Shin27) .

Fig. 1. Proposed mechanisms linking diet and liver cancer risk. T2D, type 2 diabetes; NAFLD, non-alcoholic fatty liver disease; IGF-1, insulin-like growth factor-1.
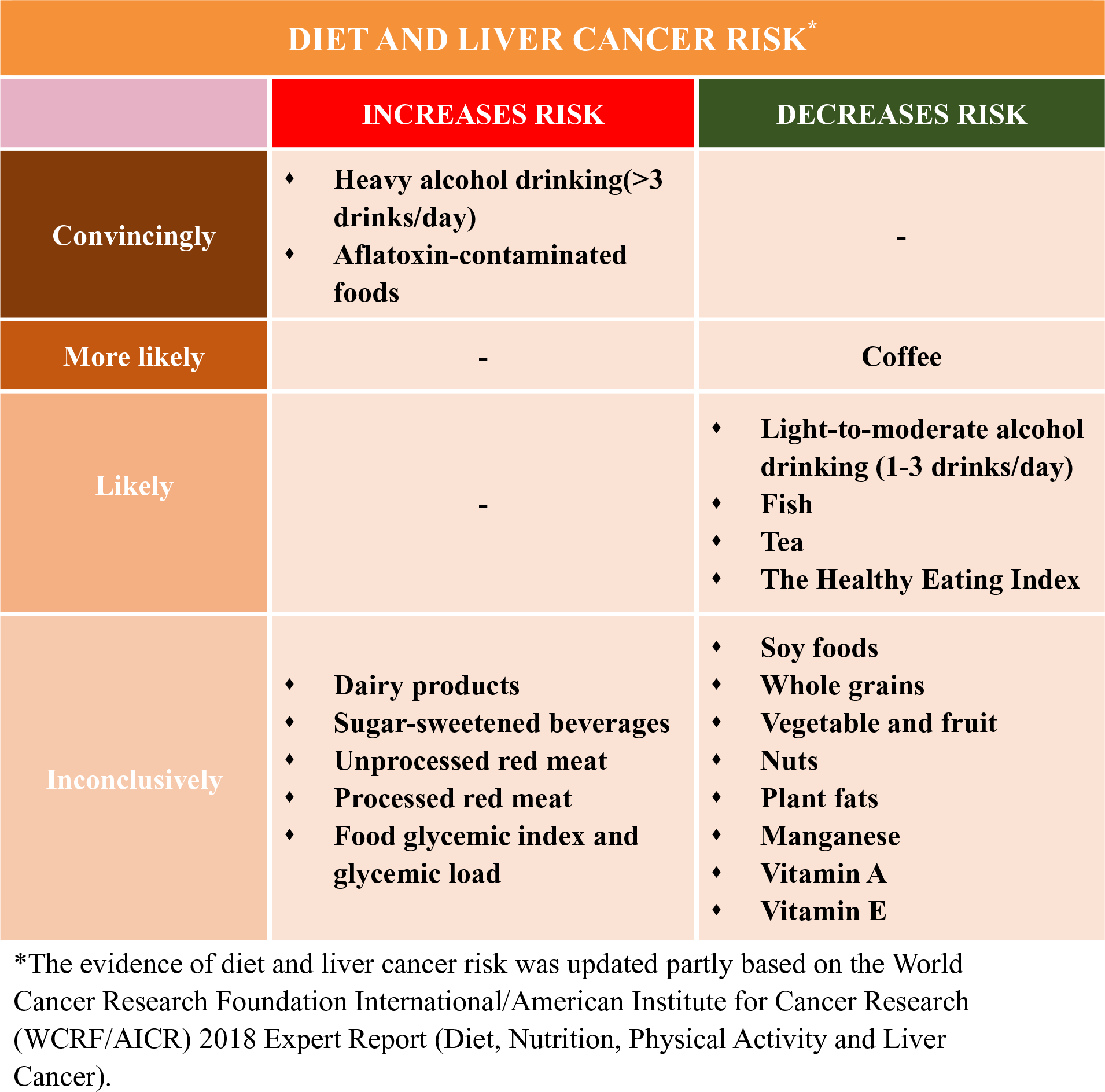
In this review, we summarised the current epidemiological evidence of diet and the risk of incident liver cancer. Compared with a recent systematic literature review (SLR) in Continuous Update Project in 2013 by WCRF/AICR(19), we summarised and evaluated relevant studies published up to August 2019. In addition to evaluating nutrients and food or food groups(19), we further described the role of different dietary patterns in liver cancer development. We did not discuss the role of aflatoxin because the recent Continuous Update Project has determined exposure to aflatoxin as a convincing cause of liver cancer, and there are few studies(Reference Chu, Yang and Wu28,Reference Chu, Yang and Wu29) on this topic since the 2013 SLR(19).
