Tourette syndrome (Tourette disorder, DSM–IV–TR) is characterised by the presence of multiple motor and one or more vocal/phonic tics present for more than a year. 1,2 Tics wax and wane over time and do not necessarily occur concurrently. The diagnosis of Tourette syndrome is relatively straightforward, but there is considerable clinical variability across individuals. The importance of evaluating the individual elements of a complex disorder such as Tourette syndrome cannot be overstated in terms of research into the underpinnings of the disorder and its clinical course. Although the familial nature of Tourette syndrome and chronic tics has been well-documented, Reference Pauls3 and twin studies implicate genetic and non-genetic factors in the expression of Tourette syndrome, Reference Price, Kidd, Cohen, Pauls and Leckman4,Reference Hyde, Aaronson, Randolph, Rickler and Weinberger5 specific, replicable findings for genetic loci have been somewhat elusive. To date, ten genetic linkage/association studies have been published. Reference Curtis, Robertson and Gurling6–15 Regions of interest have been reported for chromosomes 2, 3, 4, 5, 8, 9, 10, 11, 13, 17 and 19. All of these findings are potentially very important, but none has yet been replicated. Given strong evidence that Tourette syndrome is genetic and the lack of significant replicable linkage, combined with association findings showing that the underlying genetic mechanisms are complex and likely to be heterogeneous, the question arises as to whether there may be a way to disaggregate this heterogeneity to allow for clearer targets for clinical and genetic research.
Investigators have employed a variety of methods that address multiple quantitative phenotypic dimensions, making it possible to examine distinct components of complex phenotypes. Studies of reading disability, Reference Grigorenko, Wood, Meyer, Hart, Speed, Shuster and Pauls16 attention-deficit hyperactivity disorder (ADHD), Reference Todd, Rasmussen, Neuman, Reich, Hudziak, Bucholz, Madden and Heath17,Reference Neuman, Heath, Reich, Bucholz, Madden, Sun, Todd and Hudziak18 obsessive–compulsive disorder (OCD) Reference Alsobrook, Leckman, Goodman, Rasmussen and Pauls19 and autism Reference Bradford, Haines, Hutcheson, Gardiner, Braun, Sheffield, Cassavant, Huang, Wang, Vieland, Folstein, Santangelo and Piven20 have used various methods to discern possible underlying phenotypic constructs. There have been few attempts to formally classify people with Tourette syndrome on the basis of their tic phenomenology. Reference Jagger, Prusoff, Cohen, Kidd, Carbonari and John21,Reference Robertson, Trimble and Lees22 Robertson et al Reference Robertson, Trimble and Lees22 reported that coprolalia and echophenomena were related to obsessional symptoms and increased severity. To date, three cluster and/or factor analytic investigations of tic phenomenology have been undertaken. Reference Alsobrook and Pauls23–Reference Robertson and Cavanna25 Alsobrook & Pauls Reference Alsobrook and Pauls23 conducted a principal components factor analysis on a cohort of 85 individuals with Tourette syndrome and reported four factors: Factor 1 (APF1) was characterised by behaviours that included coprolalia, ‘aggressive’ and self-injurious behaviours; Factor 2 (APF2) was characterised by simple and complex motor tics and simple vocal/phonic tics (e.g. noises, but no actual words); Factor 3 (APF3) was characterised by ‘compulsive-like’ behaviours such as forced touching, repetitive behaviours, echo- and paliphenomena; and Factor 4 (APF4) was characterised by the absence of grunting tics and the presence of finger and hand tapping that was distinguished from finger and hand tics or forced touching. Robertson & Cavanna Reference Robertson and Cavanna25 reported the results of a principal components factor analysis completed on 69 individuals with Tourette syndrome and chronic tics, all of whom were members of a large extended Tourette syndrome pedigree. Reference Robertson and Gourdie26 It should be noted that these investigators included symptoms of other psychopathology (that are generally not considered to be tics, such as symptoms of inattention, hyperactivity, obsessionality, compulsivity, depression and anxiety) in their principal components factor