Introduction
Gypsum soils are nutrient-poor soils that have a low water-holding capacity and a high concentration of calcium and sulfate ions (Bolukbasi et al. Reference Bolukbasi, Kurt and Palacio2016; Mota et al. Reference Mota, Garrido-Becerra, Merlo, Medina-Cazorla, Sánchez-Gómez and Loidi2017), exerting strong phenotypic selection pressure on edaphically endemic plants (Muller et al. Reference Muller, Moore, Feder, Tiley and Drenovsky2017), and impacting regional floristic diversity (Damschen et al. Reference Damschen, Harrison, Ackerly, Fernandez-Going and Anacker2012). Gypsum soils are considered ‘natural laboratories’ for studying the evolution and ecophysiology of one of the most conspicuous, diverse plant communities. Gypsum plant communities comprise mainly stress-tolerant subshrubs, scattered shrubs, herbaceous perennials, and annuals (Palacio et al. Reference Palacio, Escudero, Montserrat-Martí, Maestro, Milla and Albert2007). The endemics include rare and/or threatened species with very restricted distributions (Matesanz et al. Reference Matesanz, Ramos-Muñoz, Blanco-Sánchez, García-Fernández, Sánchez and Escudero2019). However, little is known about these communities, especially in the tropics, despite including threatened, rare, and restricted species that are a priority for global biodiversity conservation (Palacio et al. Reference Palacio, Escudero, Montserrat-Martí, Maestro, Milla and Albert2007).
Currently, there is considerable confusion regarding the definition of gypsophily (Mota et al. Reference Mota, Garrido-Becerra, Pérez-García, Salmerón-Sánchez, Sánchez-Gómez and Merlo2016). By some, gypsophily has been defined as the exclusive growth (or strong preference) of a plant species in gypsum-rich soils (e.g., Merlo et al. Reference Merlo, Mota, Sánchez-Gómez, Mota, Sánchez-Gómez and Guirado2011; Salmerón-Sánchez et al. Reference Salmerón-Sánchez, Martínez-Nieto, Martínez-Hernández, Garrido-Becerra, Mendoza-Fernández, Gil de Carrasco, Ramos-Miras, Lozano, Merlo and Mota2014). However, others define gypsophily as the association of plants with gypsum soils (Mota et al. Reference Mota, Garrido-Becerra, Pérez-García, Salmerón-Sánchez, Sánchez-Gómez and Merlo2016). Terminology controversy along with subclassification of plants living in gypsum employing different attributes (i.e., degree of gypsophily, distribution, ecological behaviour, etc.) hampers gypsophile flora studies. In the simplest classification based on the extent of plant restriction to gypsum soils, first proposed by Escudero et al. (Reference Escudero, Palacio, Maestre and Luzuriaga2015), plants can be classified as (a) gypsophiles, exclusive to gypsum soils or (b) gypsovags, found in gypsum soils, but with better growth in other soils. Although other intermediate types, e.g., gypsocline and waif species, have been recognized, more field data are needed to define these types. Here, we use the dichotomous classification of Escudero et al. (Reference Escudero, Palacio, Maestre and Luzuriaga2015) based on its simplicity.
In the last decade, there has been a notable increase in ecological and evolutionary research focused on the plant life associated with gypsum soils (e.g. Bolukbasi et al. Reference Bolukbasi, Kurt and Palacio2016; Merlo et al. Reference Merlo, Garrido-Becerra, Mota, Salmerón-Sánchez, Martínez-Hernández, Mendoza-Fernández and Pérez-García2019; Muller et al. Reference Muller, Moore, Feder, Tiley and Drenovsky2017; Palacio et al. Reference Palacio, Johnson, Escudero and Montserrat-Martí2012). Gypsophile plants also have attracted interest for improving crop production in low-productivity environments (Mota et al. Reference Mota, Garrido-Becerra, Pérez-García, Salmerón-Sánchez, Sánchez-Gómez and Merlo2016) and for remediating soils (Merlo et al. Reference Merlo, Mota, Sánchez-Gómez, Mota, Sánchez-Gómez and Guirado2011; Mota et al. Reference Mota, Garrido-Becerra, Pérez-García, Salmerón-Sánchez, Sánchez-Gómez and Merlo2016). Nevertheless, only in the last 5 years have studies even considered gypsophily in humid climates, despite gypsum outcrops supporting floristic peculiarities and acting as refugia for xerothermophilic species (Casby-Horton et al. Reference Casby-Horton, Herrero, Rolong and Sparks2015; Herrero & Porta Reference Herrero and Porta2000; Parsons Reference Parsons1976; Pérez-García et al. Reference Pérez-García, Akhani, Parsons, Silcock, Kurt, Özdeniz, Spampinato, Musarella, Salmerón-Sánchez, Sola, Merlo, Martínez-Hernández, Mendoza-Fernández, Garrido-Becerra and Mota2018a). Even fewer studies have focused entirely on gypsophily in tropical regions, in part because gypsum outcrops are difficult to locate. In many countries, information for locating and delimiting gypsum deposits is sparse, usually being limited to mining geology (Pérez-García et al. Reference Pérez-García, Spampinato, Musarella, Moore, Palacio, Flores Olvera, Ochoterena, Vogiatzakis, Akhani, Kurt, Özdeniz, Sola, Mendoza-Fernández, Cabello, Guirado, Martínez-Hernández, Merlo, Salmerón-Sánchez, Mota, Musarella and Spampinato2019).
Gypsum soils have been reported in 14 Mexican states (Ortiz-Brunel et al. Reference Ortiz-Brunel, Ochoterena, Moore, Aragón-Parada, Flores, Munguía-Lino, Rodríguez, Salinas- Rofríguez and Flores-Olvera2023), mainly in arid and semi-arid regions in northern Mexico, the arid Puebla-Oaxacan zone (east-central to southern Mexico), and western Pacific Coast (Cervantes-Maldonado et al. Reference Cervantes-Maldonado, Flores-Olvera and Valdés2001), with more recently reports in other desert areas of the northwestern Mexico and in some tropical regions of Mexico (Ochoterena et al. Reference Ochoterena, Flores-Olvera, Gómez-Hinostrosa, Moore, Mandujano, Pisanty and Eguiarte2020; Pérez-García et al. Reference Pérez-García, Martínez-Hernández, Mendoza-Fernández, Merlo, Sola, Salmerón-Sánchez, Garrido-Becerra and Mota2017). Here, we focused on a tropical Mexican region where gypsophily has been barely studied.
