Se, as an essential micronutrient, is required for a number of metabolically important enzymes, and its importance for human health and prevention of disease is well established(Reference Rayman1). Se deficiency predisposes to a variety of major human diseases including cancer(Reference Schrauzer, White and Schneider2); conversely, intakes of Se above the normal recommended nutritional intake (supra-nutritional) are associated with reduced risk for a range of cancers(Reference Naithani3). In fact, Se intakes in many parts of the world are below the present dietary reference values because commonly consumed foods are often poor Se sources(Reference Kafai and Ganji4). Consequently, Se-enriched foods are likely to be beneficial for increasing human Se intake, and perhaps, reducing cancer risk. For instance, Se-enriched plant foods have been shown to significantly protect against colorectal cancer (CRC) in animal models(Reference Finley, Davis and Feng5, Reference Davis, Zeng and Finley6).
An Se-enriched milk protein product (dairy-Se) has recently been developed as a novel food product by Tatura Milk Industries of Australia. Milk is not normally a major dietary source of Se, but Se concentration in milk proteins can be readily increased to 5 parts per million (ppm) by feeding appropriate Se sources to cows(Reference Heard, Stockdale and Walker7, Reference Ortman and Pehrson8). We have shown that such a dairy source of Se produced higher plasma Se levels and significantly suppressed colon cancer incidence and relevant biomarkers of CRC risk, for example, aberrant crypt foci relative to equivalent Se amounts as Se yeast (yeast-Se)(Reference Hu, McIntosh and Le Leu9). This suggests that delivery of Se through dairy products potentially provides a good opportunity for safely improving human Se status and in the longer term reducing the risk of CRC.
Se is essential for a wide range of biological functions, which are mediated by at least twenty-five selenoproteins(Reference Gromadzinska, Reszka and Bruzelius10); some selenoproteins are particularly relevant to anticancer function in the gastrointestinal tract(Reference Pagmantidis, Meplan and van Schothorst11, Reference Mork, Lex and Scheurlen12), such as cytosolic glutathione peroxidase (GPx-1), gastrointestinal glutathione peroxidase (GPx-2), selenoprotein P (SeP) and thioredoxin reductase-1 (TrxR-1). For instance, a link between selenoproteins and colon cancer risk has been reported by genetic data and animal models(Reference Early, Hill and Burk13–Reference Irons, Carlson and Hatfield16) and functional polymorphisms in selenoprotein genes have also been linked to human cancer risk(Reference Meplan, Crosley and Nicol17). It has been proposed that genetic variation in selenoprotein genes could affect their function(s), their response to dietary Se intake and cancer risk(Reference Peters, Chatterjee and Hayes14). As far as Se and selenoproteins are concerned, studies so far have mostly relied on the assessment of blood or plasma SeP concentration and GPx-1 activity, or focused on Se deficiency and alteration in selenoprotein level(Reference Pagmantidis, Bermano and Villette18), or compared selenoprotein expression pattern between cancers and normal tissues(Reference Pagmantidis, Meplan and van Schothorst11, Reference Rayman19–Reference Brigelius-Flohe, Muller and Menard21). How dietary supplementation of Se may influence selenoprotein activity and expression in the colon has been examined only in a few animal studies(Reference Kipp, Banning and van Schothorst22); those studies did not include giving animals diets with higher Se levels than the normal recommended nutritional range. Activity or expression of specific selenoproteins in target tissues is likely to provide considerable insights into the possible involvement of those selenoproteins in health benefits including cancer prevention(Reference Rayman19). The purpose of the present study was to compare a dairy-Se with a yeast-Se for their effects on SeP, GPx-1, GPx-2 and TrxR-1 expression and GPx-1 activity in the mouse colon.
Materials and methods
Selenium supplements
Dairy-Se (TaturaBio®Se) was produced by Tatura Milk Industries (Tatura, VIC, Australia). It is a milk protein isolate with a high Se concentration (about 5 ppm), compared with control milk proteins (0·34 ppm); Yeast-Se (Sel-Plex®; 1800 μg Se/g dry weight) was provided by Alltech Biotechnology P/L (Dandenong South, VIC, Australia).
Animals
A total of forty-eight wild-type male mice of the C57BL/6J strain were obtained from the Animal Resource Centre, Perth, Australia. Animal protocols were approved by the Animal Welfare Committee at Flinders University of South Australia (reference 593/04). Mice were divided randomly into four equal experimental groups, housed in cages (four per cage) and maintained in a temperature- and humidity-controlled animal facility with a 12 h light–dark cycle at 22 ± 2°C temperature and 80 ± 10 % humidity. Mice were given free access to water.