analysis. Three factors were observed: Factor 1 (RCF1) consisted of predominantly ‘pure tics’ (both simple and complex motor and vocal/phonic tics); Factor 2 (RCF2) included ‘ADHD and aggressive behaviours’, and complex motor and vocal/phonic tics, including coprophenomena; and Factor 3 (RCF3) was characterised by ‘depression–anxiety–obsessional symptoms and self-injurious behaviour’ and compulsive-like tics, including counting and ‘evening-up’. Finally, Mathews et al Reference Mathews, Jang, Herrera, Lowe, Budman, Erenberg, Naarden, Bruun, Schork, Freimer and Reus24 reported results of hierarchical agglomerative cluster analysis (HACA) from two genetically isolated populations (Costa Ricans and Ashkenazi Jews) consisting of 254 individuals with Tourette syndrome. These investigators observed two clusters which were essentially the same in the two genetic isolates: one characterised by simple motor and vocal/phonic tics and the other by complex motor and vocal/phonic tics. Thus, the results of all of these studies suggest that Tourette syndrome is not a unitary disorder and can be disaggregated into more homogeneous components. However, these studies had relatively small samples for principal components analyses. Furthermore, one study included only related individuals Reference Robertson and Cavanna25 and another Reference Mathews, Jang, Herrera, Lowe, Budman, Erenberg, Naarden, Bruun, Schork, Freimer and Reus24 examined genetically isolated populations. All of these call into question the generalisablity of their results and speak to the need for a larger study of singleton individuals with the syndrome.
The primary aim of the current investigation was to identify quantitative components of Tourette syndrome symptomatology using a large, well-characterised sample of singleton individuals with the syndrome. As noted by Verkerk et al, Reference Verkerk, Cath, van der Linde, Both, Heutink, Breedveld, Aulchenko and Oostra27 it is crucial in future genetic studies of Tourette syndrome to search for more homogeneous phenotypes of the syndrome. These investigators suggest that the use of factor-analysed quantitative symptom scores might prove useful. If such components can be identified and can be examined in genetically informative data-sets, it could lead to a major advance in future studies of Tourette syndrome.
Methods
Participants
The sample for this study consisted of 410 people with Tourette syndrome (75.9% male) who were seen at the National Hospital for Neurology and Neurosurgery (NHNN), Queen Square, London, UK. This research was approved by the ethics committee at the NHNN for assessing both children and adults. Participants ranged in age from 3 to 59 years with a mean of 20.4 (s.d.=12.3). All individuals over the age of 18 years gave informed consent. For those individuals under the age of 18, parents gave informed consent for the children to be part of the study. All individuals were assessed and/or diagnosed with Tourette syndrome by the first author (M.M.R.) using DSM–III 28 or DSM–IV–TR 2 criteria.
Measures
The National Hospital Interview Schedule (NHIS) Reference Robertson and Eapen29 was used to characterise the motor and vocal/phonic tics. The NHIS is a semi-structured diagnostic interview shown to be reliable and valid that allows for the collection of detailed information on over 100 specific motor and vocal/phonic tics in 32 different categories. For example, specific content is collected for coprolalic utterances as well as other vocal/phonic tics. In addition, Yale Global Tic Severity Scale (YGTSS) Reference Leckman, Riddle, Hardin, Ort, Swartz, Stevenson and Cohen30 scores were obtained from 402 individuals. Percentage scores on the YGTSS ranged from 3 to 100% with a mean of 44.6% (s.d.=22.2), covering the entire range of severity, but with the mean in the moderate range. Additional information was obtained through diagnostic interview concerning age at onset of motor and vocal/phonic tic symptoms and co-occurring DSM–IV diagnoses including ADHD, substance misuse and OCD. Moreover, data were coded for the presence or absence of obsessive–compulsive behaviours without full-criterion OCD, self-injurious behaviour and aggression, along with the presence or absence of a family history of Tourette syndrome or chronic tics, ADHD and/or OCD.
