Background
Pharmacologic agents are the cornerstone of treatment for schizophrenia and bipolar I disorder, with atypical antipsychotics representing a first-line treatment option. 1 - Reference McIntyre, Berk and Brietzke 5 Treatment with many atypical antipsychotics is associated with an increased risk of weight gain and adverse metabolic effects; however, the magnitude of observed effects varies among different agents.Reference Zhang, Wang and Reynolds 6 , Reference Pillinger, McCutcheon and Vano 7 Olanzapine is associated with a lower rate of all-cause discontinuation, as well as discontinuation owing to inefficacy, compared with other atypical antipsychotics,Reference Lieberman, Stroup and McEvoy 8 - Reference Meftah, Deckler, Citrome and Kantrowitz 11 but it is associated with a significant risk for weight gain and metabolic sequelae.Reference Lieberman, Stroup and McEvoy 8 - Reference Citrome, Holt, Walker and Hoffmann 13 In clinical trials and population-based studies, treatment with olanzapine was associated with both short-term and long-term increases in weight, waist circumference, and body mass index (BMI), along with an increased risk for dyslipidemia and glucose dysregulation.Reference Pillinger, McCutcheon and Vano 7 , Reference Lieberman, Stroup and McEvoy 8 , Reference Bazo-Alvarez, Morris, Carpenter, Hayes and Petersen 14 - Reference Koro, Fedder and L’Italien 16 For some patients, these weight and metabolic consequences may offset the clinical benefits of olanzapine despite its established value as a highly efficacious medicine.
Although the effects of olanzapine on a range of measures related to body weight and metabolic function have been characterized in preclinical and clinical studies, the mechanisms underlying these effects are not well established. Research into mechanisms underlying antipsychotic-associated weight gain has focused on receptor interactions involving serotoninergic, dopaminergic, histaminergic, adrenergic, cannabinoid, and muscarinic receptors.Reference Roerig, Steffen and Mitchell 17 Additionally, the endogenous opioid system plays a role in weight and metabolic regulation,Reference Bodnar 18 , Reference Czyzyk, Nogueiras and Lockwood 19 and evidence from both preclinical and clinical studies provides a rationale for targeting this system to mitigate weight-related side effects of antipsychotic treatment.Reference Czyzyk, Nogueiras and Lockwood 19 - Reference Correll, Newcomer and Silverman 23
The aim of this review is to describe the role of the opioid system in regulation of weight and metabolism, and to examine the effects of opioid receptor antagonism on those functions. A PubMed literature search was conducted for preclinical and clinical studies examining the potential effects of opioid receptor antagonism on weight and metabolism. We included additional search terms that were selected to identify articles assessing opioid antagonists for their potential to mitigate antipsychotic-associated weight gain in general, and olanzapine-associated weight gain and/or metabolic effects specifically, as well as any other effects on weight gain, lipids, insulin, and glucose regulation. This review focuses on 3 identified opioid antagonists (naltrexone, samidorphan, and LY255582) with effects on weight and metabolic dysregulation described in the literature, and how differences in their respective pharmacokinetic profiles, opioid-receptor binding, and functional activity characteristics may underly differential metabolic treatment effects.
Opioid receptors associated with effects on body weight and metabolism: types, distribution, and function
Three opioid receptor types, mu opioid receptors (μ-ORs; MORs), delta opioid receptors (δ-ORs; DORs), and kappa opioid receptors (κ-ORs; KORs), are distributed throughout the body in both the central nervous system (CNS) and the periphery,Reference Peng, Sarkar and Chang 24 where they contribute to multiple functions, including mood and well-being, pain modulation, and reward response, as well as physiological processes such as respiration and endocrine and immune function.Reference Benarroch 25 - Reference van Steenbergen, Eikemo and Leknes 27 Each of the 3 receptor types contributes to the maintenance of body weight and metabolic function,Reference Czyzyk, Nogueiras and Lockwood 19 , Reference Czyzyk, Romero-Pico and Pintar 20 , Reference Tabarin, Diz-Chaves and Carmona Mdel 22 , Reference Wen, Peng and Pintar 28 but they are differentiated based on their primary associated endogenous peptides (β-endorphin, enkephalins, and dynorphins for MOR, DOR, and KOR, respectively),Reference Benarroch 25 the distribution of their expression throughout the body,Reference Peng, Sarkar and Chang 24 , Reference Burns, Kroll and Feldman 29 and their specific functions with respect to weight and metabolic regulation.Reference Czyzyk, Nogueiras and Lockwood 19 , Reference Czyzyk, Romero-Pico and Pintar 20 , Reference Tabarin, Diz-Chaves and Carmona Mdel 22 , Reference Wen, Peng and Pintar 28
The opioid system influences appetite and satiety via both central reward circuitryReference Nummenmaa, Saanijoki and Tuominen 30 , Reference Valbrun and Zvonarev 31 and the peripheral gastrointestinal neural system.Reference Pupovac and Anderson 32 , Reference De Vadder, Gautier-Stein and Mithieux 33 Food intake may be modified by opioid receptor agonists and antagonists through effects on palatability or satiety cues,Reference Pupovac and Anderson 32 - Reference Smith, Walker, Leeboonngam, McKinley, Denton and Lawrence 37 and there is evidence for differential involvement of receptor types centrally and peripherally.Reference De Vadder, Gautier-Stein and Mithieux 33 - Reference Cline and Sliwa 35 , Reference Gulati, Ray and Sharma 38 Opioid receptors can also influence dietary preference, altering response to salty or fatty foods,Reference Katsuura, Heckmann and Taha 36 , Reference Smith, Walker, Leeboonngam, McKinley, Denton and Lawrence 37 , Reference Na, Morris and Johnson 39 - Reference Haghighi, Melka and Bernard 42 and evidence of the involvement of opioid receptors in binge eating and obesity has been reported.Reference Valbrun and Zvonarev 31 , Reference Tuominen, Tuulari and Karlsson 43 - Reference Jonas and Gold 45 Furthermore, studies in which opioid receptors are inactivated pharmacologically or by genetic deletion (eg, murine knockout models), as well as genotype association studies, provide evidence that the endogenous opioid system is involved in the regulation of body weight and adiposity, with roles in fat and energy utilization and storageReference Czyzyk, Nogueiras and Lockwood 19 , Reference Czyzyk, Romero-Pico and Pintar 20 , Reference Tabarin, Diz-Chaves and Carmona Mdel 22 , Reference Wen, Peng and Pintar 28 ; control of insulin secretion, insulin sensitivity, and glucose productionReference Tudurí, Beiroa and Stegbauer 21 , Reference Wen, Peng and Pintar 28 , Reference Gallagher, Gordon and Langefeld 46 , Reference Verspohl, Berger and Ammon 47 ; and food intake,Reference Czyzyk, Romero-Pico and Pintar 20 , Reference Levine, Grace and Billington 48 , Reference Statnick, Tinsley, Eastwood, Suter, Mitch and Heiman 49 as detailed below.