Diets
The experimental diets fed to the mice were based on a modified form of the American Institute of Nutrition (AIN)-76A diet for rodents(23) and have been described by us previously(Reference Hu, McIntosh and Le Leu9). Control milk proteins and dairy-Se were used as protein sources; however, because the dairy protein sources have relatively high Ca concentrations, Ca was not included in the diets. The four diet groups were: (1) milk protein control diet (Se at 0·068 ppm); (2) dairy-Se diet (Se at 0·5 ppm); (3) dairy-Se diet (Se at 1 ppm); (4) milk protein control+yeast-Se diet (Se at 1 ppm). Details of the diets are provided in Table 1.
Table 1 Composition of experimental diets (g/100 g diet)
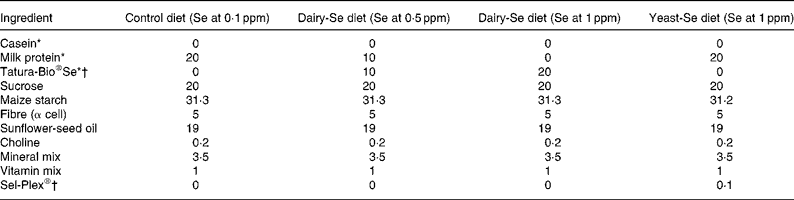
ppm, Parts per million; dairy-Se, Se-enriched milk proteins; yeast-Se, Se yeast.
* Milk protein was used as the protein source for the control diet and the yeast-Se diet; Tatura-Bio®Se (Tatura Milk Industries, Tatura, VIC, Australia) was used as the protein source for the dairy-Se diets (Se at 0·5 and 1 ppm).
† Tatura-Bio®Se was used as the Se source for the dairy-Se diets (Se at 0·5 and 1 ppm); Sel-Plex® (Alltech Biotechnology P/L, Dandenong South, VIC, Australia) was used as the Se source for the yeast-Se diet.
Experimental procedures
Mice, aged 10 weeks, were assigned to each of the four diets (twelve mice per group). After 4 weeks on the diet, mice were killed by cardiac puncture after ketamine–xylazine anaesthesia. Part of the colon was placed in RNAlater® (Ambion, Austin, TX, USA) solution at 4°C for 24 h, and stored at − 80°C until real-time PCR analysis; the remaining colon was fresh frozen immediately in liquid N2 and stored at − 80°C for assay of GPx-1 activity.
Assay of glutathione peroxide-1 activity
GPx-1 activity in the mouse colon was measured by a commercially available Glutathione Peroxidase Cellular Activity Assay Kit (Sigma, Sydney, NSW, Australia), using cumene hydroperoxide as a substrate. The colon was cut open, the mucosa scraped off and homogenised in a buffer containing 1 m-2-amino-2-hydroxymethyl-propane-1,3-diol (Tris) (pH 7·6) and 0·5 m-EDTA and centrifuged at 9391 g for 20 min at 4°C. The protein concentration was quantified using a Protein Assay Kit (Bio-Rad, Hercules, CA, USA). GPx-1 activity was determined in duplicate using 2·5–5 μl of the supernatant fraction (15–30 μg of proteins), assayed in a 100 μl reaction volume containing 5 mm-NADPH, 30 mm-H2O2 and 42 mm-reduced glutathione. The oxidation of NADPH to NADP was monitored at 340 nm on a UV-Vis spectrophotometer. A quantity of 1 unit of glutathione peroxidase will cause the formation of 1 μmol of NADP from NADPH per min in the presence of reduced glutathione, glutathione reductase, and tert-butyl hydroperoxide. GPx-1 activity was expressed as U/mg protein.
RNA isolation and cDNA synthesis
Total RNA was extracted from the mouse colon (30 mg) using a QIAGEN RNeasy Mini Kit (QIAGEN, Hilden, Germany). The concentration and purity of the total RNA was estimated using a NanoDrop® ND-1000 UV-Vis spectrophotometer by measuring the absorbance at 260 and 280 nm. All RNA samples had a 260:280 absorbance ratio between 1·9 and 2·1. First-strand cDNA (20 μl) was synthesised from 0·3 μg total RNA for each sample using a QIAGEN QuantiTect Reverse Transcription Kit (QIAGEN). cDNA was diluted 1:30 with nuclease-free water and used for real-time PCR.