Statistical analysis
All analyses were performed using SPSS 15.0 for Windows. Cluster analyses were initially performed to determine whether any of the dichotomous symptoms could be combined into pseudo-continuous clusters. Here, as in previous studies of people with Tourette syndrome, Reference Alsobrook and Pauls23–Reference Robertson and Cavanna25 tic symptoms were combined into pseudo-continuous measures by performing an initial data reduction of the entire array of dichotomous symptom variables into clusters. This reduction was achieved by an HACA Reference Gilles de la Tourette31 analysis on 32 tic symptom categories obtained from the NHIS. The HACA method progressively combines variables into related clusters until all variables are subsumed into a single cluster, using the average linkage between-groups method to evaluate the Euclidean-squared cluster distances. The HACA does not rely on preconceived characterisations concerning the number of clusters (such as specifying the number of clusters) or the relationships between them (such as specifying the distances between clusters). The stages of agglomeration are displayed as a dendrogram, with the formation of clusters at each stage plotted along a scaled stage distance axis. For the current study, the best set of clusters required to adequately represent the data was determined by inspection of the dendrogram. There is no test of significance for clustering results other than their use in subsequent analyses. For each resulting cluster, a score was then generated for each participant equal to the sum of the symptom variables contained therein: symptom variables were scored 0 for never present and 1 for ever present.
The resultant cluster scores were then used as input variables for the principal components factor analysis. Principal components factor analyses are typically based upon non-dichotomous variables; however, the variables that did not fall into a cluster on the dendrogram were entered as dichotomous variables. The use of dichotomous variables can be justified in exploratory approaches such as reported here. Using Kaiser–Guttman's rule, after factor extraction promax rotation was performed on factors with eigenvalues greater than 1.0 (factors with eigenvalues less than 1.0 are generally spurious and non-reproducible) and compared. This procedure minimised the number of variables with high loadings on each extracted factor and allowed for a more straightforward interpretation; other rotations were not explored. Symptom loadings, with coefficient absolute values greater than 0.375, were used to describe the factors.
Relations among extracted factors, age at onset of tics, gender, and co-occurring symptoms or disorders were then examined by comparing the mean factor score on each factor with each variable. Pearson correlations were computed for age at onset of the vocal/phonic and motor tics compared with factor scores for each factor. Because this resulted in 15 significance tests, a P-value of <0.003 was considered to be significant. Relations between factors and co-occurring disorders were made by comparing mean factor scores of individuals with and without the co-occurring diagnoses. Because this examination resulted in 25 significance tests, a P-value of <0.002 was considered to be significant.
Results
Heirarchical agglomerative cluster analysis on the 32 tic symptoms identified 7 clusters of symptoms; 13 symptoms remained as lone variables but were treated as clusters in subsequent analyses. The dendrogram illustrating the progression of cluster formation and the final resulting clusters is shown in online Fig. DS1. The clusters were characterised by the following behaviours: tics of the head and face, including throat clearing and shoulder shrugging; touching self; leg and foot movements; grunting; hopping, skipping, and jumping; forced touching; coughing; arm movements; adjusting clothing and compulsive looking; coprolalia and copropraxia, hitting, palipraxia, kicking and mental coprolalia; spitting; palilalia; echolalia and echopraxia; self-injurious behaviour; random words; tensing body; tensing abdomen; touching chin to chest and shoulders; torso and hip movements; and finger tapping.
For each of these clusters, a score was generated equal to the sum of the elemental symptom variables. These scores were then included in the factor analysis. After factor extraction and promax rotation of the cluster variables, five factors resulted which accounted for 46.6% of the symptomatic variance in the sample (see online Table DS1). Factor 1, which accounted for 22.9% of the variance, includes coprolalia, copropraxia, echolalia, echopraxia, palilalia, palipraxia, hitting, spitting, kicking, random words, forced touching and self-injurious behaviour. Factor 2, which accounted for 7.4% of the variance, is characterised by complex motor tics (e.g. arm, leg, foot movements, hopping, skipping, jumping and torso movements). Factor 3, which accounted for 5.8% of the variance, includes coughing, tensing of the body, grunting and simple motor and vocal/phonic tics (e.g. eye blinking, facial tics, head tics, noises, and throat clearing). Factor 4, which accounted for 5.4% of the variance is characterised by compulsive-like behaviours such as repetitive looking, adjusting clothing, finger tapping, leg and foot movement, and tensing of the abdomen. Finally, Factor 5, which accounted for 5.1% of the variance, also included simple motor and vocal/phonic tics as well as touching one's self.