Macro-/micronutrients and liver cancer risk
Carbohydrates
Over the past decades, sugar-sweetened beverages consumption has increased dramatically worldwide(Reference Bleich, Wang and Wang30). Although still inconclusive, this rising trend in sugar consumption, practically simple sugar (mainly fructose consumption), has been positively associated with weight gain and obesity(Reference Rebollo, Roglans and Alegret31), insulin resistance and T2D(Reference Vila, Roglans and Perna32), and NAFLD(Reference Roglans, Vila and Farre33–Reference Rebollo, Roglans and Baena35). As mentioned above, insulin resistance, obesity and NAFLD may lead to the establishment of HCC. Thus, sugar-sweetened beverage consumption, mainly fructose, could be thereby linked to HCC development. This putative mechanism was supported by animal studies(Reference Laguna, Alegret and Roglans36). Likewise, food glycaemic index, glycaemic load, insulinaemic index and insulinaemic load, indicators of the glucose and insulin response to different dietary sugars, were positively associated with an increased risk of obesity(Reference Gaesser37), insulin resistance and T2D(Reference Lau, Faerch and Glumer38), liver steatosis(Reference Valtuena, Pellegrini and Ardigo39), and NAFLD(Reference Le and Bortolotti40).
However, only few observational studies have investigated the association between glycaemic index and glycaemic load (Reference Fedirko, Lukanova and Bamia41–Reference Vogtmann, Li and Shu46) and dietary sugar(Reference Fedirko, Lukanova and Bamia41,Reference Vogtmann, Li and Shu46–Reference Tasevska, Jiao and Cross48) in relation to liver cancer (online Supplementary Table S1). For glycaemic index and glycaemic load, the results among current epidemiological studies were inconclusive, varying from a positive(Reference George, Mayne and Leitzmann42,Reference Lagiou, Rossi and Tzonou44,Reference Rossi, Lipworth and Maso45) , null(Reference Fedirko, Lukanova and Bamia41,Reference Hu, La Vecchia and Augustin43,Reference Vogtmann, Li and Shu46) , to an inverse(Reference George, Mayne and Leitzmann42) association. Several studies(Reference George, Mayne and Leitzmann42,Reference Hu, La Vecchia and Augustin43,Reference Tasevska, Jiao and Cross48) have not considered the possible influence of chronic HBV and/or HCV infection in the analysis since they were primarily designed for cancer in general, and only two prospective studies(Reference Fedirko, Lukanova and Bamia41,Reference Rossi, Lipworth and Maso45) have adjusted for HBV and HCV infections in the analysis. In addition, the potential reverse causality and recall bias in retrospective case–control design(Reference Hu, La Vecchia and Augustin43,Reference Lagiou, Rossi and Tzonou44) may partially interpret such a discrepancy.
Similarly, there has been limited epidemiological evidence for the association between sugar-sweetened beverage intake and risk of HCC, with inconsistent results. Only two cohort studies(Reference Fedirko, Lukanova and Bamia41,Reference Vogtmann, Li and Shu46) have investigated the association between total carbohydrate consumption and liver cancer risk and yielded a null association, in which the chronic HBV and/or HCV infection have not been considered. Surprisingly, an inverse association of sucrose and fructose intake with the risk of HCC has been suggested in a case–control study nested within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort(Reference Fedirko, Lukanova and Bamia41) and in the National Institutes of Health-American Association of Retired Persons (NIH-AARP) cohort(Reference George, Mayne and Leitzmann42), respectively. However, the results could have been by chance given the limited number of liver cancer cases among the two studies. Overall, the relation has not been well defined based on current limited evidence. Given carbohydrates as a main energy source for human, future research should offer more insights on dietary carbohydrate quantity especially its quality with HCC risk, which may help individuals choose foods with ‘good’ carbohydrates. For example, in addition to glycaemic index, glycaemic load, insulinaemic index, and insulinaemic load, a carbohydrate:fibre ratio as a new measure of carbohydrate quality was shown to be more superior to other consumer-oriented methods for selecting more healthful foods(Reference Mozaffarian, Lee and Kennedy49), and a low carbohydrate:fibre ratio in diet was found to be associated with reduced risk of T2D(Reference AlEssa, Bhupathiraju and Malik50) and CHD(Reference AlEssa, Cohen and Malik51), though its association with liver cancer has not been studied.