Opioid receptor distribution
MOR, DOR, and KOR are expressed in both the CNS and periphery, in sites that are associated with regulation of food intake and metabolism.Reference Bodnar 18 , Reference Benarroch 25 , Reference Wen, Peng and Pintar 28 Distributions in the CNS and periphery are overlapping, but distinct, as illustrated in Figure 1.Reference Peng, Sarkar and Chang 24 , Reference Burns, Kroll and Feldman 29 , Reference Light, Bieliauskas and Zubieta 50 In the CNS, opioid receptor expression has been observed in the mesocorticolimbic pathway, which projects from the substantia nigra and ventral tegmental area into the striatum and prefrontal cortex and is associated with dopaminergic reward circuitry.Reference Peng, Sarkar and Chang 24 , Reference Benarroch 25 , Reference Light, Bieliauskas and Zubieta 50 , Reference Arias-Carrión, Stamelou, Murillo-Rodríguez, Menéndez-González and Pöppel 51 MOR is widely distributed in the human CNS, with the highest levels detected in the cerebellum, nucleus accumbens, caudate nucleus, and prefrontal cortex.Reference Peng, Sarkar and Chang 24 , Reference Baldo 52 DOR is highly expressed in the human cerebral cortex, prefrontal cortex, nucleus accumbens, caudate nucleus, temporal lobe, and hippocampus,Reference Peng, Sarkar and Chang 24 , Reference Burns, Kroll and Feldman 29 and the greatest KOR expression has been observed in human prefrontal cortex, ventral tegmental area, nucleus accumbens, and the caudate nucleus.Reference Peng, Sarkar and Chang 24 , Reference Burns, Kroll and Feldman 29

Figure 1. Distribution: areas of greatest expression of MOR, DOR, and KOR in the CNS and periphery.Reference Peng, Sarkar and Chang 24 , Reference Burns, Kroll and Feldman 29 , Reference Light, Bieliauskas and Zubieta 50 Abbreviations: CNS, central nervous system; DOR, delta opioid receptor; KOR, kappa opioid receptor; MOR, mu opioid receptor. ©Alkermes, Inc. 2021.
In the periphery, MOR, DOR, and KOR are expressed in tissues that play a role in regulation of metabolic function and body weight, again with distinct patterns of expression for each receptor type.Reference Czyzyk, Romero-Pico and Pintar 20 , Reference Tabarin, Diz-Chaves and Carmona Mdel 22 , Reference Peng, Sarkar and Chang 24 , Reference Wen, Peng and Pintar 28 , Reference Verspohl, Berger and Ammon 47 , Reference Schleicher 53 The 3 receptor types are expressed in pancreas and small intestine with MOR expression delimited to these structures.Reference Peng, Sarkar and Chang 24 DOR is also found in lung, heart, kidney, thymus, and skeletal muscle, and KOR is expressed in the lung, heart, kidney, spleen, thymus, skeletal muscle, and liver (Figure 1).Reference Peng, Sarkar and Chang 24
Opioid receptor gene deletion studies
The observed receptor expression patterns suggest that MOR, DOR, and KOR could contribute differentially to the regulation of weight and metabolism and are supported by the observed effects of respective opioid receptor gene deletions from murine knockout models. In high-fat diet models, mice fed a calorie-rich diet have increases in body weight and fat mass over time compared with mice fed a standard laboratory diet.Reference Czyzyk, Nogueiras and Lockwood 19 , Reference Czyzyk, Romero-Pico and Pintar 20 , Reference Tabarin, Diz-Chaves and Carmona Mdel 22 In this model, genetic deletion of each opioid receptor type resulted in a reduction in fat mass accumulation and diet-associated weight gain compared with that observed in wild-type animals.Reference Czyzyk, Nogueiras and Lockwood 19 , Reference Czyzyk, Romero-Pico and Pintar 20 , Reference Tabarin, Diz-Chaves and Carmona Mdel 22 However, specific effects of genetic deletion differed for MOR, DOR, and KOR (Figure 2).