In the states of Campeche, Quintana Roo, and Yucatan (southeast Mexico in the Yucatan Peninsula), gypsum outcrops are widely documented geologically for the Icaiche Formation (Martínez et al. Reference Martínez, Sousa and Ramos-Álvarez2001; Martínez & Galindo-Leal, Reference Martínez and Galindo-Leal2002; Fragoso-Servón Reference Fragoso-Servón2015; Perry et al. Reference Perry, Velazquez-Oliman, Leal-Bautista and Dunning2019; Servicio Geológico Mexicano 2007), the oldest exposed geological formation (Palaeocene) mainly in the state of Campeche. The formation contains gypsum beds that cover a minimum of 10,000 km2 (Perry et al. Reference Perry, Velazquez-Oliman, Leal-Bautista and Dunning2019). However, gypsum has not been recognized yet as an edaphic category in any of the soil profiles registered for the state of Campeche. Gypsum outcrops extend along hillsides and hilltops within the Zoh-Laguna Plateau (Martínez & Galindo-Leal, Reference Martínez and Galindo-Leal2002; Šprajc, Reference Šprajc2008) and can reach 40 m thick with a purity between 40 and 96% (Martínez & Galindo-Leal, Reference Martínez and Galindo-Leal2002). The part of the plateau with the gypsum outcrops and the adjacent flood-prone lowlands (locally known as ‘bajos’), however, remains unknown or unexplored (Martínez et al. Reference Martínez, Sousa and Ramos-Álvarez2001) and it may extend into Belize and Guatemala (Perry et al. Reference Perry, Velazquez-Oliman, Leal-Bautista and Dunning2019). We also lack complete, accurate information on the distribution and delimitation of gypsum soils in these areas and elsewhere in the rest of the state of Campeche and Yucatan Peninsula in part due to the lack of road access, dense vegetation, and intense weathering of gypsum-containing rock that limit study of the Icaiche Formation outcrops (Perry et al. Reference Perry, Velazquez-Oliman, Leal-Bautista and Dunning2019).
In Campeche, since gypsum edaphism in the region was first discussed in the botanical work of Martínez et al. (Reference Martínez, Sousa and Ramos-Álvarez2001), gypsum soil and its association with vegetation have been poorly documented (e.g., Gunn et al. Reference Gunn, Foss, Folan, Domínguez-Carrasco and Faust2002; Salas-Orozco Reference Salas-Orozco1974). Although gypsum accentuates the aridity of the Zoh-Laguna Plateau area, leading to strong selective pressure and adaptations to water shortage in plants (Galindo-Leal Reference Galindo-Leal1999), only a few studies have documented the plant–gypsum relationship on a more conceptual basis (e.g. Pérez-García et al. Reference Pérez-García, Martínez-Hernández, Mendoza-Fernández, Merlo, Sola, Salmerón-Sánchez, Garrido-Becerra and Mota2017), more often only noting that certain plant species occur exclusively in areas with gypsum (e.g. Borhidi & Martínez-Salas Reference Borhidi and Martínez-Salas2012; Martínez & Galindo-Leal Reference Martínez and Galindo-Leal2002; Trejo-Casanova Reference Trejo-Casanova2015). In a pioneering approach to exploring gypsum outcrops in a tropical zone, Trejo-Casanova (Reference Trejo-Casanova2015) determined the richness and plant diversity of some gypsum outcrops in the Calakmul Biosphere Reserve, Campeche. The composition of endemic plants and other vegetation in these areas also has been attributed to the influence of gypsum (Pérez-García et al. Reference Pérez-García, Martínez-Hernández, Mendoza-Fernández, Merlo, Sola, Salmerón-Sánchez, Garrido-Becerra and Mota2017; Villaseñor Reference Villaseñor2016).
New technology now available to map and analyze gypsum soils (Casby-Horton et al. Reference Casby-Horton, Herrero, Rolong and Sparks2015) enables the global study of this edaphism across a range of climates to develop a consistent, broadly applicable theory of gypsophily (Mota et al. Reference Mota, Garrido-Becerra, Pérez-García, Salmerón-Sánchez, Sánchez-Gómez and Merlo2016). Published data (geological maps, data on mining, and other documents obtained from the literature), georeferenced herbarium collections, and remote sensing can be integrated to summarize the available information and determine a reliable starting point to locate areas without having a prior report of gypsum. In fact, Ochoterena et al. (Reference Ochoterena, Flores-Olvera, Gómez-Hinostrosa, Moore, Mandujano, Pisanty and Eguiarte2020) highlight the importance of combining published data with georeferenced botanical collections to generate lists of plants growing in gypsum, which already has proven to be useful in the case of Cuatro Ciénegas, Coahuila, Mexico.
Defining the precise location of gypsum outcrops in tropical regions is a crucial first step for identifying and characterizing the gypsophile flora. Characterizing this plant diversity is important because a significant number of endemic species have been reported to be associated with gypsum soils in other regions (Mota et al. Reference Mota, Garrido-Becerra, Merlo, Medina-Cazorla, Sánchez-Gómez and Loidi2017), even in areas with higher humidity, e.g., tropical deciduous forest in the states of Jalisco, Colima, Michoacán, Guerrero, and Oaxaca (Harker et al. Reference Harker, Hernández-López and Muñiz-Castro2021). In addition, the limited information on gypsum soils in these tropical environments comes mainly from reports on mining (Pérez-García et al. Reference Pérez-García, Martínez-Hernández, Mendoza-Fernández, Merlo, Sola, Salmerón-Sánchez, Garrido-Becerra and Mota2017), an activity that directly threatens biodiversity. Therefore, knowledge of the location and biodiversity of gypsum soils in tropical areas of the world is also a prerequisite for their protection and conservation.
In the present study, we sought to (a) identify sites in Campeche with gypsum outcrops based on a systematic literature review and herbarium collections; (b) define polygons of potential gypsum outcropping with remote sensing tools; (c) provide a list of vascular plant species associated with gypsum soils in the Campeche state; and (d) measure the gypsum content in soils from potential gypsum areas.
Materials and methods
Study area
The study area in the state of Campeche in the western Yucatan Peninsula, Mexico (Figure 1) has a warm humid climate (Instituto Nacional de Estadística y Geografía 2017). Natural vegetation includes tropical forest (25.1%), tropical dry forest (16%), hydrophilic vegetation (7.4%), and halophilic grassland–savanna (1.7%) (Instituto Nacional de Estadística y Geografía 2016).

Figure 1. Map of the study area with symbols indicating sites where gypsum was reported in a publication from the literature review (a) and an example of how a polygon was identified with remote sensing: visualization of potential gypsum outcrops with Landsat 8 (channels 7, 6, and 4) imagery (turquoise colour) (b) and Google Earth imagery (whitish colour) (c). Photograph of study area in the polygon shown in images b and c (d).
Geologically, the Yucatan Peninsula has a sedimentary structure formed by carbonate, evaporitic, and clastic rocks, such as limestone, dolomite, gypsum, and sandstone, which overlay the metamorphic basement of the Paleozoic age (Fragoso-Servón et al. Reference Fragoso-Servón, Bautista-Zuñiga, Pereira and Fraustro2016; García-Gil et al. Reference García-Gil, Palacio-Prieto and Ortiz-Pérez2002).
Literature review
The literature search was designed following the PRISMA 2020 criteria (Page et al. Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennan, Chou, Glanville, Grimshaw, Hróbjartsson, Lalu, Li, Loder, Mayo-Wilson, McDonald, McGuinness, Stewart, Thomas, Tricco, Welch, Whiting and Moher2021; Method S1), and a full list of references is included in the Supplementary Materials (References S1). We focused on two main topics for gypsophiles and gypsum environments: biological (plants that live on gypsum soils) and edaphic (gypsum soils). We searched for literature using the following criteria: (a) full text was accessible, (b) keywords for the search were present in the body of the text and referring to the study area of this work, (c) the scope including biological sciences or earth sciences (i.e., sources from medicine, dentistry, architecture, etc. were discarded), (d) information on the location of gypsum outcrops and/or vegetation that tends to grow on gypsum soils within the study area, and (e) studies published in both English and Spanish in the search process. We assessed studies published from 1955 to 2019 (64 years) and retrieved using 18 keywords (Table S1). The search yielded 2,586 results, and a total of 100 references (3.9% of the total; Table S1) met the selection criteria. After duplicates were removed, we ended with a total of 73 records (Figure S1), which were used to generate a list of species reported in gypsum soils and gypsum sites.