Dietary fats/fatty acids
To date, the association between dietary fat intake and risk of HCC has not been well studied, and the existing epidemiological evidence is limited and inconclusive(Reference Kuper, Tzonou and Lagiou47,Reference Duarte-Salles, Fedirko and Stepien52–Reference Yang, Sui and Ma57) . In current existing three case–control studies that were all conducted in European populations, two(Reference Kuper, Tzonou and Lagiou47,Reference Polesel, Talamini and Montella54) reported a null association between total fats and HCC risk, while another one(Reference Hadziyannis, Tabor and Kaklamani55) showed a significant positive association (OR 1·4). In a hospital-based case–control study from Italy, participants with higher intake of PUFA, but not MUFA or SFA, had a significant lower risk of HCC (OR 0·60, 95 % CI 0·40, 0·88)(Reference Polesel, Talamini and Montella54). Prospective studies include one conducted in the NIH-AARP Diet and Health cohort, which showed a statistically significant positive association (hazards ratios (HR) per 5 % increase in energy) for total (HR 1·10) and saturated fat (HR 1·38), and a null association for monounsaturated (HR 1·18) or polyunsaturated fats (HR 1·06)(Reference Freedman, Cross and McGlynn53). Likewise, a cohort study among Singapore Chinese showed similar patterns with HR (the highest v. lowest quartile) of 1·26 for total, 1·40 for saturated and 0·90 for monounsaturated fats (all P values for trend across quartiles were >0·05)(Reference Koh, Dan and Goh56). In contrast, a prospective study from the EPIC(Reference Duarte-Salles, Fedirko and Stepien52) showed a suggestive inverse HCC association for total fats (highest v. lowest quartile, HR 0·74, 95 % CI 0·45, 1·23), monounsaturated fats (HR 0·53, 95 % CI 0·27, 1·01) and polyunsaturated fats (HR 0·86, 95 % CI 0·55, 1·35).
The discrepancy among cohort studies of fat and HCC could be partly explained by different dietary sources and intake levels of fat. For example, for total dietary fat, the average intake levels for participants in the NIH-AARP cohort(Reference Freedman, Cross and McGlynn53) were lower than those in the EPIC(Reference Duarte-Salles, Fedirko and Stepien52) and higher than those from the Singapore cohort(Reference Koh, Dan and Goh56). Additionally, the main food sources of monounsaturated fat within the NIH-AARP are meat and meat products (>20 %). Unlike the NIH-AARP cohort, the main food sources of monounsaturated fat in the EPIC study were olive oil and nuts (about 20 %). The vegetable (plant) fat including fats from olive oil and nuts was shown to be associated with a lower risk of HCC.
A large number of laboratory studies indicate that n-3 PUFA possessed anti-inflammatory activity by inhibiting IL-1 and TNF synthesis(Reference Endres, Ghorbani and Kelley58,Reference James, Gibson and Cleland59) , which can contribute in HCC prevention, given that chronic inflammation plays a central role in HCC development. n-3 PUFA might exert anticancer effects also through their ability to induce apoptosis, to modulate cell cycle and eicosanoid production(Reference Fauser, Prisciandaro and Cummins60). In particular, n-3 PUFA have been shown to inhibit HCC growth in vitro through the blockage of β-catenin and cyclo-oxygenase-2(Reference Lim, Han and Dai61). Furthermore, it is observed that n-3 PUFA can improve hepatic steatosis and insulin sensitivity and reduce inflammation in patients with NAFLD(Reference Capanni, Calella and Biagini62–Reference Di Minno, Russolillo and Lupoli64). Taken together, this evidence suggests a molecular mechanism of n-3 PUFA in HCC prevention. Conversely, metabolism of n-6 PUFA leads to increased levels of pro-inflammatory products, such as PG E2, thromboxane, and indirectly C-reactive protein, plasminogen activator inhibitor-1, TNF-a and IL-6(Reference Calder65,Reference Monteiro, Leslie and Moghadasian66) , which have been implicated in causing advanced fibrosis in non-alcoholic steatohepatitis, and subsequently to cirrhosis and ultimately to HCC as discussed above.
However, little is known about the relationship between dietary n-3 and n-6 PUFA and liver cancer in population studies. Only one study to date has investigated the association of n-6 PUFA with the risk of incident HCC in a cohort of 63 257 Chinese adults aged 45–74 years in Singapore and suggested a significantly positive association (HR 1·49)(Reference Koh, Dan and Goh56). This positive association was further confirmed among individuals who were free of chronic infection with HBV or HCV(Reference Koh, Dan and Goh56). Interestingly, they also found that higher n-6:n-3 PUFA ratio is associated with the increased risk of HCC, although with no association for the n-3 PUFA(Reference Koh, Dan and Goh56). Few studies have assessed the relation between n-3 PUFA and liver cancer risk, one in a Japanese cohort(Reference Sawada, Inoue and Iwasaki67) and one in a Italian hospital-based case–control study(Reference Polesel, Talamini and Montella54), both with an inverse association. Although there could be a potential beneficial role of plant fats and unsaturated fats especially n-3 PUFA in liver cancer development, no firm conclusion was reached based on current evidence. More epidemiological studies in this direction, together with considering the fat subtypes, fat food sources (animal, vegetable and dairy fat), and the possible substituting effect(Reference Song and Giovannucci68) of different fat subtypes such as substituting plant fat for animal or dairy fat, or substituting unsaturated fat for saturated fat in hepatocarcinogenesis are warranted, which may have both public health and aetiological importance.