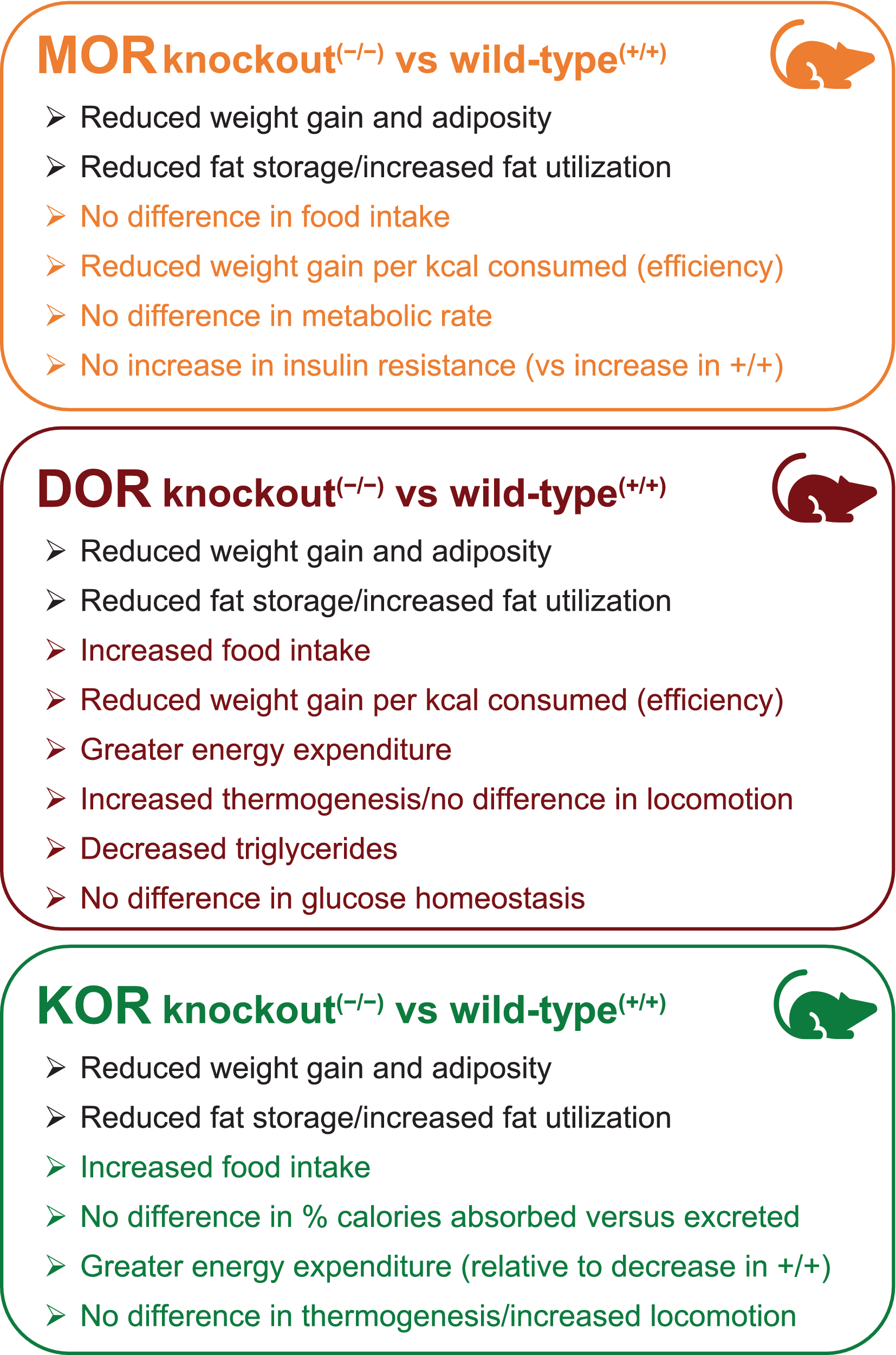
Figure 2. Metabolism: effects of opioid receptor deletion in diet-induced obesity; murine knockout high-fat diet models.Reference Czyzyk, Nogueiras and Lockwood 19 , Reference Czyzyk, Romero-Pico and Pintar 20 , Reference Tabarin, Diz-Chaves and Carmona Mdel 22 Abbreviations: DOR, delta opioid receptor; KOR, kappa opioid receptor; MOR, mu opioid receptor. ©Alkermes, Inc. 2021.
Reported effects of MOR deletion in mice fed a standard diet differ across studies. In one set of studies, MOR knockout mice given a standard diet for 180 days after birth had greater weight gain and increased fat tissue compared with wild-type mice.Reference Wen, Peng and Pintar 28 The MOR knockout mice also had enhanced glucose tolerance, which resulted from insulin hypersecretion, although the insulin hypersecretion was not associated with any increased propensity for developing type II diabetes as the MOR knockout mice aged.Reference Wen, Peng and Pintar 28 In a second set of studies, MOR knockout mice fed a standard diet had no increase in body weight, food intake, or total energy expenditure; however, a shift in fuel utilization to relatively greater fat utilization was observed. In contrast to the Wen 2009 study,Reference Wen, Peng and Pintar 28 this second study used only male mice that were 16 to 28 weeks of age at the onset of the study, but the period over which weight gain was assessed was not specified. On a high-fat diet (Figure 2), MOR knockout mice had similar caloric intake and no evidence of a change in metabolic rate, but a significantly lower ratio of body weight gained per kilocalorie consumed, compared with wild-type mice.Reference Tabarin, Diz-Chaves and Carmona Mdel 22 MOR gene deletion was protective against insulin resistance and glucose intolerance in aging mice fed a high-fat diet, and was associated with the upregulation of enzymes involved in fatty acid oxidation in liver and skeletal muscle, reducing the efficient storage of ingested fat.Reference Tabarin, Diz-Chaves and Carmona Mdel 22
In contrast, DOR inactivation was associated with an increase in both body weight-normalized food intake and total energy expenditure and was associated with a significantly reduced ratio of weight gain to food consumed.Reference Czyzyk, Romero-Pico and Pintar 20 The greater energy expenditure in DOR knockout mice appears to be driven at least in part by activation of thermogenesis, as evidenced by an increased body surface temperature and up-regulation of thermogenesis-associated genes in brown adipose tissue.Reference Czyzyk, Romero-Pico and Pintar 20 No increase in locomotor activity was observed relative to wild-type mice. DOR inactivation in the high-fat diet model was also associated with reduced liver triglyceride levels and hepatic fat storage, elevated fatty acid turnover in white adipose tissue, and decreased plasma triglyceride and leptin levels.Reference Czyzyk, Romero-Pico and Pintar 20 DOR deletion was protective against glucose intolerance in mice fed standard chow, and no difference was observed in body weight or adiposity relative to wild-type littermates.Reference Czyzyk, Romero-Pico and Pintar 20 Interestingly, this is in contrast with MOR knockout results, in which the protective effects on glucose intolerance were observed only in mice fed a high-fat diet.Reference Tabarin, Diz-Chaves and Carmona Mdel 22
Standard diet-fed KOR knockout mice did not differ from wild-type mice in body weight, fat mass, food intake, or energy expenditure, yet these mice had significantly less fat-free mass.Reference Czyzyk, Nogueiras and Lockwood 19 KOR knockout mice fed a high-fat diet had reduced weight gain, fat mass, and fat-free mass, despite an increase in food intake and no difference in the percentage of calories absorbed vs excreted.Reference Czyzyk, Nogueiras and Lockwood 19 Unlike wild-type mice, which reduced energy expenditure when shifted from a standard to high-fat diet, mice lacking the KOR gene maintained their energy expenditure on a high-fat diet.Reference Czyzyk, Nogueiras and Lockwood 19 KOR knockout mice had elevated levels of spontaneous locomotor activity, but no evidence of increased thermogenesis in brown adipose tissue or skeletal muscle.Reference Czyzyk, Nogueiras and Lockwood 19 KOR inactivation was also associated with reduced lipid storage in liver and white adipose tissue, and increased fatty acid oxidation in the liver.Reference Czyzyk, Nogueiras and Lockwood 19
Taken together, genetic deletion studies indicate that while MOR, DOR, and KOR each contribute to regulation of body weight, their specific roles differ regarding regulation of energy intake, storage, and utilization. Consequently, the effects of opioid receptor modulation may be expected to differ, depending on which opioid receptors are targeted, as well as the type of diet consumed.