Herbarium databases
Search strategy
We built another list of vascular plants from Campeche using herbaria databases of vascular plant specimens from CICY, MEXU (https://www.gob.mx/sgm/articulos/consulta-los-informes-tecnicos-publicaciones-y-tesis?idiom=es), UADY, and GBIF (https://www.gbif.org/occurrence/search), which included approximately 35 herbaria. The database in Excel (Microsoft, Redmond, WA, USA) included georeferenced botanical information of 120,332 records from the study area containing species name, genus, family, order, endemicity, soil fidelity, and locality. Information about the vascular plants that followed the search terms ‘Gypsum’ AND ‘Campeche’ was extracted from this database.
Screening and eligibility criteria
We screened the above-mentioned database for all specimens for which gypsum was either included in the collector’s description of the collection site, e.g., gypsum quarry, or indicated as the substrate at the site. This database included 280 records and collector information for each record. When any records lacked spatial coordinates or that were clearly erroneous but included the location or a description of the locality and met one of the two criteria described above, we adjusted the geographic coordinates as best as possible. After removing duplicates, this database had a total of 144 records.
Selection process
The scientific names were standardized based on the International Plant Name Index (https://www.ipni.org/), The Plant List (http://www.theplantlist.org/), and mainly the Flora Mesoamericana project (http://legacy.tropicos.org/project/fm), except for the record Lantana dwyeriana Moldenke, for which we followed Villaseñor (Reference Villaseñor2016). In the final checklist, we classified species as probable gypsophiles if they were present on a potential gypsum outcrop (polygon) and those described as gypsophiles in the literature, except for Holographis websteri T.F. Daniel, which we found is not restricted to gypsum soils and shows a broader distribution throughout the Yucatan Peninsula. The remaining species were classified as gypsovags.
Remote sensing
We scanned and visualized the study area for gypsum outcrops using remote sensing techniques and Landsat 7 and Landsat 8 imagery focused on short-wave infrared spectra as described by Ochoterena et al. (Reference Ochoterena, Flores-Olvera, Gómez-Hinostrosa, Moore, Mandujano, Pisanty and Eguiarte2020). Gypsum contains trapped water molecules, so gypsum areas reflect a turquoise colour. We used this method to validate gypsum areas reported in the literature and to identify and delimit potential gypsum outcrops for later botanical exploration.
First, the collected information on georeferenced areas with gypsum from the literature review was displayed on a map of the Mexican state of Campeche generated in the open-source Geographic Information System (GIS) QGIS 3.10 A Coruña (from the Open-Source Geospatial Foundation). For general visualization of the studied area (state of Campeche) and evaluating the points displayed on the map, we used an open-access version of ArcGIS Landsat 8 imagery (https://www.arcgis.com/home/webmap/viewer.html?useExisting=1) with channels 7, 6, and 4 focused on short-wave infrared spectra (WRS path: 020, WRS row: 047), which will colour gypsum areas turquoise (Figure 1b). We also compared ArcGIS Landsat imagery and Google Earth imagery (gypsum has a whitish appearance; Figure 1c) to identify potential gypsum outcrops, which were then traced using the polygon tool in Google Earth (Figure 2).

Figure 2. Left (a): Polygons generated in the present study to identify areas with gipsophylic flora in tropical Mexico. Polygons with gypsum in which georeferenced herbarium specimens occur are indicated in green triangles (A); and polygons in which georeferenced herbarium specimens were found, but did not mention gypsum, are in pink triangles (B).
After exporting polygon layer to QGIS on the previously generated map, we incorporated into the same QGIS project the collected information of herbarium specimens, i.e., records with a gypsum report and those that did not have such information, to identify those that occurred within gypsum polygons. We then evaluated the collection sites of the specimens for which a gypsum locality or substrate was reported and, if applicable, proposed a new polygon delimitation.
Vascular plant list for Campeche
Remote sensing, botanical collection data, and literature sources were then used to generate a final vascular plant checklist. The species that were collected in any of the polygons and those that appeared in any of the three sources (remote sensing, database of herbarium specimens with a gypsum indication, and preliminary list from the literature) were placed in our final checklist of vascular plant species reported in gypsum soils in the state of Campeche (hereafter ‘final checklist’; Table 2 and S2).
Fieldwork soil sampling
We focused on collecting soil samples instead of collecting plants to validate gypsum areas, so we could reliably estimate plant biodiversity associated with gypsum.
Soils were sampled during the dry season (February 22–26, 2020) at seven sites in Calakmul municipality in Campeche (Figure 3 and S2). This municipality had the most reported gypsum locations based on the literature review of sites, including reports of gypsum in soils at lower depths. Sampling sites were chosen based on georeferenced gypsum reports from the literature, gypsum reports from herbaria databases with or without remote sensing confirmation, or potential gypsum outcrops, with or without a previous report in literature or herbaria databases, that were verified by remote sensing (Table S3).
Soils were sampled considering the topography of each site (Figure 3), as gypsum has been found on slopes, hilltops, and in areas called ‘lows’ [generally used to refer to cumulative plains that tend to flood; (Martínez & Galindo-Leal Reference Martínez and Galindo-Leal2002; Palacio-Aponte et al. Reference Palacio-Aponte, Noriega-Trejo and Zamora-Crescendo2002)]. At each study site, we sampled soils at three locations with contrasting topographic positions, i.e., low, mid-point, and high, and collected three replicates at each location (7 sites x 3 locations/site x 3 replicates/location = 63 samples). On average, there was 29 m between each sampling site. Samples were taken from the first 30 cm of the soil, and each one weighed around 2 kg. These samples were placed in hermetically sealed plastic bags and labelled. Samples were oven-dried at approximately 40°C and analyzed at the Laboratorio Central Universitario from Universidad Autónoma de Chapingo (Central University Laboratory from Autonomous University of Chapingo). Gypsum content (%) was measured using the OMRAN GypSim method (Omran Reference Omran2016).

Figure 3. Examples of areas with gypsum soils in Calakmul municipality, Campeche, Mexico. Soil samples were collected across a range of topographic positions, (a): low point (L), mid-point (M), and high point (H). Gypsum landscapes in El Carmen II (b) and in San Miguel (c), correspond to polygons 8 and 10, respectively.
Different combinations of the three criteria already mentioned (literature, herbarium material, and remote sensing), used to evaluate the accuracy of predicting the presence of gypsum, were correlated with the gypsum percentage in the studied soils (Table S3).