Dietary proteins/amino acids
The three BCAA, leucine, isoleucine and valine, are among the nine essential amino acids for humans. They have been shown to affect gene expression, protein metabolism, apoptosis and regeneration of hepatocytes and insulin resistance(Reference Takami, Yamasaki and Saeki69). They have also been shown to inhibit the proliferation of liver cancer cells in vitro (Reference Sugiyama, Yu and Nagasue70). In addition, all three BCAA were found to accelerate insulin-induced vascular endothelial growth factor mRNA degradation at the post-transcriptional level, down-regulating vascular endothelial growth factor expression during the development of HCC(Reference Miuma, Ichikawa and Arima71). BCAA were also shown to induce apoptosis of liver cancer cell lines by inhibiting insulin-induced PI3K/Akt and NFκB pathways through mTORC1- and mTORC2-dependent mechanisms(Reference Hagiwara, Nishiyama and Ishizaki72). Moreover, BCAA may inhibit obesity-related hepatocarcinogenesis by suppressing the stimulatory effect of visfatin, an adipokine with a critical role in HCC proliferation(Reference Ninomiya, Shimizu and Imai73). In line with experimental studies, clinical studies showed that BCAA supplementation can help in the management of HCC(Reference Takami, Yamasaki and Saeki69), including reducing early recurrence of HCC(Reference Ichikawa, Okabayashi and Maeda74), improving quality of life(Reference Okabayashi, Iyoki and Sugimoto75,Reference Kuroda, Ushio and Miyamoto76) and liver functions(Reference Meng, Leung and Ho77) among patients undergoing liver resection for HCC, and increasing serum albumin levels during radiotherapy(Reference Lee, Seong and Bae78).
Interestingly, a recent meta-analysis of seven cohort and ten case–control studies indicated a potential protective association between BCAA and HCC risk(Reference Luo, Yang and Liu79). However, to date, there has been no epidemiological research to directly investigate the putative association between dietary BCAA intake and liver cancer risk to our knowledge.
Dietary trace elements and vitamins
In the two cohorts of Shanghai Men’s and Women’s Health Study with 132 765 Chinese adults and over 500 liver cancer cases, it suggested that dietary Mn intake was inversely associated with liver cancer risk (highest v. lowest quintile, HR 0·51, 95 % CI 0·35, 0·73; P trend = 0·001)(Reference Ma, Yang and Li80). This result was repeated in a nested case–control subset that adjusted for HBV infection(Reference Ma, Yang and Li80). However, we did not find any significant associations for folic acids or other trace elements including Se, Fe, Zn or Cu, although higher serum Cu and Cu:Zn ratio(Reference Fang, Chen and Wang81), and lower serum folate concentration(Reference Fang, Liu and Liao82) was suggested to be associated with poor prognosis among liver cancer patients. This is the first cohort design to investigate the association between trace elements and liver cancer risk, although two previous case–control studies have been conducted with limited cancer cases. One case–control study (ninety-seven cancer cases and 128 controls)(Reference Kuper, Tzonou and Lagiou47) showed a null association between trace elements and HCC, and another (185 cancer cases and 412 controls)(Reference Polesel, Talamini and Montella54) showed an inverse association for β-carotene intake and a suggestive inverse association with vitamin E intake. In the same cohorts, with 267 incident liver cancer cases, dietary vitamin E intake was inversely associated with liver cancer risk (highest v. lowest quintile, HR 0·60, 95 % CI 0·40, 0·89; P trend = 0·001)(Reference Zhang, Shu and Li83). Similar association was found for vitamin E supplement use (HR 0·52, 95 % CI 0·30, 0·90)(Reference Zhang, Shu and Li83).
In terms of vitamin A, interestingly, a case–control study in China showed a U-shaped association with HCC risk(Reference Lan, Zhang and Liao84). An intake level of 1000 μg retinol equivalent per d or more has shown beneficial association. Consistently, in another Shanghai male cohort(Reference Yuan, Gao and Ong85) and a Finnish cohort of middle-aged male smokers(Reference Lai, Weinstein and Albanes86), higher pre-diagnostic serum β-carotene and retinol levels were associated with lower risk of HCC. Although there is no observational studies investigating vitamin D intake in relation to HCC risk, a clinical cohort showed that only higher bioavailable (not bound to vitamin D-binding protein), rather than total circulating, 25-hydroxyvitamin D (25OHD) levels were associated with improved survival among HCC patients, indicating a potential utility of bioavailable 25OHD as a prognostic biomarker in HCC patients(Reference Fang, Long and Zhang87). Whether bioavailable but not total serum 25OHD is a useful biomarker in liver cancer risk prediction remains unknown.