Differential effects of opioid antagonists
Given the metabolic-related functions associated with the endogenous opioid system, drugs that interact with MOR, DOR, or KOR have the potential to produce effects on body weight and metabolism by regulating activity at those receptors. Whereas endogenous opioid receptor agonists bind to and activate receptors, opioid antagonists bind to and block receptor activation. While MOR, DOR, or KOR agonists each have been reported to stimulate food or caloric intake in ratsReference Gosnell, Levine and Morley 54 and humans,Reference Morley, Parker and Levine 55 administration of opioid antagonists conversely has been associated with attenuated food consumption and reduced fat accumulation in preclinical and clinical studies.Reference Correll, Newcomer and Silverman 23 , Reference Cunningham, Eyerman and Todtenkopf 56 - Reference Kurbanov, Currie, Simonson, Borsook and Elman 58
Representative compounds that act as antagonists at MOR, DOR, and/or KOR identified in this focused review (eg, naltrexone, samidorphan, and LY255582) can be differentiated based on a number of characteristics, including ease of access to the systemic circulation and opioid receptors (pharmacokinetic profile), binding affinities, and/or the ability to inhibit agonist-induced activation of each receptor type.Reference Shaw, Mitch, Leander, Mendelsohn and Zimmerman 59 - Reference Bidlack, Knapp and Deaver 61 Each of these properties could potentially impact their effects on regulation of body weight and metabolism, with distinctions between compounds observed both preclinically and clinically. Naltrexone is used in the treatment of patients with alcohol or opioid use disorder 62 and has been assessed in several other preclinical and clinical models.Reference Thorsell 63 - Reference Tek, Ratliff, Reutenauer, Ganguli and O’Malley 73 In animal models of obesity, treatment with naltrexone has been associated with at least transient reductions in food intake and/or body weight gain.Reference Recant, Voyles, Luciano and Pert 69 - Reference Panigrahi, Meece and Wardlaw 72 Clinically, the effects of naltrexone on body weight or BMI have been reported in small studies enrolling overweight patients with polycystic ovary syndrome (PCOS),Reference Ahmed, Duleba, El Shahat, Ibrahim and Salem 66 , Reference Fruzzetti, Bersi, Parrini, Ricci and Genazzani 67 binge eating,Reference Raingeard, Courtet, Renard and Bringer 68 or schizophrenia.Reference Tek, Ratliff, Reutenauer, Ganguli and O’Malley 73 Samidorphan in combination with olanzapine (OLZ/SAM; Lybalvi, Alkermes, Inc.) is approved in the United States for the treatment of adults with schizophrenia or bipolar I disorder, including acute treatment of manic or mixed episodes as monotherapy and as an adjunct to lithium or valproate, or as maintenance monotherapy treatment. 74 In clinical studies, OLZ/SAM treatment mitigated olanzapine-associated weight gain while maintaining antipsychotic efficacy.Reference Correll, Newcomer and Silverman 23 , Reference Potkin, Kunovac and Silverman 75 LY255582 has been associated with reduced weight gain vs vehicle-treated controls in animal models of genetic and diet-induced obesityReference Statnick, Tinsley, Eastwood, Suter, Mitch and Heiman 49 , Reference Ibrahim, Ibrahim, Aziz and Rahman 57 , Reference Shaw, Mitch, Leander, Mendelsohn and Zimmerman 59 , Reference Shaw 70 ; however, to the best of our knowledge, no data from human studies have been reported.
Pharmacokinetic parameters related to how much and the rate at which a drug is absorbed after oral administration (bioavailability; C max), the time taken to reach maximum plasma concentration (T max) after dosing, and how long effective plasma drug concentrations persist (half-life) are of interest for understanding a drug’s clinical potential. The pharmacokinetics of naltrexone and samidorphan have been assessed in human studiesReference Meyer, Straughn, Lo, Schary and Whitney 76 , Reference Kumar, Lu, Hard and von Moltke 77 ; the pharmacokinetics of LY255582 have been examined in animal models (rat and dog).Reference Swanson, Catlow, Pohland, Chay and Johnson 78 The reported oral bioavailability of naltrexone is variable, with estimates ranging from 5% to 40% (Table 1). 62 , Reference Meyer, Straughn, Lo, Schary and Whitney 76 The bioavailability of samidorphan is 69%,Reference Kumar, Lu, Hard and von Moltke 77 whereas for LY255582, it is <1% (in dogs).Reference Swanson, Catlow, Pohland, Chay and Johnson 78 The half-life of samidorphan (7-9 hoursReference Kumar, Lu, Hard and von Moltke 77 ; Table 1) is approximately twice as long as the half-lives of naltrexone and LY255582 (4 hours 62 and 3.2 hours,Reference Swanson, Catlow, Pohland, Chay and Johnson 78 respectively). Pharmacokinetic parameters such as these are of interest because a larger fraction of administered drug available to systemic circulation and greater persistence of that drug in circulating plasma increase the window for any clinically relevant receptor interactions.