Real-time PCR
Real-time quantitative PCR of the four genes was performed in triplicate on a Rotor-Gene 3000 Cycler (Corbett, Sydney, NSW, Australia). Oligonucleotide primers were designed using Primer Express software v. 1.5 (Applied Biosystems, Inc., Foster City, CA, USA), based on sequences from the Genbank database (Table 2). All PCR reagents were purchased from QIAGEN. The PCR reaction was determined in a 20 μl final volume containing 6 μl of diluted cDNA and 2 × QuantiTect SYBR Green PCR Kit. The primer concentration for each gene was 10 μm (forward and reverse primer). The cycling protocol started with an initial hot-start at 95°C for 15 min, followed by forty-five cycles at 94°C for 15 s, 60°C for 30 s and 72°C for 30 s, and finished with a final extension at 72°C for 4 min. The specificity of PCR was confirmed by melting-curve analysis with only one peak being present for PCR products of SeP, GPx-1, GPx-2 and TrxR-1 genes, and of the housekeeping gene of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). For each PCR run, a non-template reaction was included as negative controls.
Table 2 Oligonucleotide primers used for real-time PCR
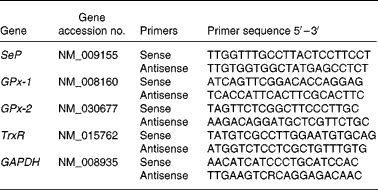
SeP, selenoprotein P; GPx-1, cytosolic glutathione peroxidase-1; GPx-2, gastrointestinal glutathione peroxidase-2; TrxR, thioredoxin reductase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Cycle thresholds were determined using the relative quantification analysis module in the Rotor-Gene 3000 Series software (Corbett). The amplification efficiency of each primer pair was estimated from a real-time PCR dilution curve generated using serial dilutions of cDNA. Real-time quantitative PCR analysis was then performed using Q-Gene software(Reference Simon24); with the amplification efficiency applied to the relative concentration analyses of both the genes of interest and the housekeeping gene (GAPDH). Gene of interest expression data were normalised by dividing the corresponding levels of GAPDH for each sample.
Statistical analyses
Statistical analyses were performed using SPSS for Windows, version 14.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as mean values with their standard errors. Between-group comparisons for each gene were assessed using one-way ANOVA with correction for multiple comparisons by Tukey's post hoc test. Differences between groups were considered significant when P < 0·05.
Results
Effects of dietary selenium intake on cytosolic glutathione peroxidase-1 activity in mouse colon
The effects of dietary Se intake on mouse colon GPx-1 activity are shown in Table 3. After 4 weeks on the diets with different Se forms and concentrations, GPx-1 activity differed in mouse colon across the four diets. GPx-1 activity in mice fed the yeast-Se diet with Se at 1 ppm was significantly higher (8·12 (sem 0·63) U/mg protein) than in those on the control diet (5·85 (sem 0·63) U/mg protein) (P < 0·01). However, GPx-1 activity in mice fed dairy-Se diets did not differ significantly across the doses tested; it was 6·15 (sem 0·57) U/mg protein in mice fed at 0·5 ppm and 5·95 (sem 0·75) U/mg protein in mice fed at 1 ppm.
Table 3 Effects of dietary supplementation of selenium on cytosolic glutathione peroxidase-1 (GPx-1) activity in mouse colon
(Mean values with their standard errors)

ppm, Parts per million; dairy-Se, Se-enriched milk proteins; yeast-Se, Se yeast.
* Mean value was significantly different from that of the control diet (P < 0·01; ANOVA).
Expression of selenoprotein P, cytosolic glutathione peroxidase-1, gastrointestinal glutathione peroxidase-2 and thioredoxin reductase-1 mRNA in mouse colon
Selenoprotein gene expression was analysed by quantitative real-time PCR. The expression pattern of SeP, GPx-1, GPx-2 and TrxR-1 in the mouse colon was comparable with those reported in previous studies in human subjects and rodents(Reference Hill, Zhou and McMahan25, Reference Hoffmann, Hoge and Li26). Our data showed that SeP was the major selenoprotein expressed in mouse colon with a relative expression level of 2·56 (sem 0·33), followed by GPx-1 and GPx-2, with a relative expression level of 0·93 (sem 0·21) and 0·88 (sem 0·22), respectively. TrxR-1 was also observed in mouse colon but expressed at a relative lower level of 0·062 (sem 0·04) (Table 4).