The current experimental evidence supports the roles of several vitamins or trace elements (e.g. vitamins A, C and E, Se, Fe, Zn, Mn and Cu) as antioxidants in protection against oxidative stress (e.g. lipid peroxidation, DNA and protein damage)(Reference Wolonciej, Milewska and Roszkowska-Jakimiec88) which may generate reactive oxygen species and oxygen-free radicals, thereby relates to carcinogenesis(Reference Puertollano, Puertollano and de Cienfuegos89). Nonetheless, given the limited existing observational studies, all of which were conducted in China (high-risk country) except a cohort study in Finland(Reference Lai, Weinstein and Albanes86), and multiple comparisons among those studies(Reference Ma, Yang and Li80,Reference Zhang, Shu and Li83) , more related studies, especially studies in low-risk countries (e.g. European or Northern American countries) are warranted.
Foods or food groups and liver cancer risk
Dairy products
Previous studies suggested that high dairy product intake may increase the levels of plasma IGF-1(Reference Ma, Giovannucci and Pollak90–Reference Heaney, McCarron and Dawson-Hughes93). The increased concentration of IGF-1, an important factor in the regulation of cell proliferation, differentiation, apoptosis and carcinogenesis, might contribute to the development of several cancers including HCC in experimental studies(Reference Malaguarnera and Belfiore21,Reference Hopfner, Huether and Sutter94) . In line with the evidence, two cohort studies(Reference Duarte-Salles, Fedirko and Stepien95,Reference Yang, Sui and Ma96) but not all(Reference Kuper, Tzonou and Lagiou47,Reference Fukuda, Shibata and Hirohata97–Reference Park, Leitzmann and Subar101) showed that higher total dairy product intake (three serving per d or more) was associated with a statistically significant higher risk of HCC, while yogurt consumption was suggestively associated with lower HCC risk, and these associations remained within participants who were free of HBV and HCV infections (online Supplementary Table S2). Although dietary Ca, vitamin D, fat and protein from dairy sources were associated with increased HCC risk in EPIC cohort(Reference Duarte-Salles, Fedirko and Stepien95), we did not find such associations in the Nurses’ Health Study (NHS) and Health Professionals Follow-Up Study (HPFS)(Reference Yang, Sui and Ma96). Potential biologic mechanisms linking the positive associations between dairy products and HCC might include circulating IGF-1, but this needs further investigation in experimental and prospective settings. Future studies that comprehensively assess the association of dairy products according to different food groups (total dairy products, milk, cheese and yogurt) as well as their major micro- and macro-nutrient components (Ca, vitamin D, fats, lactose and protein) are needed to fully characterise the dairy products–liver cancer association.
Whole grains, fruit, vegetable and nuts
As mentioned above, we found that a vegetable-based dietary pattern was associated with a lower risk of liver cancer(Reference Zhang, Xiang and Li102). Consistently, total fibre and vegetable fibre, especially cereal fibre, were possibly associated with lower HCC risk, while fibre from fruit did not seem to be associated with HCC risk(Reference Fedirko, Lukanova and Bamia41,Reference Yang, Ma and Liu103) . These findings are less likely due to chance, since compared with fruit fibre, cereal fibre (possibly vegetable fibre) has been shown to be more consistently associated with lower risk of other health outcomes including total mortality(Reference Kim and Je104,Reference Aune, Keum and Giovannucci105) , CVD(Reference Aune, Keum and Giovannucci105,Reference Threapleton, Greenwood and Evans106) , T2D(Reference Aune, Keum and Giovannucci105,Reference Yao, Fang and Xu107) and colorectal cancer(Reference Aune, Chan and Lau108–Reference Schatzkin, Mouw and Park112). The exact reasons for such a difference remain unknown. A potential explanation is that fruits, particularly fruit juice, contain sugar or added sugar (e.g. fructose and sucrose), which may cause hepatic damage and NAFLD(Reference DiNicolantonio, Subramonian and O’Keefe113), thereby masking the possible benefit of fibre intake.
Nuts are rich in unsaturated fatty acids, vegetable protein, vitamins, folate, fibre, minerals and other bioactive compounds(Reference Ros114,Reference Ros and Hu115) . Nut consumption was associated with reduced risk of NAFLD(Reference Han, Jo and Lee116). We did not find any protective association between total nut or walnut consumption and HCC risk in the two USA cohorts, although increasing intake of tree nuts was found to possibly reduce the HCC risk(Reference Sui, Yang and Ma117). These findings need to be validated due to limited HCC cases and relatively low intake levels of tree nuts in the study.