Table 1. Opioid Antagonist Pharmacokinetic Profiles: Naltrexone, 62 , Reference Wentland, Lu, Lou, Bu, Knapp and Bidlack 80 Samidorphan,Reference Kumar, Lu, Hard and von Moltke 77 and LY255582Reference Swanson, Catlow, Pohland, Chay and Johnson 78

Abbreviations: C max, maximum plasma concentration; t ½, terminal elimination half-life; T max, time to maximum plasma concentration.
Naltrexone, samidorphan, and LY255582 have distinct binding affinities at MOR, DOR, and KOR and also differ in their ability to block activation of those receptors in an assay measuring functional effectsReference Wentland, Lou and Lu 79 (Table 2). Naltrexone binds with subnanomolar affinity at MOR (Ki = 0.11 nM) and KOR (Ki = 0.19 nM), but has much lower affinity for DOR (Ki = 60 nM).Reference Wentland, Lu, Lou, Bu, Knapp and Bidlack 80 - Reference Tan, Gajipara, Zhou, Namchuk and Cunningham 82 Samidorphan has higher affinity for each of the 3 opioid receptors compared with naltrexone (MOR: Ki = 0.052 nM; DOR: Ki = 2.6 nM; KOR: Ki = 0.23 nM),Reference Bidlack, Knapp and Deaver 61 , Reference Wentland, Lu, Lou, Bu, Knapp and Bidlack 80 , Reference Tan, Gajipara, Zhou, Namchuk and Cunningham 82 and LY255582 is a pan-opioid antagonist with a modest degree of selectivity for MOR (Ki = 0.41 nM) over DOR and KOR (Ki = 5.2 nM and Ki = 2.0 nM, respectively).Reference Carroll and Dolle 83 In the functional assay, both naltrexone and samidorphan are antagonists at MOR (>90% maximal inhibition of agonist-stimulated receptor activation) and mixed agonists/antagonists at DOR and KOR, stimulating or inhibiting opioid receptor activation under different conditions (Table 2).Reference Bidlack, Knapp and Deaver 61 , Reference Wentland, Lou and Lu 79 , Reference Wentland, Lou and Lu 84 However, at clinically relevant concentrations, samidorphan likely functions as an antagonist at both MOR and DOR, while naltrexone likely functions as an MOR antagonist.Reference Cunningham, Eyerman and Todtenkopf 56 LY255582 is a pan-opioid antagonist at MOR, DOR, and KORReference Emmerson, McKinzie, Surface, Suter, Mitch and Statnick 85 (Table 2); however, LY255582 has not been characterized (or published, to the best of our knowledge) using the same assays reported here for naltrexone or samidorphan.
Table 2. Opioid Antagonist Receptor Binding and Functional Activity: Naltrexone,Reference Carroll, Chaudhari and Thomas 60 , Reference Wentland, Lou and Lu 79 , Reference Wentland, Lu, Lou, Bu, Knapp and Bidlack 80 Samidorphan,Reference Wentland, Lou and Lu 79 , Reference Wentland, Lu, Lou, Bu, Knapp and Bidlack 80 and LY255582Reference Shaw, Mitch, Leander, Mendelsohn and Zimmerman 59 , Reference Carroll, Chaudhari and Thomas 60 , Reference Carroll and Dolle 83 , Reference Emmerson, McKinzie, Surface, Suter, Mitch and Statnick 85

Abbreviations: CHO, Chinese hamster ovary; DAMGO, (D-Ala2,MePhe4,Gly-ol5)enkephalin; DOR, delta opioid receptor; DPDPE, cyclo(D-Pen2,D-Pen5)enkephalin; E max, maximal effect when all receptors are occupied by drug; EC50, half-maximal effective concentration; I max, maximal inhibition; IC50, concentration of drug producing 50% inhibition; Ke , functional inhibition constant; Ki , inhibitory constant; KOR, kappa opioid receptor; MOR, mu opioid receptor; SEM, standard error of the mean.
a Values for each parameter are mean (SEM) from at least 3 experiments performed in triplicate.
b Receptor activation was assessed using a [35S]GTPγS binding assay. Membrane protein from CHO cells that stably expressed one type of the human opioid receptor were incubated with the compound in the presence of either [3H]DAMGO (MOR), [3H]naltrindole or DPDPE (DOR), or [3H]U69,593 (KOR).
c SEM not reported.
Because these opioid antagonists differentially bind to and inhibit activation of MOR, DOR, and KOR, and the specific effects of MOR, DOR, and KOR gene deletions on regulation of body weight and metabolism also differ, it is reasonable to expect that the preclinical and clinical effects of the individual opioid antagonists might differ as well. Differential effects of naltrexone, samidorphan, and LY255582 on body weight and metabolism observed in preclinical and clinical studies (Table 3) are summarized below. Preclinical and clinical data related specifically to olanzapine-associated weight gain are also highlighted.
Table 3. Summary of Studies Examining the Effects of Opioid Antagonists on Weight, Metabolism, and Appetite: Naltrexone, Samidorphan, and LY255582

Notes: ![]() reported effect;
reported effect; ![]() no reported effect; cells with no symbol shown indicate that the measure was not assessed.
no reported effect; cells with no symbol shown indicate that the measure was not assessed.
Abbreviations: BMI, body mass index; OLZ, olanzapine; PCOS, polycystic ovary syndrome.
a Transient effect only.
b Not tested statistically.