In an attempt to provide evidence for the external validity of these factors, additional analyses were undertaken in which the relationship between these five factors and age at onset, gender, the presence of co-occurring conditions (e.g. ADHD, OCD, substance misuse, self-injurious behaviour and aggression) and the presence of a positive family history for Tourette syndrome, OCD/obsessive–compulsive behaviour and ADHD was examined. The results are presented in Tables 1, 2, 3, 4, 5. All comparisons were corrected for multiple testing. There were significant negative correlations between Factor 1 and Factor 3 scores and age at onset (Factor 1: r=–0.177, P<0.0001; Factor 3: r=–0.168, P<0.001) (Table 1). When individuals were dichotomised according to whether their factor score was ≤0 or >0, significant differences for age at onset were observed for Factors 1 and 3 (Table 2). The mean age at onset of Tourette syndrome for individuals with a score >0 for Factor 1 was 6.29 compared with the mean of 7.4 for individuals with a score of ≤0 (P<0.005). For Factor 3, the mean age at onset for individuals with a factor score >0 was 6.43 compared with 7.39 for individuals with a score ≤0 (P<0.015).
Table 1 Correlation with between factor scores and age at onset
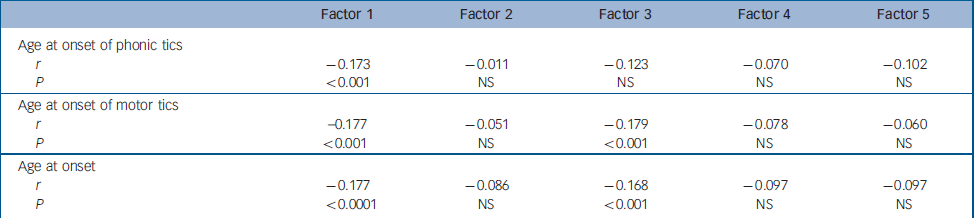
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | |
|---|---|---|---|---|---|
| Age at onset of phonic tics | |||||
| r | -0.173 | -0.011 | -0.123 | -0.070 | -0.102 |
| P | <0.001 | NS | NS | NS | NS |
| Age at onset of motor tics | |||||
| r | -0.177 | -0.051 | -0.179 | -0.078 | -0.060 |
| P | <0.001 | NS | <0.001 | NS | NS |
| Age at onset | |||||
| r | -0.177 | -0.086 | -0.168 | -0.097 | -0.097 |
| P | <0.0001 | NS | <0.001 | NS | NS |
Table 2 Mean age at onset for factor scores
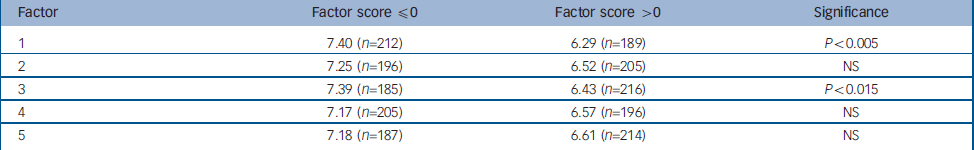
| Factor | Factor score ≤0 | Factor score >0 | Significance |
|---|---|---|---|
| 1 | 7.40 (n=212) | 6.29 (n=189) | P<0.005 |
| 2 | 7.25 (n=196) | 6.52 (n=205) | NS |
| 3 | 7.39 (n=185) | 6.43 (n=216) | P<0.015 |
| 4 | 7.17 (n=205) | 6.57 (n=196) | NS |
| 5 | 7.18 (n=187) | 6.61 (n=214) | NS |
Table 3 Mean factor scores of individuals with and without co-occurring diagnoses
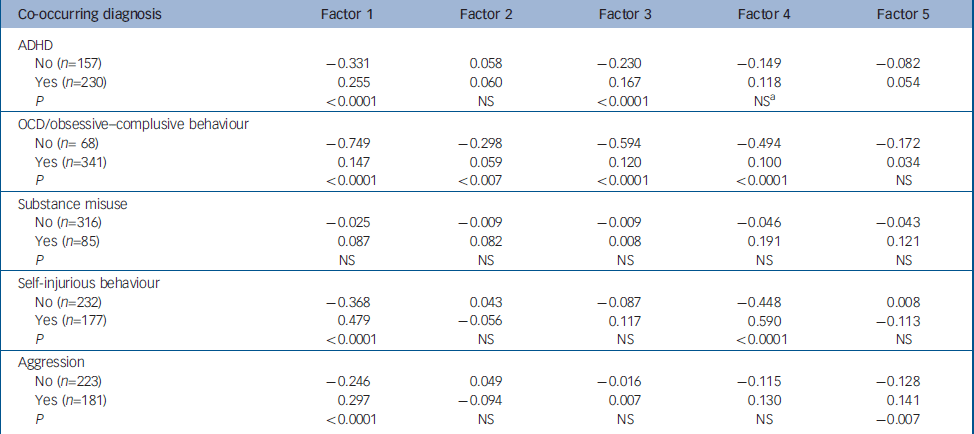
| Co-occurring diagnosis | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 |