Meats
In 2015, the International Agency for Research on Cancer classified consumption of red meats as ‘probably carcinogenic to humans’ (Group 2A), while consumption of processed red meats was classified as ‘carcinogenic to humans’ (Group 1)(Reference Bouvard, Loomis and Guyton118). In addition to high levels of saturated fat and haem Fe found in meats, possible underlying mechanisms include the potentially carcinogenic chemicals N-nitroso compounds formed endogenously from nitrate or nitrite during meat processing or preservation, and heterocyclic amines (HCA) during meat cooking. By contrast, white meats (i.e. poultry and fish), particularly fish intake, have been shown to decrease cancer risk(Reference Luo, Yang and Liu79,Reference Huang, Duan and Hu119) , possibly due to long-chain n-3 PUFA present in fish, particularly fatty fish. Also, white meat including fish and poultry is a rich source of BCAA, which may play a protective role in hepatocarcinogenesis (see Section 1.3).
There have been few cohort studies of the association between red meat intake and HCC risk(Reference Freedman, Cross and McGlynn53,Reference Fedirko, Trichopolou and Bamia120–Reference Ma, Yang and Li122) . For unprocessed red meat, two USA cohort studies, the NHS and HPFS(Reference Ma, Yang and Li122) as well as a European cohort study, the EPIC(Reference Fedirko, Trichopolou and Bamia120) did not observe an association between unprocessed red meat intake and HCC, which was consistent with findings from a recent meta-analysis(Reference Luo, Yang and Liu79), although another USA cohort, the NIH-AARP(Reference Freedman, Cross and McGlynn53), showed a suggestive positive association for unprocessed red meat. A non-significant association for unprocessed red meat with HCC was also seen in the Japanese Ministry of Education cohort(Reference Kurozawa, Ogimoto and Shibata121), showing no significant association between beef or pork intake and HCC mortality without adjustment for any risk factors(Reference Kurozawa, Ogimoto and Shibata121). Most current studies seemed to support a null association for processed meat(Reference Freedman, Cross and McGlynn53,Reference Luo, Yang and Liu79,Reference Fedirko, Trichopolou and Bamia120,Reference Kurozawa, Ogimoto and Shibata121) , or heterocyclic amines(Reference Freedman, Cross and McGlynn53), although a positive association between processed red meat and HCC risk was found in the NHS and HPFS(Reference Ma, Yang and Li122). Notably, cohort studies(Reference Freedman, Cross and McGlynn53,Reference Fedirko, Trichopolou and Bamia120,Reference Ma, Yang and Li122) and the meta-analyses(Reference Luo, Yang and Liu79,Reference Huang, Duan and Hu119) consistently suggested a protective association of white meat intake, particularly fish intake with HCC development, which was consistent with the findings form the most recent report by WCRF/AICR(19). Overall, intake of red meat particularly processed red meat may increase, while white meat possibly fish may decrease the risk of HCC. These findings need to be validated in more studies, ideally further considering the preparation or cooking methods of meat as well as the meat-derived mutagenicity or heterocyclic amines in liver cancer development.
Soya
Evidence showed that soya isoflavones may prevent liver cancer both in vitro and in vivo (Reference Su, Chow and Kung123). Compared with those in Europe and North America, Asia has one of the world’s highest intake levels of soya foods. There have been few observational studies of soya or soya isoflavones and liver cancer risk with inconclusive findings(Reference Zhang, Shu and Li83,Reference Sharp, Lagarde and Mizuno124–Reference Zamora-Ros, Fedirko and Trichopoulou127) . In a Japanese nested case–control study (176 HCC cases and 560 controls among Japanese A-bomb survivors), consumption of miso soup and tofu was significantly inversely associated with HCC risk accounting for HBV and HCV infections(Reference Sharp, Lagarde and Mizuno124). This finding was consistent with our cohort study in China showing that increasing soya intake may decrease liver cancer risk(Reference Zhang, Shu and Li83). On the contrary, soya isoflavones (genistein or daidzein) showed a positive association with HCC risk in women and showed a null association in men in another Japanese cohort (101 HCC cases), which also carefully considered the HBV and HCV infections(Reference Kurahashi, Inoue and Iwasaki125). There have been two studies of dietary flavonoids and HCC risk in non-Asian countries with relatively low intake levels of soya(Reference Lagiou, Rossi and Lagiou126,Reference Zamora-Ros, Fedirko and Trichopoulou127) . One is a hospital-based case–control study in Greece(Reference Lagiou, Rossi and Lagiou126) showing a null association between dietary flavonoids from fresh beans and whole-grain bread and HCC risk; another is a prospective study in Europe(Reference Zamora-Ros, Fedirko and Trichopoulou127) showing a non-significant association between isoflavone intake (about 40 % from soya foods) and HCC risk, although an inverse association between total flavonoid intake and HCC risk was observed in the two studies(Reference Lagiou, Rossi and Lagiou126,Reference Zamora-Ros, Fedirko and Trichopoulou127) . These contradict results would be partly interpreted by challenge in assessing soya food intake.