Naltrexone
Naltrexone is used as a treatment in patients with alcohol or opioid use disorder. 62 , 86 Naltrexone in combination with bupropion is approved for chronic weight management in overweight or obese adults as an adjunct to a reduced-calorie diet and increased physical activity. 87 However, it should be noted that in clinical studies, naltrexone monotherapy has not been found to reduce weight in patients who are overweight or obese.Reference Rebello and Greenway 64 , Reference Greenway, Dunayevich and Tollefson 88
In animal models, naltrexone reduces weight but not food intake in genetically obese mice and rats,Reference Recant, Voyles, Luciano and Pert 69 , Reference Shaw 70 and decreases weight, fat mass, and caloric intake in rats fed a high-fat or sucrose-supplemented diet.Reference Ibrahim, Ibrahim, Aziz and Rahman 57 , Reference Marks-Kaufman, Balmagiya and Gross 71 , Reference Panigrahi, Meece and Wardlaw 72 In mice fed a high-fat diet, transient effects on weight and food intake were reported.Reference Panigrahi, Meece and Wardlaw 72 Naltrexone’s effects on antipsychotic-induced weight gain in rodents have been equivocal (Table 3). Assessed in a standard rodent model using female rats, naltrexone did not mitigate olanzapine-associated weight gain in 1 study,Reference Todtenkopf 89 but did reduce body weight gain and food intake in a second study.Reference Kurbanov, Currie, Simonson, Borsook and Elman 58 Naltrexone had no effect on weight gain associated with the antipsychotic sulpiride in rats.Reference Baptista, Lacruz and Acosta 90
Reductions in body weight have been observed with naltrexone in several small clinical studies for different conditions associated with weight gain, for example, in PCOS,Reference Ahmed, Duleba, El Shahat, Ibrahim and Salem 66 , Reference Fruzzetti, Bersi, Parrini, Ricci and Genazzani 67 binge eating,Reference Raingeard, Courtet, Renard and Bringer 68 and in patients taking antipsychotics for schizophrenia.Reference Tek, Ratliff, Reutenauer, Ganguli and O’Malley 73 In overweight or obese females with PCOS, naltrexone was associated with a reduction in BMI and with improvement in fasting insulin levels in 1 small study (N = 30),Reference Ahmed, Duleba, El Shahat, Ibrahim and Salem 66 and with weight loss, reduced BMI, and reduced insulin levels, particularly in females with low baseline glucose:insulin ratios, in a second study (N = 10).Reference Fruzzetti, Bersi, Parrini, Ricci and Genazzani 67 Females with binge eating and type 1 diabetes treated with naltrexone (N = 3) had reductions in body weight and binging episodes, and improvement in hemoglobin A1c levels.Reference Raingeard, Courtet, Renard and Bringer 68
The reported effects of naltrexone on antipsychotic-induced weight gain have also varied in clinical studies (Tables 3 and 4). In a pilot study of overweight females with schizophrenia or schizoaffective disorder who were taking antipsychotic medication at enrollment (N = 24), naltrexone treatment was associated with weight loss vs placebo over 8 weeks.Reference Tek, Ratliff, Reutenauer, Ganguli and O’Malley 73 Of note, based on a subgroup analysis, weight loss was observed only in nondiabetic (n = 5) patients; no weight loss was observed in patients with diabetes (n = 6).Reference Tek, Ratliff, Reutenauer, Ganguli and O’Malley 73 There were no significant changes in waist circumference or metabolic parameters observed.Reference Tek, Ratliff, Reutenauer, Ganguli and O’Malley 73 In a second study enrolling obese or overweight patients on a stable dose of olanzapine for the treatment of schizophrenia or schizoaffective disorder (N = 30), there were no significant changes in body weight or BMI from baseline to week 12 in either naltrexone or placebo groups, although a significant reduction in fat mass and fat-free mass was observed at week 12 with naltrexone.Reference Taveira, Wu and Tschibelu 91 No differences in lipids were observed between treatment groups.Reference Taveira, Wu and Tschibelu 91
Table 4. Clinical Studies Assessing Opioid Antagonist Mitigation of Antipsychotic-Associated Weight Gain
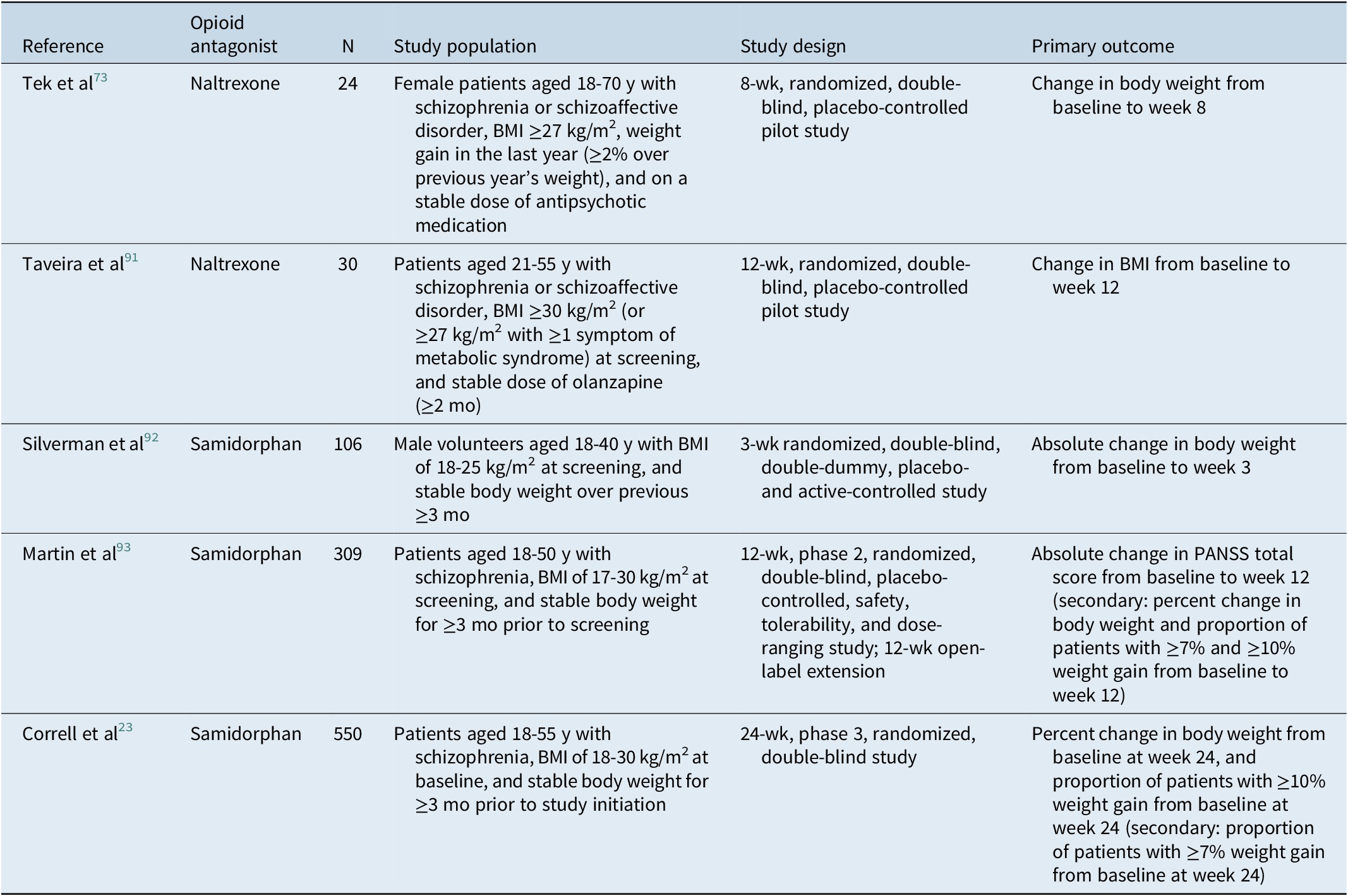
Abbreviations: BMI, body mass index; PANSS, Positive and Negative Syndrome Scale.
Samidorphan