Table 4 Relative expression of selenoprotein P (SeP), cytosolic glutathione peroxidase-1 (GPx-1), gastrointestinal glutathione peroxidase-2 (GPx-2) and thioredoxin reductase-1 (TrxR-1) mRNA in mouse colon*
(Mean values with their standard errors)
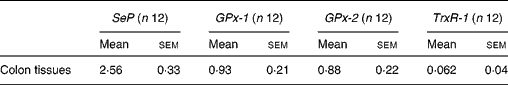
* The gene expression of the four selenoprotein genes is related to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a reference gene.
Effects of dietary selenium on selenoprotein P, cytosolic glutathione peroxidase-1, gastrointestinal glutathione peroxidase-2 and thioredoxin reductase-1 mRNA in mouse colon
Fold changes of colonic SeP, GPx-1, GPx-2 and TrxR mRNA in response to dietary Se supplementation relative to control are shown in Fig. 1. After 4 weeks of Se supplementation, selenoprotein gene expression in the mouse colon responded differently depending on the Se supplement. SeP mRNA level increased in a dose-dependent manner in response to dairy-Se diets, being significantly higher (> 2-fold) in mice fed dairy-Se with Se at 1 ppm than those on the control diet (P < 0·05) (Fig. 1(a)). A trend to increased SeP mRNA was also found in mice fed equivalent 1 ppm Se as yeast-Se, but it was not significantly different compared with that of the control diet (P = 0·068). Increases in GPx-2 mRNA levels in response to dairy-Se diets were also dose-dependent, with a significantly higher level of GPx-2 mRNA (1·9-fold) found in mice fed dairy-Se with Se at 1 ppm, compared with mice fed the control diet (P < 0·05) (Fig. 1(b)); however, yeast-Se did not significantly affect GPx-2 mRNA expression in the mouse colon. In the case of GPx-1, a significantly higher GPx-1 mRNA level was found in mice fed the yeast-Se diet with Se at 1 ppm; it was 2·7-fold higher than those on the control diet (P < 0·05) (Fig. 1(c)). A trend of higher GPx-1 mRNA was also found in mice fed the dairy-Se diet at 1 ppm, but it was not significant compared with those on the control diet (P = 0·060). Our data indicated that the increased expression of GPx-1 mRNA in the mouse colon was reflected in that of GPx-1 activity after dietary Se supplementation. However, TrxR-1 mRNA level was not changed by dietary Se supplementation either from the dairy or yeast source (Fig. 1(d)).

Fig. 1 Effects of dietary supplementation of Se on selenoprotein P (SeP) (a), gastrointestinal glutathione peroxidase-2 (GPx-2) (b), cytosolic glutathione peroxidase-1 (GPx-1) (c) and thioredoxin reductase-1 (TrxR-1) (d) mRNA expression in the mouse colon. Data are fold changes of colonic SeP, GPx-2, GPx-1 and TrxR-1 mRNA expression in response to dietary Se supplementation, relative to control, with control expression set at 1. Gene expression values have been normalised against the reference gene of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Dairy-Se 0·5, Se-enriched milk proteins (Se at 0·5 parts per million (ppm)); Dairy-Se 1, Se-enriched milk proteins (Se at 1 ppm); Yeast-Se 1, Se yeast (Se at 1 ppm). Values are means (n 12), with standard errors represented by vertical bars. * Mean value was significantly different from that for the control diet (P < 0·05; ANOVA).
Discussion
There is evidence from in vitro and in vivo studies that dietary supplementation of Se across a significant concentration range may regulate selenoproteins in target tissues(Reference Weiss, Evenson and Thompson27–Reference Behne and Wolters30). In the case of the colon, the expression of several selenoprotein genes is significantly affected in animals fed Se-deficient diets compared with those fed Se-adequate diets(Reference Pagmantidis, Bermano and Villette18, Reference Kipp, Banning and van Schothorst22). However, little is known about whether selenoproteins in the colon are regulated by increasing Se intake beyond what is considered nutritionally adequate (1·5 to 10 times recommended adequate dietary intake), and whether the regulation is dependent on Se form. In the present study, we showed for the first time that colonic selenoprotein levels, namely SeP, GPx-1 and GPx-2 in the mouse colon were regulated differently depending on the Se form. We found that dairy-Se at 1 ppm significantly increased expression of colonic SeP and GPx-2 mRNA but did not affect GPx-1, in particular GPx-1 activity, whereas yeast-Se at 1 ppm significantly increased colonic GPx-1 mRNA and GPx-1 activity without affecting SeP and GPx-2 mRNA. Studies from human clinical trials and animal experiments indicated that the chemical form of Se and not Se per se was the critical determinant of Se bioavailability and Se efficacy(Reference Facompre and El-Bayoumy31–Reference Whanger and Butler34). Our data support this concept, for while Se in both dairy and yeast sources is present as selenomethionine at 83 % and as selenocysteine at about 5 %, dairy-Se does not contain low-molecular-weight Se compounds due to the preparative procedure (filtration at 10 kDa), whereas yeast-Se contains 3 % of selenite. Additionally, dairy-Se also contains 4 % unknown components(Reference Rayman35); thus future studies are needed to identify organic species existing in dairy-Se. It is possible that the different Se forms may account for their different effects by affecting Se metabolism, Se delivery to target tissues, and subsequent selenoprotein synthesis, expression and function.