Results
Gypsum outcrops based on literature review
Most of the reports of gypsum soil came from deep horizon samples of Gleysols and Vertisols in lowlands in central and southern Campeche (Aguilar-Nogales Reference Aguilar-Nogales1981; Bautista et al. Reference Bautista, Palacio-Aponte, Quintana and Zinck2011; Bautista & Palacio Reference Bautista, Palacio, Krasilnikov, Jiménez, Reyna and García2011; Beach et al. Reference Beach, Luzzadder-Beach, Cook, Dunning, Kennett, Krause, Terry, Trein and Valdez2015; Brewer Reference Brewer2017; Dunning Reference Dunning and Liendo-Stuardo2008; Dunning et al. Reference Dunning, Anaya-Hernández, Beach, Carr, Griffin, Jones, Lentz, Luzzadder-Beach, Reese-Taylor and Šprajc2019; Faust & Folan Reference Faust and Folan2015; Folan et al. Reference Folan, Domínguez-Carrasco, Gunn, Morales-López, González-Heredia, Villanueva-García, Torrescano-Valle and Cucina2013; Geovannini-Acuña Reference Geovannini-Acuña, Reese-taylor and Anaya-Hernández2013; Gunn et al. Reference Gunn, Foss, Folan, Domínguez-Carrasco and Faust2002, Reference Gunn, Folan, Day and Faust2012; LaRocque et al. Reference LaRocque, Leblon and Ek2019; Martínez & Galindo-Leal Reference Martínez and Galindo-Leal2002; Parker Reference Parker2015; Salas-Orozco Reference Salas-Orozco1974) and hillsides and hilltops (Martínez & Galindo-Leal, Reference Martínez and Galindo-Leal2002; Šprajc & Flores-Esquivel Reference Šprajc2008; Šprajc et al. Reference Šprajc, Flores-Esquivel, Čaval and García-López2014). Gypsum was also mentioned in studies on soil development in Campeche (Bautista et al. Reference Bautista, Palacio-Aponte, Quintana and Zinck2011; Fragoso-Servón Reference Fragoso-Servón2015; Zamora-Crescencio Reference Zamora-Crescencio1999) and on the distribution of gypsum outcrops or gypsum soils within the Zoh-Laguna Plateau (Borhidi & Martínez-Salas Reference Borhidi and Martínez-Salas2012; Galindo-Leal Reference Galindo-Leal1999; Martínez et al. Reference Martínez, Sousa and Ramos-Álvarez2001; Pérez-García et al. Reference Pérez-García, Martínez-Hernández, Mendoza-Fernández, Merlo, Sola, Salmerón-Sánchez, Garrido-Becerra and Mota2017).
Ninety-four geographical areas were recovered from 73 documents included in the literature review as having gypsum, but geographical coordinates or specific collection sites were reported for only 66 of the 94 areas. Based on these reports, the 66 gypsum areas were distributed in the municipalities of Calakmul (47 areas), Holpechén (14 areas), Champotón (three areas), and Campeche (two areas) in the Mexican state of Campeche and in that order of significance. Nearly all of these areas (97%) are within the Icaiche Formation, where layers of gypsum deposits have been reported previously (Figure 1).
Although most of the gypsum reports from 73 documents for this area had a geological context, meaning that gypsum is not necessarily present at the soil surface but may be present in deeper horizons, we found 27 documents included an edaphic context, mentioning gypsum being present at the soil surface or close to the soil surface. However, both geological and edaphic contexts were incorporated in the 66 georeferenced locations (Figure S3).
Among the 66 geographical areas, 26 were described as gypsum outcrops (39%), six as gypsum occurrence (9%), and 34 as gypsum mines (52%) in which Calakmul accounts for the majority of gypsum sources (Figure S4A). For these 66 areas, six polygons of potential gypsum outcrops (polygons 2–5, 7, and 14) were confirmed based on remote sensing and correspond to gypsum mines (Table S4).
Gypsum outcrops based on herbarium and literature data
We identified 144 records from herbaria, i.e., records with gypsum according to collection data, across 37 georeferenced locations in nine localities within Calakmul and Hopelchén.
From herbaria data (144 records) and georeferenced spots (37), five polygons (polygons 2, 7, 10, 13, and 14) were confirmed based on remote sensing. Of the five, four are gypsum mine sites (2, 7, 13, and 14) and three (2, 7, and 14) matched sites reported in the literature (Table S5). Polygons 2 and 7 had the highest amount of herbaria records (69%) and georeferenced spots (19) in two localities in Calakmul, whereas polygons 10, 13, and 14 had fewer herbaria records (23%) and georeferenced locations (10) in three localities in Calakmul and Hopelchén. The remaining herbaria records (8%) and georeferenced locations (8) were not located within any polygon, yet were located within the Zoh-Laguna Plateau (Table S5; Figure S5)
Gypsum outcrops based on herbarium data, literature review, and remote sensing
Using Google Earth and Landsat 8 imagery of georeferenced gypsum sites identified from literature, herbarium data, and overview of the Zoh-Laguna plateau area, we delimited 14 potential gypsum outcrop polygons (13 were in Calakmul, one in Holpechén; Figure 4). Three of these polygons (polygons 2, 7, and 14; Figure 2, Table S4) were confirmed based on herbarium data, literature review, and remote sensing; three (3, 4, and 5; Figure 2; Table S4) were confirmed based on literature review and remote sensing; two (polygons 10 and 13; Figure 2; Table S4) were confirmed based on herbarium data and remote sensing; and the remaining six polygons (1, 6, 8, 9, 11, and 12; Table S4) were delimited only using remote sensing (Landsat 8 and Google Earth imagery criteria). Of the 14 potential gypsum outcrops, seven of these were located within gypsum mines (polygons 2–5, 7, 13, and 14) documented by the Mexican Geological Services (SGM, 2007).
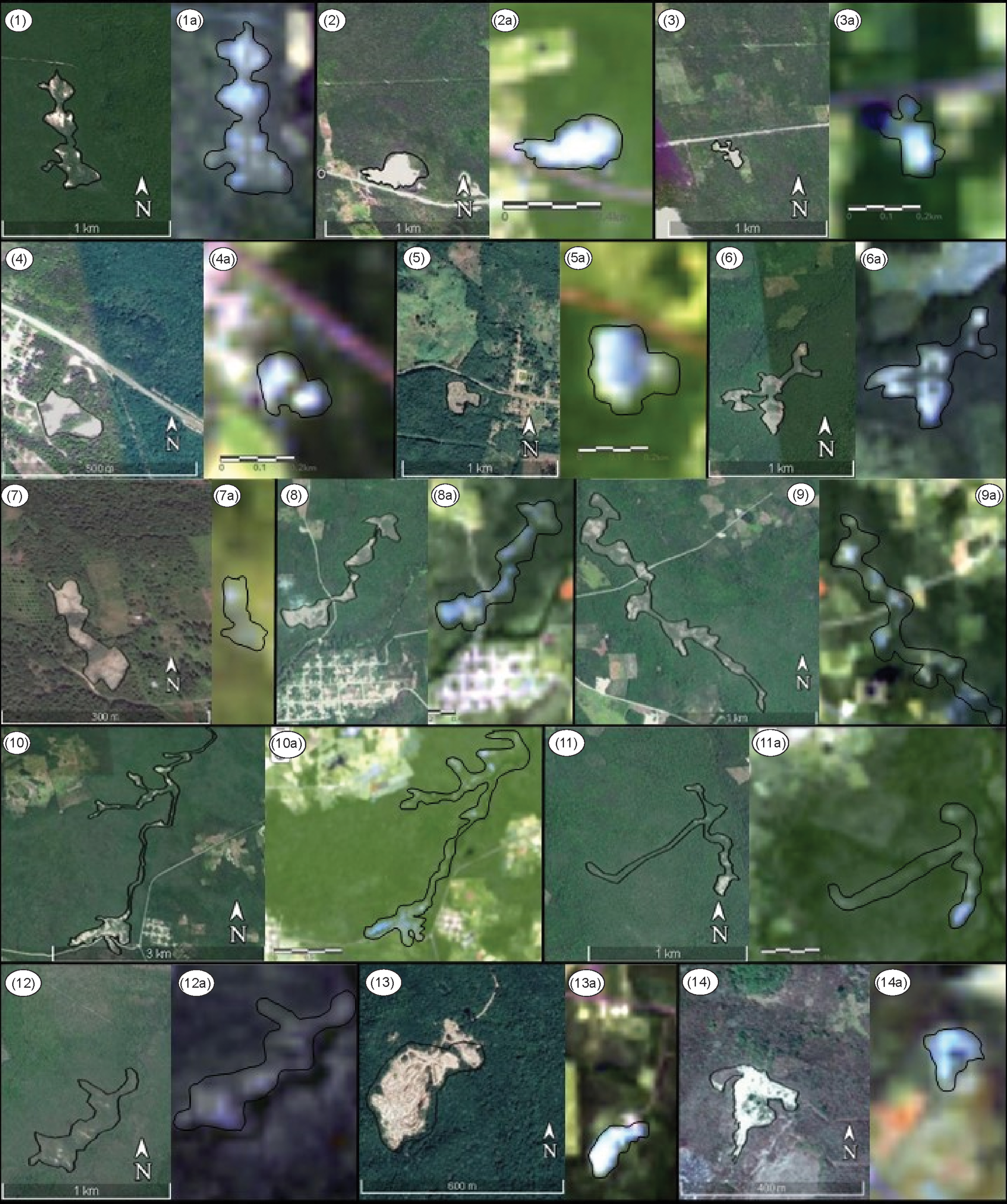
Figure 4. Polygons generated in the present study in Campeche, Mexico that are visualized with Google Earth (left image: 1–14) and Landsat 8 images bands 7, 6, and 4 (right image: 1a-14a) across gypsum landscapes, as indicated by areas in blue.