The effects of samidorphan (previously referred to as ALKS 33 and as RDC-0313) on body weight and metabolic parameters have focused on animal and human studies of olanzapine-associated weight gainReference Correll, Newcomer and Silverman 23 , Reference Cunningham, Eyerman and Todtenkopf 56 , Reference Silverman, Martin, Memisoglu, DiPetrillo, Correll and Kane 92 , Reference Martin, Correll and Weiden 93 (Table 3); based on preclinical and clinical observations, samidorphan monotherapy would not be expected to result in weight loss.Reference Cunningham, Eyerman and Todtenkopf 56 , Reference Silverman, Martin, Memisoglu, DiPetrillo, Correll and Kane 92 In preclinical studies, samidorphan attenuated olanzapine-associated increases in weight and adiposity in both rats and nonhuman primates.Reference Cunningham, Eyerman and Todtenkopf 56 A transient decrease in food intake was observed in rats after treatment with olanzapine plus samidorphan.Reference Cunningham, Eyerman and Todtenkopf 56 Olanzapine administered alone decreased hepatic insulin sensitivity and glucose utilization in muscle while increasing glucose utilization in adipose tissue. Samidorphan attenuated the olanzapine-associated effects on glucose utilization but did not restore hepatic insulin sensitivity.Reference Cunningham, Eyerman and Todtenkopf 56 In nonhuman primates fed a high-fat diet, food intake was reduced after treatment with olanzapine plus samidorphan vs olanzapine.Reference Cunningham, Eyerman and Todtenkopf 56 Olanzapine treatment was associated with a decrease in insulin sensitivity in monkeys, which was prevented by coadministration of samidorphan.Reference Cunningham, Eyerman and Todtenkopf 56
In humans, samidorphan mitigated olanzapine-associated weight gain in both healthy volunteersReference Silverman, Martin, Memisoglu, DiPetrillo, Correll and Kane 92 and in patients with schizophrenia in phase 2 and phase 3 clinical studies (Table 4).Reference Correll, Newcomer and Silverman 23 , Reference Martin, Correll and Weiden 93 , Reference Citrome, Graham and Simmons 94 Increased waist circumference, which has been associated with greater risk of cardiovascular disease and diabetes,Reference Klein, Allison and Heymsfield 95 was also attenuated in patients treated with OLZ/SAM compared with patients treated with olanzapine in a phase 3 study.Reference Correll, Newcomer and Silverman 23 Changes from baseline in mean serum insulin, glucose, or triglyceride levels were similar after treatment with olanzapine plus samidorphan vs olanzapine in healthy subjectsReference Silverman, Martin, Memisoglu, DiPetrillo, Correll and Kane 92 and in patients with schizophreniaReference Martin, Correll and Weiden 93 ; levels were also similar after 24-week treatment with OLZ/SAM vs olanzapine in patients with schizophrenia.Reference Correll, Newcomer and Silverman 23 Treatment with olanzapine in combination with samidorphan was associated with a smaller increase in total cholesterol levels compared with olanzapine in healthy subjectsReference Silverman, Martin, Memisoglu, DiPetrillo, Correll and Kane 92 ; however, no difference in total cholesterol levels was observed after treatment with OLZ/SAM vs olanzapine in patients with schizophrenia.Reference Correll, Newcomer and Silverman 23
LY255582
To date, LY255582 has not been assessed in human or animal studies assessing effects on antipsychotic-associated weight gain, but it has been examined in animal models of obesityReference Statnick, Tinsley, Eastwood, Suter, Mitch and Heiman 49 , Reference Ibrahim, Ibrahim, Aziz and Rahman 57 , Reference Shaw, Mitch, Leander, Mendelsohn and Zimmerman 59 , Reference Shaw 70 (Table 3). In genetically obese ratsReference Shaw, Mitch, Leander, Mendelsohn and Zimmerman 59 , Reference Shaw 70 and in rats with diet-induced obesity,Reference Statnick, Tinsley, Eastwood, Suter, Mitch and Heiman 49 , Reference Ibrahim, Ibrahim, Aziz and Rahman 57 LY255582 reduced food intake, body weight gain, and fat mass; LY255582 also reduced body weight and food intake in normal weight rats.Reference Levine, Grace, Billington and Zimmerman 96 No significant differences were observed between LY255582 and vehicle control groups in glucose, triglycerides, total cholesterol, or insulin after 68 days of treatment in genetically obese rats.Reference Shaw, Mitch, Leander, Mendelsohn and Zimmerman 59 LY255582 reduced insulin levels compared with the vehicle control in rats with diet-related obesityReference Statnick, Tinsley, Eastwood, Suter, Mitch and Heiman 49 , Reference Ibrahim, Ibrahim, Aziz and Rahman 57 ; glucose levels were unchanged vs control on day 11,Reference Statnick, Tinsley, Eastwood, Suter, Mitch and Heiman 49 but significantly reduced after 4 weeks of treatmentReference Ibrahim, Ibrahim, Aziz and Rahman 57 . LY255582 in rats fed a high-fat diet was also associated with significant improvement in lipid profileReference Ibrahim, Ibrahim, Aziz and Rahman 57 and with changes in energy utilization (a shift from fat to carbohydrate utilization).Reference Statnick, Tinsley, Eastwood, Suter, Mitch and Heiman 49
Discussion
This review summarizes the potential role of the opioid system in regulating metabolic sequelae, including weight, as well as a potential role in antipsychotic-associated weight gain and metabolic dysregulation. While the published data for naltrexone, samidorphan, and LY255582 support a role for the endogenous opioid system in the regulation of metabolism and weight, they also suggest that the specific effects of opioid antagonists on weight gain, fat mass accumulation, food intake, and regulation of energy utilization vary, likely owing to differential pharmacokinetic, binding, and functional activity profiles. However, animal models and clinical studies indicate that opioid antagonism can be employed to mitigate the adverse effects of antipsychotics on metabolic function.