Recently, in a case–control study nested within the Shanghai Men’s Health Study and Shanghai Women’s Health Study, we found that urinary excretion of genistein (a metabolite of soya flavonoids) was associated with lower risk of liver cancer in women, but not in men(Reference Zhang, Wang and Gao128). Future epidemiological study using ‘omics’ technology (e.g. metabolomics) to develop a metabolic signature reflecting soya intake (internal exposure) is needed. This approach using high-throughput metabolomics profiling to measure intermediate molecules and products of metabolism; metabolomic profiles reflects individuals’ dietary intakes and other sources of variability in metabolism (e.g. genetic variations(Reference Illig, Gieger and Zhai129) and diet–gut microbiome interplay(Reference Shoaie, Ghaffari and Kovatcheva-Datchary130)), thereby objectively assesses the diet and metabolic responses to diet intake(Reference Guertin, Moore and Sampson131–Reference Guasch-Ferré, Bhupathiraju and Hu133).
Beverages
Tea
Tea is one of the most popular beverages consumed worldwide especially in China. Animal and in vitro studies showed a protective effect against cancer initiation and its subsequent development, which is largely owing to the polyphenolic antioxidants present in tea(Reference Yang, Wang and Lu134). We previously conducted a meta-analysis of six case–control and seven cohort studies and found a suggestive inverse association between total tea drinking and liver cancer risk (relative risk 0·77, 95 % CI 0·57, 1·03) and a significant inverse association for green tea (relative risk 0·79, 95 % CI 0·68, 0·93)(Reference Fon Sing, Yang and Gao135). After that, observational studies of tea drinking(Reference Bamia, Lagiou and Jenab136–Reference Tamura, Wada and Konishi138) consistently showed a protective association with HCC risk. Although these findings were not supported by a nested case–control study (211 cases and 1067 matched controls in Shanghai cohort)(Reference Butler, Huang and Wang139) showing that higher urinary levels of catechins (partly reflect green tea drinking) may not decrease but increase risk of HCC development (online Supplementary Table S3). Future prospective studies that account for different characteristics of tea such as tea types, preparation methods and tea strength are needed to fully characterise such an association, ideally with useful biomarkers that can better reflect tea consumption in a subset of participants to confirm the consistency and to adjust for measurement error if possible.
Coffee and alcohol
The evidence that coffee protects against liver cancer has been determined as ‘probable’ but not ‘convincing’ by WCRF/AICR in their 2013 SLR(19). After 2013 SLR, a total of sixteen relevant studies including eight meta-analyses(Reference Bravi, Bosetti and Tavani140–Reference Tamura, Hishida and Wakai147) and eight cohort studies(Reference Bamia, Lagiou and Jenab136,Reference Tamura, Wada and Konishi138,Reference Jang, Jeong and Lee148–Reference Loftfield, Rothwell and Sinha153) were published. All above studies support an inverse association between coffee and liver cancer risk. Current meta-analyses(Reference Bravi, Bosetti and Tavani140,Reference Bravi, Tavani and Bosetti144,Reference Godos, Micek and Marranzano145) consistently showed that increasing coffee consumption by one cup/d was associated with a 15–20 % risk reduction of HCC. The protective association between regular coffee intake and liver cancer risk appeared stronger in men, in Asian populations, and in case–control studies compared with those in women, in non-Asian populations, and in cohort studies, respectively. Compared with caffeinated coffee, decaffeinated coffee showed a weaker non-significant inverse association with liver cancer risk(Reference Kennedy, Roderick and Buchanan146). For cancer subtypes, there seemed no association between coffee consumption and biliary tract cancers including intrahepatic cholangiocarcinoma (ICC)(Reference Godos, Micek and Marranzano145). The above differential associations according to sex, type of coffee consumed (caffeinated v. decaffeinated coffee) and liver cancer subtype (HCC v. ICC) were replicated in the Liver Cancer Pooling Project, a consortium of over ten US-based cohorts with 860 HCC cases and 260 ICC cases(Reference Petrick, Freedman and Graubard151). Further, the inverse association between coffee and HCC risk was consistent across strata of HBV and HCV infection status without significant heterogeneity(Reference Bamia, Lagiou and Jenab136,Reference Aleksandrova, Bamia and Drogan150) . Overall, these results strengthen the existing evidence regarding the inverse association between coffee and HCC risk. Future research in this direction needs to offer more mechanistic insights, although two recent large prospective studies suggested that the possible pathways linking coffee-HCC association include reduction of inflammation and hepatocellular injury(Reference Aleksandrova, Bamia and Drogan150) and regulation of metabolism of bile acids and glycerophospholipids(Reference Loftfield, Rothwell and Sinha153).