Polygons ranged in area from 0.73 to 67.12 ha (Table 1). The smallest polygon is located within a gypsum mine (polygon 7) and the largest polygon in an area of apparently undisturbed natural vegetation (polygon 10). All polygons were located within the limits of the Icaiche Formation and/or the Zoh-Laguna Plateau.
Table 1. List of potential gypsum outcrops in the state of Campeche

Soil gypsum content
We found gypsum in the 63 soil samples from the seven sample sites, with gypsum content ranging from 4% to 51% (Table S3). Sampling locations in San Miguel (Table S3) had the highest gypsum content, reaching an average of 44%, followed by samples from El Carmen II (Table S3) with an average content of 31% and Eugenio Echeverria Castellot (Table S3) with an average content of 26% (Figure S6A). La Valeriana and El Manantial had the lowest gypsum content (11%) (Table S3). Thus, although all sampling sites had gypsum, those with the highest gypsum content were those that included areas with natural vegetation (Figure S6). For the seven tested sites, the remote sensing verification colours (turquoise and white) corresponded to high gypsum soil concentrations (Figure S6). Gypsum content was not correlated with the topographic relief (Table S3).
Vascular plant diversity
Our final checklist added 112 species and 21 families to those already obtained from the literature review (39 spp. and 25 families), including 18 species and six families reported here for the first time as growing in gypsum soils in the studied area and representing species that coincide with a gypsum polygon proposed in this study, despite the lack of a soil description for the collected plants (Table S2). Based on the literature review, we identified 39 species of angiosperms distributed among 36 genera and 25 families that grow on gypsum soils in Campeche (Table S2). From the herbaria database, we found 103 species distributed among 84 genera and 36 families that occur in areas with gypsum.
We found a total of 120,332 herbarium records that were associated with the potential gypsum outcrops (polygons) identified using remote sensing (Figure 2a). Among these records, we found 59 records (45 without gypsum specification) in four of 14 polygons (Figure 2a), representing 45 species. Yet seven species from the checklist that were based on the polygons are epiphytic and were thus removed from the list, yielding a total of 38 species (34 genera and 25 families).
The final checklist, including species from remote sensing (39 spp.), herbarium (103 spp.), and literature reports (39 spp.), contains 151 species that potentially grow on gypsum (Table S2), belonging to 121genera and 46 families (Table 2). Fabaceae had the most species present (19), followed by Rubiaceae (13) and Euphorbiaceae (12) (Table 2; Figure S7). Among the 46 families, 21 had only one species present (14%) (Figure S7E). Only 38 species from 25 families (25% of all species, 54% of all families) coincided with a gypsum outcrop polygon defined in this study (Table S2).
Table 2. Number of vascular plant species for each family in the final checklist of vascular plants reported to grow in gypsum in the state of Campeche, Mexico. NSL: Total number of species potentially growing on gypsum in final checklist. NSP: Number of species growing on delimited gypsum polygons identified in this study

Endemism
Only four species of the 151 in the final checklist are recognized explicitly as gypsophiles according to the literature (Table S2): Holographis websteri T.F. Daniel (Acanthaceae), Mitracarpus gypsophilus Borhidi & E. Martínez (Rubiaceae), Lantana dwyeriana Moldenke (Verbenaceae), and Fuirena stephani Ramos & Diego (Cyperaceae). Fuirena stephani was even proposed to be a hygrogypsophyte by Pérez-García et al. (Reference Pérez-García, Martínez-Hernández, Mendoza-Fernández, Merlo, Sola, Salmerón-Sánchez, Garrido-Becerra and Mota2017). Among these four gypsophiles, only M. gypsophilus was found in a delimited potential polygon.
Among the 151 species, 38 were found in one or more of the 14 polygons. One of the 38 is the gypsophyte, M. gypsophilus. We postulate that it and L. dwyeriana Moldenke and F. Ramos & Diego are probable gypsophiles, whereas the remaining 148 are gypsovags including H. websteri T.F. Daniel, which was reported as a gypsophile by Pérez-García et al. (Reference Pérez-García, Martínez-Hernández, Mendoza-Fernández, Merlo, Sola, Salmerón-Sánchez, Garrido-Becerra and Mota2017).
Among the 148 gypsovags, 37 are confirmed as gypsovags based on botanical criteria and reports that they either grow on gypsum mines, gypsum outcrops, or in habitats that include gypsum environments (Cervantes-Maldonado et al. Reference Cervantes-Maldonado, Flores-Olvera and Valdés2001; Martínez & Galindo-Leal Reference Martínez and Galindo-Leal2002; Ortiz-Díaz et al. Reference Ortiz-Díaz, Arnelas, Cerros-Tlatilpa, Siqueiros-Delgado and Tun-Garrido2015; Powell Reference Powell1978). The remaining 111 are probable gypsovags based on literature reports (25 spp.), herbarium data (80 spp.), or both (6 spp.), but their presence in a delimited polygon could not be confirmed (Table S2). Therefore, since we could not confirm gypsum presence using either soil analysis or remote sensing where these species occur, we classified them as probable gypsovags.
Among the 151 species in the final checklist, 17 were reported as endemic to the Yucatan Peninsula Biotic Province (Duno de Stefano et al. Reference Duno de Stefano, Carnevali Fernández-Concha, Ramírez Morillo, Tapia Muñoz, Can Itzá, Hernández-Aguilar and Embray2010), 14 of the 17 are gypsovags, and the remaining three are probable gypsophiles.