The beneficial effects of Se are thought to be mediated through the function of selenoproteins. Our particular interest is the potential regulation of SeP, GPx-1, GPx-2 and TrxR-1 in the colon by Se supplementing due to their potential relevance to CRC prevention. Our data support the view that SeP and GPx-2 along with GPx-1 are three selenoproteins of major functional significance in the mouse colon(Reference Mork, Lex and Scheurlen12, Reference Hoffmann, Hoge and Li26). As they responded significantly to supra-nutritional levels of Se intake, they may represent intestinal targets for Se supplementation aimed not at correcting deficiency but at achieving levels thought sufficient to contribute to cancer prevention. A recent human study showed that Se supplementation predominantly affected the genes that function in protein biosynthesis, which were linked to increased selenoprotein expression in the target tissues(Reference Pagmantidis, Meplan and van Schothorst11). Others have proposed that selenoprotein levels in targeted tissues may better reflect the functional selenoprotein activity than the plasma selenoprotein levels(Reference Sunde, Paterson and Evenson36), and be more relevant to the beneficial anticancer effects of Se.
SeP is a major plasma selenoprotein with a crucial role in Se transport(Reference Rayman19). The presence of SeP is thought to be vital in terms of influencing individual selenoprotein expression in different tissues(Reference Hill, Zhou and McMahan25, Reference Hoffmann, Hoge and Li26, Reference Esworthy, Swiderek and Ho37). Given its transport function, this might explain the effective influence of SeP on other individual selenoproteins in the colon. In addition, SeP can also function as antioxidative defence and cancer prevention. For instance, SeP knock-out mice were linked to increased cancer development(Reference Diwadkar-Navsariwala, Prins and Swanson38), a significant reduction or loss of SeP mRNA expression was observed in CRC(Reference Al-Taie, Uceyler and Eubner39) and some genetic variants in SeP were associated with human advanced colorectal adenoma(Reference Persson-Moschos, Stavenow and Akesson40). But the potential role of SeP to CRC prevention remains speculative and further studies are needed.
GPx-1 and GPx-2 are the major proteins responsible for 70 % GPx enzyme activity in the gastrointestinal tract. Unlike GPx-1 that is expressed in almost all tissues in the human body(Reference Chu, Esworthy and Doroshow41), GPx-2 is expressed exclusively in the gastrointestinal tract, providing 50 % of GPx activity(Reference Esworthy, Swiderek and Ho37). GPx-1 and GPx-2 have diverse biological roles that involve antioxidant function, inhibition of hydroperoxide, balance oxidative stress and associated inflammation. Their roles in CRC prevention have also received much attention because GPx-1/GPx-2 double knock-out mice progressively developed colitis and subsequent intestinal cancer(Reference Chu, Esworthy and Chu15, Reference Irons, Carlson and Hatfield16, Reference Hu, Benya and Carroll42). The present study showed that supra-nutritional intakes of dairy-Se significantly increased colonic GPx-2 mRNA, and slightly increased GPx-1 mRNA, but failed to increase colonic GPx-1 activity, whereas yeast-Se significantly increased colonic GPx-1 mRNA and GPx-1 activity without increasing GPx-2 mRNA. Since our previous animal studies showed that it was dairy-Se that protected against CRC rather than yeast-Se(Reference Hu, McIntosh and Le Leu9), up-regulation of GPx-2 may be of importance in terms of CRC prevention, particularly with regard to its tissue specificity and stability in Se deficiency (i.e. selenoprotein hierarchy)(Reference Persson-Moschos, Stavenow and Akesson40, Reference Chu, Esworthy and Ho43).