The data summarized here are from analyses that did not directly compare these 3 compounds, and given the noted heterogeneity among opioid antagonists, generalizations to other opioid antagonists cannot be made. Observed effects on antipsychotic-associated weight gain and metabolic dysregulation vary among the opioid antagonists examined, and are likely due to their differing pharmacokinetics and pharmacodynamic profiles at MOR, DOR, and KOR. Results from preclinical and clinical studies with these representative molecules illustrate that opioid receptor antagonism can modify metabolic regulation, while also suggesting that unique characteristics of individual opioid antagonists may result in different clinical effects in terms of their ability to mitigate weight gain and metabolic sequelae. Additionally, these findings are based on limited evidence obtained from the published literature, which may have been skewed by publication bias; we were unable to locate results of any clinical study for LY255582.
Conclusions
Preclinical and clinical data suggest that the endogenous opioid system is involved in regulating aspects of metabolism, including weight regulation, and that the opioid system is a viable target to address antipsychotic-associated weight gain and metabolic dysregulation. Antipsychotic-associated weight gain can reduce patients’ quality of life and satisfaction with care, and may negatively impact treatment adherence.Reference Velligan, Weiden and Sajatovic 97 , Reference McIntyre 98 Opioid receptor antagonism may be a strategy to mitigate/limit the weight and metabolic-related effects, and its use could thereby increase the clinical utility of otherwise highly efficacious pharmacologic treatments that are associated with weight gain. Differences in observed preclinical and clinical weight-related effects of 3 opioid receptor antagonists, each with distinct pharmacokinetic characteristics, binding, and functional activity profiles with respect to MOR, DOR, and KOR, underscore that the unique properties of individual opioid receptor antagonists may yield important differences in their effectiveness for mitigating antipsychotic-associated weight gain.
Acknowledgements
Medical writing and editorial support were provided by Kathleen M. Dorries, PhD, and John H. Simmons, MD, of Peloton Advantage, LLC, an OPEN Health company, and funded by Alkermes, Inc.
Financial support
This work was sponsored by Alkermes, Inc., the company that developed samidorphan in combination with olanzapine (Lybalvi) to mitigate olanzapine-associated weight gain in the treatment of adults with schizophrenia or bipolar I disorder.
Author contributions
All authors collaborated in the preparation of this manuscript, and critically reviewed and provided revisions to the manuscript. All authors assume responsibility for the completeness and accuracy of the data presented here, based on the data in the literature. All authors granted final approval of the manuscript for submission.
Disclosures
R.S.M. has received research grant support from the CIHR/GACD/National Natural Research Foundation of China, and the Milken Institute; has served as a speaker or consultant for AbbVie, Alkermes, Atai Life Sciences, Axsome, Bausch Health, Biogen, Boehringer Ingelheim, Eisai, Intra-Cellular Therapies, Janssen, Kris Pharma, Lundbeck, Mitsubishi Tanabe, Neumora Therapeutics, Neurocrine, NewBridge Pharmaceuticals, Novo Nordisk, Otsuka, Pfizer, Purdue, Sage, Sanofi, Sunovion, and Takeda; and is the CEO of Braxia Scientific Corp. L.C. has served as a consultant for AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Eisai, Enteris BioPharma, HLS Therapeutics, Impel, Intra-Cellular Therapies, Janssen, Karuna, Lundbeck, Lyndra, Medavante-ProPhase, Merck, Neurocrine, Novartis, Noven, Otsuka, Ovid, Relmada, Reviva, Sage, Sunovion, the University of Arizona, and Teva; has participated in one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research; has served as a speaker for AbbVie/Allergan, Acadia, Alkermes, Angelini, Eisai, Intra-Cellular Therapies, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Sage, Sunovion, Takeda, and Teva; has participated in CME activities organized by medical education companies, including Medscape, NACCME, NEI, and Vindico, as well as for universities and professional organizations/societies; owns stock in Bristol Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, and Pfizer; and has received royalties from Wiley, UpToDate, Springer Healthcare, and Elsevier. H.C., M.S.T., L.A.T., M.W., and S.A. are employees of Alkermes, Inc., and may own stock/options in the company.