Discussion
Gypsum outcrops in Campeche, Mexico
The 14 polygons that delimited gypsum areas based on remote sensing were fewer than the total number of records (66) that reported or predicted the presence of gypsum in the literature. Using remote sensing to delimit polygons thus avoids overestimating the presence of gypsum sites and may more accurately show associations between soil and plant species. In this sense, seven polygons (1, 6, 8, 9, 10, 11, and 12) did not correspond to gypsum mines, and field observations of two of those seven (8 and 10) allowed us to infer that remote sensing was useful for identifying and delimiting sites that were not previously described as having gypsum (Table S4).
The gypsum polygons had characteristics reported in the literature that are consistent with those of gypsum outcrops in more arid climates, including an abrupt discontinuity in the surrounding vegetation and the density and the appearance of the soil, such as colouration and low organic material content (Pérez-García et al. Reference Pérez-García, Salmerón-Sánchez, Martínez-Hernández, Mendoza-Fernandez, Merlo, Mota, Bevilacqua, Calabrò and Della-Spina2021). Future work in Campeche and other tropical regions should incorporate botanical exploration, satellite remote sensing, geological and ecological data, and factors such as water availability and seasonality to better understand these outcrops and their vegetation because their occurrence is generally underestimated and even neglected worldwide as a result of mismanagement of cultivation areas, landfills, urban planning or mining (Pérez-García et al. Reference Pérez-García, Salmerón-Sánchez, Martínez-Hernández, Mendoza-Fernandez, Merlo, Mota, Bevilacqua, Calabrò and Della-Spina2021).
Pérez-García et al. (Reference Pérez-García, Martínez-Hernández, Mendoza-Fernández, Merlo, Sola, Salmerón-Sánchez, Garrido-Becerra and Mota2017) listed several tropical countries as having gypsum outcrops but no associated floristic information is available. The method here employed could contribute to the specific delimitation of these outcrops, and the identification of associated flora. Although Pérez-García et al. (Reference Pérez-García, Martínez-Hernández, Mendoza-Fernández, Merlo, Sola, Salmerón-Sánchez, Garrido-Becerra and Mota2017) did not include Guatemala in their global distribution list of gypsum deposits, the National Commission of Protected Areas (Comisión Nacional de Áreas Protegidas (CONAP), 2010) reported a gypsum outcrop known as El Desierto in northeastern Guatemala. The gypsum deposits in this region have also been confirmed through CONAP´s photographic evidence and our remote visualization data (unpublished), which suggest that the method can be successfully applied to other tropical regions in the world.
Gypsum soils concentration
We found gypsum in all the soil samples collected, and those with the highest concentration (up to 51%) were from a site of natural, undisturbed vegetation. This result is significant, given that studies focused on gypsophile plants have mostly covered only arid and semi-arid regions, which have suggested that humid climates do not promote gypsum formation or that it can be easily removed from the surface by leaching (Parsons Reference Parsons1976; Pérez-López et al. Reference Pérez-López, Pérez-Valera, Sánchez Gómez, Mota, Sánchez-Gómez and Guirado2011). Five years ago, Pérez-García et al. (Reference Pérez-García, Akhani, Parsons, Silcock, Kurt, Özdeniz, Spampinato, Musarella, Salmerón-Sánchez, Sola, Merlo, Martínez-Hernández, Mendoza-Fernández, Garrido-Becerra and Mota2018a) published the first study to include gypsum outcrops in a tropical region. Even in the state of Campeche, where gypsum outcrops had already been reported, they have not been formally studied edaphically. Thus, our study is the first to report the percentage of gypsum in the surface layer of soils in which flora associated with this mineral could be established in Campeche.
According to certain authors (e.g. Ferrandis et al. Reference Ferrandis, Herranz and Copete2005; Mota et al. Reference Mota, Navarro, Peñas, Pérez-García, Posadas, Pujadas Salvà, Rodríguez-Tamayo, Sainz-Ollero, Salazar, Sánchez-Gómez, Segarra-Moragues, Serra, Sola, Torres, Triano, Valle, Villar, Ballesteros, Bartolomé, Cano, Del Río, Domínguez Lozano, Fabado, Fabregat, Ferrer, García-Fuentes, Garrido-Becerra, Goñi, Guirado, Gutiérrez, Guzmán, Ivorra, Jiménez Martínez, Laguna, Lahora, López Udías, Lorite, Marchal, Martínez-Hernández, Martínez Labarga, Medina-Cazorla, Mendoza, Merlo, Mota, Sánchez-Gómez and Guirado2011, Reference Mota, Garrido-Becerra, Pérez-García, Salmerón-Sánchez, Sánchez-Gómez and Merlo2016), soils with more than 5% are considered gypsic, lower than 25% are hypogypsics, and those higher than 51% are hypergypsic. As mentioned already, 25% gypsum has been considered the limit for gypsophily, which limits the ability of plants to take up nutrients and for roots to penetrate the soil. Among study sites with soil with an average gypsum content below 25%, the status of El Refugio as having gypsum could not be verified remotely, but our visit to the site clearly showed that gypsum was present. Although the area with gypsum may be too small to be detected with Landsat imagery (Ochoterena et al. Reference Ochoterena, Flores-Olvera, Gómez-Hinostrosa, Moore, Mandujano, Pisanty and Eguiarte2020), some soil samples from El Refugio had a gypsum content of 26–38% (Table S3). Sites that were positive for gypsum and verified remotely had average gypsum concentrations of 13% (La Moza), 31% (El Carmen II), 26% (Eugenio Echeverría Castellot), and 44% (San Miguel). However, we could not verify if lower gypsum concentrations reflect a turquoise colour as detected via remote sensing. We found that the variability of the gypsum content was not correlated with topographic position, which is consistent with the island-like pattern of the gypsum deposits suggested for this kind of environment in the North American deserts (e.g., Aguirre-Liguori et al. Reference Aguirre-Liguori, Scheinvar and Egiarte2014; Johnston Reference Johnston1941; Moore & Jansen Reference Moore and Jansen2007).
Species growing in the polygons of potential gypsum outcrops
In the final checklist, only 38 species were confirmed in polygons with gypsum (Table S2), including one of the four species formally recognized as a gypsophyte (Mitracarpus gypsophilus). Of the 38 species, 20 were confirmed with georeferenced specimens collected from gypsum mines. The remaining species were collected within gypsum outcrop polygons with natural vegetation, but neither these nor the specimens from gypsum mines included information about the surrounding soil.
The lack of detailed soil information on herbarium labels was one limitation that we faced when working with herbarium collections. Others have noted that the lack of critical site details in many herbarium labels makes compiling lists of gypsophiles and gipsovags difficult (e.g., Rick Reference Rick2011). The presence of gypsum can also be confused with other substrates. Johnston (Reference Johnston1941), for example, indicated that in northern Mexico labels, gypsum may be described as calcareous or even saline. However, botanical observations from collecting sites are valuable, accessible, and practical for identifying and classifying plants growing on gypsum that may encourage further research on variables such as soil and plant chemical composition and distribution that enhance our understanding of plant–gypsum relationships.
Considering only the species from georeferenced collections located within the defined polygons, the dominance of the family Rubiaceae was evident, with six species (including a gypsophyte), followed by Asteraceae with three (Table 2). Our results yielded not only a final checklist of vascular plants with possible distribution in gypsum soils based on different sources but also an overview of areas that still need botanical exploration (nine polygons remain unexplored). In the Yucatan Peninsula, the state of Campeche there has been little work to increase botanical collections, but this situation is improving. Campeche recently had the most new species and new records reported in the Yucatan Peninsula (Pérez-Sarabia et al. Reference Pérez-Sarabia, Duno de Stefano, Fernández-Concha, Ramírez Morillo, Méndez-Jiménez, Zamora-Crescencio, Gutiérrez-Baez and Cetzal-Ix2017). In particular, the central area of the Zoh-Laguna Plateau is not well known, and numerous new species and local endemism have been found in this region (Martínez et al. Reference Martínez, Sousa and Ramos-Álvarez2001). We expect that the species list generated here, including the gypsophile species and the representativeness within the families, will be further enriched and refined as unexplored areas are studied and older data are integrated.