In contrast to GPx-1, the lack of GPx-2 was more detrimental because one intact allele was sufficient to prevent intestinal inflammation(Reference Bartel, Bartz and Wolf44), thereby indicating that it has anti-cancer effects rather than acting as an anti-inflammatory. GPx-2 is involved in cell growth and differentiation, suppression of cyclo-oxygenase-2 expression(Reference Banning, Kipp and Schmitmeier45) and activation by the β-catenin–T cell factor (TCF) complex(Reference Kipp, Banning and Brigelius-Flohe46). Its expression is also regulated by Nrf2, a transcription factor that induces enzymes that are cytoprotective and tumour preventive(Reference Banning, Deubel and Kluth47). But GPx-2 may have dual roles in carcinogenesis because GPx-2 was highly expressed in human colorectal adenomas and carcinomas(Reference Al-Taie, Uceyler and Eubner39, Reference Mork, al-Taie and Bahr48, Reference Murawaki, Tsuchiya and Kanbe49); some hypothesised that a beneficial role of GPx-2 in carcinogenesis may depend on the stage of tumorigenesis(Reference Banning, Kipp and Schmitmeier45). During the initiation stage, GPx-2 can protect cells from oxidative damage and reduce cyclo-oxygenase-2 expression and PGE2 production. One notable characteristic of Se is that its protective effects are more pronounced in the early stage of carcinogenesis(Reference Finley, Davis and Feng5, Reference Fiala, Joseph and Sohn50–Reference Rao, Wang and Simi52).
Whether GPx-1 has protective effects for cancer prevention remains an interesting topic for future research(Reference Thomson53). There were reports that genetic variants of the GPx-1 were associated with increased CRC risk, and loss of heterozygosity at the GPx-1 locus was involved with malignant progression(Reference Hu, Benya and Carroll42). These data, along with the differential expression patterns reported for GPx-1 in tumour v. normal tissues(Reference Moghadaszadeh and Beggs54), support the relevance of GPx-1 in cancer prevention(Reference Diwadkar-Navsariwala and Diamond55). However, increasing evidence has suggested that GPx-1 might not act as the prime mechanism of chemoprevention(Reference Irons, Carlson and Hatfield16, Reference Bermano, Nicol and Dyer56) because it reached its maximum with adequate Se intake, and did not change appreciably when Se intake increased to the levels that were 10-fold higher; such levels are necessary to see chemopreventive effects in the animal models(Reference Ganther57). Since Se intake at a supra-nutritional level also reduced the risk of colon cancer in transgenic mice that had reduced GPx-1 expression, the chemopreventive effect of Se may not be dependent on GPx-1 expression(Reference Irons, Carlson and Hatfield16).
TrxR-1, as part of the thioredoxin system, is important in antioxidant defence, but it has dual and contradictory effects on tumour development(Reference Moghadaszadeh and Beggs54). Like GPx-2, TrxR-1 levels were highly expressed in a variety of tumour tissues in humans(Reference Al-Taie, Uceyler and Eubner39). There are reports that Se may affect TrxR-1 in two ways; increasing with excess of Se intake and declining with continued high levels of Se intake(Reference Ganther57). Since the expression of TrxR-1 in the mouse colon was very low compared with SeP, GPx-1 and GPx-2, and it did not respond to Se supplement from either the dairy or yeast source, this suggests that the function of TrxR-1 in the colon might not be as important as SeP, GPx-1 and GPx-2.
In conclusion, the present study indicates that activity and expression of selenoproteins in the mouse colon is regulated differently by different dietary sources of Se, namely dairy-Se compared with yeast-Se. We have previously shown that dairy-Se at 1 ppm, but not yeast-Se at the same level, was protective against CRC in an azoxymethane-induced CRC mouse model. The present study shows that dairy-Se but not yeast-Se up-regulates colonic GPx-2 and SeP mRNA expression, suggesting that regulation of these genes is important in the prevention of CRC.
Acknowledgements
This project was funded by the Gardiner Foundation Major Research and Development Project (Melbourne, VIC, Australia; project no. MP4/009). The authors thank the support from the Department of Primary Industries Victoria, Tatura Milk Industries Ltd (Tatura, VIC, Australia), Alltech Biotech Pty Ltd (Dandenong South, Vic. Australia), the Geoffrey Gardiner Foundation (Melbourne, VIC, Australia) and the National Health and Medical Research Council of Australia.
Y. H., G. M. and G. Y. were involved in the design of the study. Y. H. and R. L. L. were responsible for execution of the experimental work and data collection. Y. H. was responsible for the data analysis and writing of the manuscript.
None of the authors has conflicts of interest.