Heavy alcohol intake (i.e. >45 g/d, above about 3 drinks/d) has been determined as a convincing cause of liver cancer by WCRF/AICR(19), which was further confirmed by the following cohort studies(Reference Loomba, Yang and Su154,Reference Petrick, Campbell and Koshiol155) and meta-analyses(Reference Turati, Galeone and Rota156,Reference Chuang, Lee and Wu157) . However, whether light-to-moderate alcohol consumption (e.g. <3 drinks/d) is associated with liver cancer is unanswered in the 2013 SLR(19). Current epidemiological evidence including a meta-analysis of nineteen cohort studies(Reference Turati, Galeone and Rota156) and a US cohort consortium study(Reference Petrick, Campbell and Koshiol155) supports an inverse association of light-to-moderate alcohol drinking with HCC risk, but not with ICC. The moderate alcohol intake is associated with a decreased risk of T2D, possibly via increasing insulin sensitivity(Reference Schrieks, Heil and Hendriks158). Thus, light-to-moderate alcohol drinking may reduce the HCC risk through decreasing the risk of T2D. However, due to limited evidence, more studies are warranted.
Dietary pattern and liver cancer risk
Diets are complex combinations of nutrients and other compounds that act synergistically within individual foods and across food combinations(Reference Tapsell, Neale and Satija159). Thus, evaluating the association of dietary pattern with health outcomes may capture dietary effects on health more completely. Also, findings from such research might provide important data to the development of dietary guidelines. There are four studies(Reference Zhang, Xiang and Li102,Reference Chen, Fang and Wang160–Reference Ma, Yang and Simon162) , two in China(Reference Zhang, Xiang and Li102,Reference Chen, Fang and Wang160) and two in the USA(Reference Li, Park and McGlynn161,Reference Ma, Yang and Simon162) , investigating the associations between certain dietary patterns and the risk of incident liver cancer to date (online Supplementary Table S4). Our study in the Shanghai Men’s Health Study and Shanghai Women’s Health Study(Reference Zhang, Xiang and Li102) showed that a vegetable-based dietary pattern was associated with a lower risk of liver cancer. Two cohort studies in the USA(Reference Li, Park and McGlynn161,Reference Ma, Yang and Simon162) suggested that participants with higher 2010 Alternative Healthy Eating Index (2010) and Alternate Mediterranean Diet scores had a lower risk of incident HCC(Reference Li, Park and McGlynn161). Consistently, one case–control study showed an association of higher adherence to Healthy Eating Index-2015 with lower HCC risk among Chinese adults(Reference Chen, Fang and Wang160). These healthy dietary patterns share several common dietary components (such as high intake of fruits and vegetables, nuts and legumes, and whole grains and low intake of red and processed meats) and showed moderate correlation between one another(Reference Li, Park and McGlynn161,Reference Ma, Yang and Simon162) , although they were not developed specifically for cancer prevention.
To strengthen the scientific evidence based on dietary patterns and to inform the Dietary Guidelines for Americans, the National Cancer Institute has initiated the Dietary Patterns Methods Project(163). This project aimed to compare key diet quality indices and their associations with cancer and CVD. These indices were selected based on a comprehensive review of literature, including the Alternative Healthy Eating Index, the Healthy Eating Index, the Dietary Approaches to Stop Hypertension and the Alternate Mediterranean Diet. However, little is known about the association between compliance to other country-specific dietary guidelines or recommendations and health outcomes including liver cancer development. Given large variations in diet and eating patterns across countries, this issue is of great important. In China, for example, only one study has evaluated the association of the 2016 Dietary Guidelines for Chinese with the risk of liver cancer(Reference Chen, Fang and Wang160). Future studies are needed to address this issue. In addition, given a potential mediated role of inflammation, insulin resistance, and gut microbiota (Fig. 1) in diet-liver cancer association, investigating the dietary pattern based on its inflammatory or insulinaemic potential (e.g. empirical dietary inflammatory index(Reference Tabung, Smith-Warner and Chavarro164) and empirical dietary index for hyperinsulinaemia(Reference Tabung, Wang and Fung165)) in relation to liver cancer risk would elucidate biological mechanisms relating diet to liver cancer.
Conclusion
In summary, current epidemiological evidence supports an important role of diet in liver cancer development, although there are large knowledge gaps between diet and liver cancer risk. Future nutritional epidemiological studies of diet and liver cancer with large sample size are warranted and need to consider: (1) how to best measure diet; (2) the possible aetiological heterogeneity between liver cancer subtypes (HCC v. ICC); (3) the potential influence of chronic HBV or HCV infection; (4) high-risk populations (e.g. patients with NAFLD or cirrhosis) and (5) a possible interplay with host gut microbiota or genetic variations. In addition, the underlying mechanisms linking diet and liver cancer remain to be further elucidated.
Acknowledgements
This work was supported by grants of the Scientific Research of BSKY (XJ201935) from Anhui Medical University, the National Key Project of Research and Development Program of China (2016YFC1302503) and the National Key Basic Research Program ‘973 Project’ (2015CB554000).
None of the funders had any influence on the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Y.-B. X. and W.-S. Y. obtained the funding. W.-S. Y. wrote the initial draft of the manuscript. All authors critically reviewed the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520001208