|---|---|---|---|---|---|
| ADHD | |||||
| No (n=157) | -0.331 | 0.058 | -0.230 | -0.149 | -0.082 |
| Yes (n=230) | 0.255 | 0.060 | 0.167 | 0.118 | 0.054 |
| P | <0.0001 | NS | <0.0001 | NSa | |
| OCD/obsessive—complusive behaviour | |||||
| No (n= 68) | -0.749 | -0.298 | -0.594 | -0.494 | -0.172 |
| Yes (n=341) | 0.147 | 0.059 | 0.120 | 0.100 | 0.034 |
| P | <0.0001 | <0.007 | <0.0001 | <0.0001 | NS |
| Substance misuse | |||||
| No (n=316) | -0.025 | -0.009 | -0.009 | -0.046 | -0.043 |
| Yes (n=85) | 0.087 | 0.082 | 0.008 | 0.191 | 0.121 |
| P | NS | NS | NS | NS | NS |
| Self-injurious behaviour | |||||
| No (n=232) | -0.368 | 0.043 | -0.087 | -0.448 | 0.008 |
| Yes (n=177) | 0.479 | -0.056 | 0.117 | 0.590 | -0.113 |
| P | <0.0001 | NS | NS | <0.0001 | NS |
| Aggression | |||||
| No (n=223) | -0.246 | 0.049 | -0.016 | -0.115 | -0.128 |
| Yes (n=181) | 0.297 | -0.094 | 0.007 | 0.130 | 0.141 |
| P | <0.0001 | NS | NS | NS | -0.007 |
Table 4 Mean factor scores of individuals with and without family history of Tourette syndrome, OCD and ADHD
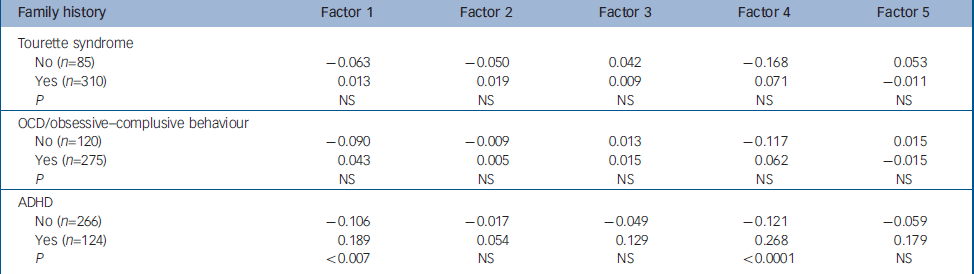
| Family history | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 |
|---|---|---|---|---|---|
| Tourette syndrome | |||||
| No (n=85) | -0.063 | -0.050 | 0.042 | -0.168 | 0.053 |
| Yes (n=310) | 0.013 | 0.019 | 0.009 | 0.071 | -0.011 |
| P | NS | NS | NS | NS | NS |
| OCD/obsessive—complusive behaviour | |||||
| No (n=120) | -0.090 | -0.009 | 0.013 | -0.117 | 0.015 |
| Yes (n=275) | 0.043 | 0.005 | 0.015 | 0.062 | -0.015 |
| P | NS | NS | NS | NS | NS |
| ADHD | |||||
| No (n=266) | -0.106 | -0.017 | -0.049 | -0.121 | -0.059 |
| Yes (n=124) | 0.189 | 0.054 | 0.129 | 0.268 | 0.179 |
| P | <0.007 | NS | NS | <0.0001 | NS |
Table 5 Mean factor scores for males and females
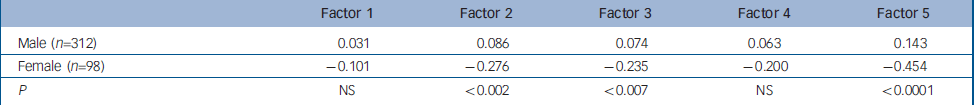
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | |
|---|---|---|---|---|---|
| Male (n=312) | 0.031 | 0.086 | 0.074 | 0.063 | 0.143 |
| Female (n=98) | -0.101 | -0.276 | -0.235 | -0.200 | -0.454 |
| P | NS | <0.002 | <0.007 | NS | <0.0001 |