Vascular plant diversity in gypsum outcrops of Campeche
The literature search based on the presence of gypsum and gypsum–vegetation relationship returned numerous results when general concepts such as ‘yeso’ and ‘gypsum’ were used and when using information from governmental sources. The only reference found that mentioned ‘gypsophily’ for plants from Campeche was by Pérez-García et al. (Reference Pérez-García, Martínez-Hernández, Mendoza-Fernández, Merlo, Sola, Salmerón-Sánchez, Garrido-Becerra and Mota2017). However, for the relationship of gypsum with vegetation, the oldest reference was by Galindo-Leal (Reference Galindo-Leal1999), who noted that the gypsum outcrops in Campeche are unique for tropical areas. Since then, only four species have been formally recognized as gypsophytes (see Borhidi & Martínez-Salas Reference Borhidi and Martínez-Salas2012; Pérez-García et al. Reference Pérez-García, Martínez-Hernández, Mendoza-Fernández, Merlo, Sola, Salmerón-Sánchez, Garrido-Becerra and Mota2017) in Campeche and just one thesis research has focused on gypsophily in the studied area (see Trejo-Casanova Reference Trejo-Casanova2015).
Before the study of Pérez-García et al. (Reference Pérez-García, Martínez-Hernández, Mendoza-Fernández, Merlo, Sola, Salmerón-Sánchez, Garrido-Becerra and Mota2017), studies on gypsophily focused on the specificity of this edaphism in arid and semi-arid areas (e.g. Escudero et al. Reference Escudero, Palacio, Maestre and Luzuriaga2015; Merlo-Calvente et al. Reference Merlo-Calvente, Gil de Carrasco, Sola-Gómez, Jiménez-Sánchez, Rodríguez-Tamayo and Mota-Poveda2009; Romão & Escudero Reference Romão and Escudero2005). Pérez-García et al. (Reference Pérez-García, Martínez-Hernández, Mendoza-Fernández, Merlo, Sola, Salmerón-Sánchez, Garrido-Becerra and Mota2017) also were the first to use the term hygrogypsophyte, because of the humid conditions in which tropical gypsophytes can be found.
Fabaceae, Rubiaceae, Euphorbiaceae, Poaceae, and Asteraceae were the families with the most species represented in the final checklist. Among these families, Fabaceae, Poaceae and Asteraceae also have the most species represented in the Yucatan Peninsula (Duno de Stefano et al. Reference Duno de Stefano, Ramírez Morillo, Tapia-Muñoz, Hernández-Aguilar, Can, Cetzal-Ix, Méndez-Jiménez, Zamora-Crescencio, Gutiérrez-Báez and Fernández-Concha2018). Except for Rubiaceae, the dominance of these families among gypsophile flora has been reported previously (e.g. Akpulat & Celik Reference Akpulat and Celik2005; Cervantes-Maldonado et al. Reference Cervantes-Maldonado, Flores-Olvera and Valdés2001; Musarella et al. Reference Musarella, Mendoza-Fernández, Mota, Alessandrini, Bacchetta, Brullo, Caldarella, Ciaschetti, Conti, Di Martino, Falci, Gianguzzi, Guarino, Manzi, Minissale, Montanari, Pasta, Peruzzi, Podda, Sciandrello, Scuderi, Troia and Spampinato2018; Ochoterena et al. Reference Ochoterena, Flores-Olvera, Gómez-Hinostrosa, Moore, Mandujano, Pisanty and Eguiarte2020; Rick Reference Rick2011).
The prevalence of species in the family Asteraceae and the order Asterales may be due to their extraordinary niche diversification (Pérez-García et al. Reference Pérez-García, Akhani, Parsons, Silcock, Kurt, Özdeniz, Spampinato, Musarella, Salmerón-Sánchez, Sola, Merlo, Martínez-Hernández, Mendoza-Fernández, Garrido-Becerra and Mota2018a). The prevalence of the order Fabales also may be due to adaptations such as the capacity to accumulate Ca and S or form a symbiosis with Rhizobium Frank that provide an adaptive advantage in N-poor soils (Pérez-García et al. Reference Pérez-García, Akhani, Parsons, Silcock, Kurt, Özdeniz, Spampinato, Musarella, Salmerón-Sánchez, Sola, Merlo, Martínez-Hernández, Mendoza-Fernández, Garrido-Becerra and Mota2018a). Many species belonging to the families Poaceae and Euphorbiaceae can also grow in stressful and diverse environments (Cervantes-Maldonado et al. Reference Cervantes-Maldonado, Flores-Olvera and Valdés2001; Musarella et al. Reference Musarella, Mendoza-Fernández, Mota, Alessandrini, Bacchetta, Brullo, Caldarella, Ciaschetti, Conti, Di Martino, Falci, Gianguzzi, Guarino, Manzi, Minissale, Montanari, Pasta, Peruzzi, Podda, Sciandrello, Scuderi, Troia and Spampinato2018), although, in the case of Euphorbiaceae, none of the species reported in Mexico grow exclusively in gypsum soils (Cervantes-Maldonado et al. Reference Cervantes-Maldonado, Flores-Olvera and Valdés2001).
We were surprised that species of Hechtia (Bromeliaceae) and Bletia (Orchidaceae) may be potential gypsophytes, as their families consist largely of epiphytic species and orchids that have not been previously reported in gypsum soils in Campeche. However, gypsophytes from both genera have been found in the state of Oaxaca (Hernández-Cárdenas et al. Reference Hernández-Cárdenas, López-Ferrari and Espejo-Serna2019; Salazar et al. Reference Salazar, Chávez-Rendón, De Ávila and Jiménez-Machorro2016), and Musarella et al. (Reference Musarella, Mendoza-Fernández, Mota, Alessandrini, Bacchetta, Brullo, Caldarella, Ciaschetti, Conti, Di Martino, Falci, Gianguzzi, Guarino, Manzi, Minissale, Montanari, Pasta, Peruzzi, Podda, Sciandrello, Scuderi, Troia and Spampinato2018) reported gypsovags from Orchidaceae in a checklist of gypsophilous vascular flora in Italy. Contrary to the assumption of Martínez and Galindo-Leal (Reference Martínez and Galindo-Leal2002), the Sapotaceae family was poorly represented (two species). Of these two, Manilkara zapota (L.) P. Royen was present in a potential gypsum outcrop polygon. The presence of dwarf phenotypes of the same species was reported in a gypsum outcrop in Guatemala (CONAP, 2010).
Although some families in our final checklist appeared in the lists of Musarella et al. (Reference Musarella, Mendoza-Fernández, Mota, Alessandrini, Bacchetta, Brullo, Caldarella, Ciaschetti, Conti, Di Martino, Falci, Gianguzzi, Guarino, Manzi, Minissale, Montanari, Pasta, Peruzzi, Podda, Sciandrello, Scuderi, Troia and Spampinato2018; Italy), Ochoterena et al. (Reference Ochoterena, Flores-Olvera, Gómez-Hinostrosa, Moore, Mandujano, Pisanty and Eguiarte2020; Cuatro Ciénegas, Coahuila, Mexico), Pérez-García et al. (Reference Pérez-García, Akhani, Parsons, Silcock, Kurt, Özdeniz, Spampinato, Musarella, Salmerón-Sánchez, Sola, Merlo, Martínez-Hernández, Mendoza-Fernández, Garrido-Becerra and Mota2018a; Paleartic and Australia), and Rick (Reference Rick2011; Australia), our list shared only four species and 13 genera with them. Ochoterena et al. (Reference Ochoterena, Flores-Olvera, Gómez-Hinostrosa, Moore, Mandujano, Pisanty and Eguiarte2020) reported Flaveria trinervia (Spreng.) C. Mohr (Asteraceae), Portulaca oleracea L. (Portulacaceae), and Karwinskia humboldtiana S. Watson (Rhamnaceae) as gypsovags and a variety of Viguiera dentata B.L. Turner as a gypsophyte. Our findings agree with Ochoterena et al. (Reference Ochoterena, Flores-Olvera, Gómez-Hinostrosa, Moore, Mandujano, Pisanty and Eguiarte2020) related to the gypsovags. Species of Ruellia (Acanthaceae), Heliotropium (Boraginaceae), Eleocharis and Fuirena (Cyperaceae), Diospyros (Ebenaceae), Euphorbia (Euphorbiaceae), Sporobolus (Poaceae), Randia (Rubiaceae), and Solanum (Solanaceae) were reported as gypsophytes or gypsovags previously by Pérez-García et al. (Reference Pérez-García, Akhani, Parsons, Silcock, Kurt, Özdeniz, Spampinato, Musarella, Salmerón-Sánchez, Sola, Merlo, Martínez-Hernández, Mendoza-Fernández, Garrido-Becerra and Mota2018a) and Ochoterena et al. (Reference Ochoterena, Flores-Olvera, Gómez-Hinostrosa, Moore, Mandujano, Pisanty and Eguiarte2020).
Endemism
Of the 151 species in the final checklist, approximately 17 (11%) have been classified as endemic to the Yucatan Peninsula Biotic Province (Durán et al. Reference Durán, Trejo-Torres and Ibarra-Manríquez1998; Villaseñor Reference Villaseñor2016), and we detected five of these growing in potential gypsum polygons. On the other hand, of the four endemic species indicated as gypsophiles in the literature, only one (Mitracarpus gypsophilus) was confirmed to be present in one or more potential gypsum polygons.
However, only two of the seven polygons with natural vegetation included sites where herbarium specimens had been collected.
Here, we identified new gypsum outcrops, compiled a list of the species documented for these areas in literature and herbaria, reported gypsum concentrations in some of these outcrops, and confirmed the presence of endemic vegetation on gypsum soils in a tropical region in Mexico (Campeche). This information can be used to determine conservation priorities for these regions and highlight the need for characterizing them.
Gypsum mining represents a threat to the biodiversity of gypsum outcrops in the region. Recent expansion of livestock farming in the region might also become a serious threat to biodiversity, even in areas considered unsuitable for milpa (a traditional agriculture system) (Špirić et al. Reference Špirić, Vallejo and Ramírez2022). An even more important driver of deforestation in this region is mechanised agriculture, which is replacing the milpa in Campeche (Špirić et al. Reference Špirić, Vallejo and Ramírez2022). Other agricultural practices such as the use of fire and changes in the agricultural cycles also have contributed to forest fragmentation in the region (Špirić et al. Reference Špirić, Vallejo and Ramírez2022).
Land-use changes, such as migration, have extended human settlements into the forests of Campeche in locations such as Narciso Mendoza and El Carmen II, where we found some of the gypsum outcrops, and small populations of migrants have organized as ejidos within the buffer zone of the Calakmul Biosphere Reserve (Neulinger et al. Reference Neulinger, Vogl and Alayón-Gamboa2013). A governmental urbanization project seeks to increase tourism in the region (Pérez-Ortega & Gutiérrez-Jaber Reference Pérez-Ortega and Gutiérrez-Jaber2022); railway construction, increased human activities, and new potential settlements around train stations are a few of the activities that may modify the region and could threaten biodiversity in the region.
In this study, we identified seven potential gypsum polygons with natural vegetation, excluding seven polygons that correspond to mining sites. The spatial distribution of gypsum outcrops enables the study of plant speciation, metapopulation dynamics, and evolutionary subjects (Escudero et al. Reference Escudero, Palacio, Maestre and Luzuriaga2015). However, some studies suggest that gypsophile floras may have evolved independently (e.g., Moore et al. Reference Moore, Mota, Douglas, Flores-Olvera, Ochoterena, Rajakaruna, Boyd and Harris2014) and that gypsophily could have appeared several times (e.g., Escudero et al. Reference Escudero, Palacio, Maestre and Luzuriaga2015). Species of cosmopolitan genera such as Euphorbia, which include gypsophiles, are hard to find in more than one gypsum region (Moore et al. Reference Moore, Mota, Douglas, Flores-Olvera, Ochoterena, Rajakaruna, Boyd and Harris2014). Pérez-García et al. (Reference Pérez-García, Martínez-Hernández, Mendoza-Fernández, Merlo, Salmerón-Sánchez and Mota2018b) remarked on the great differences in species between the list of tropical gypsophytes in Africa with respect to the list for the Palearctic zone.
Conclusions
We found that gypsum outcrops in the Mexican state of Campeche, as indicated in the literature, are associated with the Icaiche Formation and the Zoh-Laguna Plateau. The use and integration of different sources are useful for compiling information on known gypsum outcrops and locating and delimiting gypsum areas that have not been reported. Four of the polygons that we identified in this study have gypsum concentrations from 5% to 51% in the first 30 cm below the soil surface, and represent areas for future studies of gypsophily in this tropical area.
The final checklist achieved in this study contained 151 species. The best-represented families were Fabaceae, Rubiaceae, Euphorbiaceae, Poaceae, and Asteraceae, and 18 species and six families are reported for the first time as growing in gypsum within the study area.
Integrating data from botanical collections with soil analyses of gypsum outcrops and other sites should increase the number of known gypsophile species in the state of Campeche. The sources used in this study can also help in the exploration of other regions that may harbour gypsophile plants in other environments in Mexico and the world, particularly tropical regions.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0266467423000287
Acknowledgements
We are grateful to Hilda Flores Olvera (IBUNAM), Helga Ochoterena (IBUNAM), and Carlos Gómez Hinostrosa (IBUNAM) for help with remote sensing methodology. We thank the curators of the herbaria CICY, GBIF, MEXU, and UADY for providing the databases that contributed enormously to this study. We are indebted to CONANP for the necessary permit (No. D-RBC 030/2020) for fieldwork in Calakmul. We thank Demetrio Álvarez, Juan Rosado, Marcos Quiroz, Sandra Díaz, and Alejandro Suárez for fieldwork support. We are grateful to Héctor Estrada Medina (UADY) for help with soil sampling design and José Luis Tapia (CICY) for help with final checklist of vascular plant species revision. We are grateful to the two anonymous reviewers and editors who helped us improve this manuscript.
Competing interests
None
Financial support
We thank CICY for the financial support to PMCN, with scholarship number 1041300004.