The next set of analyses compared the mean factors scores for individuals with co-occurring ADHD, OCD or obsessive–compulsive behaviour, substance misuse, self-injurious behaviour and aggression (Table 3). For individuals with co-occurring ADHD, there were significant differences in mean factor scores observed for Factor 1 (P<0.0001) and Factor 3 (P<0.0001), and there was a trend for Factor 4 (P<0.010) (note that a P<0.003 was required for statistical significance after correction for multiple comparisons). For individuals with OCD/obsessive–compulsive behaviour, there were significant differences in mean factor scores for Factors 1 (P<0.0001), 2 (P<0.007), 3 (P< 0.0001) and 4 (P<0.0001). For individuals with co-occurring substance misuse, no significant differences were observed. Individuals with self-injurious behaviour had significantly different mean factor scores for Factors 1 (P<0.0001) and 4 (P<0.0001). This was not surprising since both factors included self-injurious behaviour as a tic. Finally, individuals with aggression had mean factor score differences for Factors 1 (P<0.0001) and 5 (P<0.007).
With respect to family history of Tourette syndrome, OCD/obsessive–compulsive behaviour and ADHD, there were few significant differences in mean factor scores (Table 4). The only significant differences observed were for individuals with ADHD. The mean factor scores for Factor 1 were significantly higher (P<0.007) for individuals with both Tourette syndrome and a family history of ADHD, as were mean factor scores for Factor 4 (P<0.0001). It should be noted that the majority of individuals in this study had positive family histories for both Tourette syndrome (79%) and OCD/obsessive–compulsive behaviour (71%), thus the fact that neither showed any relation to any factors scores could reflect the fact that there was little variability in family history for both Tourette syndrome and OCD/obsessive–compulsive behaviour in this sample.
Finally, when males and females were compared (Table 5), females had lower scores for all five factors and the differences for Factors 2 (P<0.002), 3 (P<0.007) and 5 (P<0.0001) were statistically significant.
Discussion
A unitary disorder?
The current findings are consistent with the growing body of evidence that Tourette syndrome is not a unitary condition as suggested by all international diagnostic criteria. Two investigations using principal components factor analysis Reference Alsobrook and Pauls23,Reference Robertson and Cavanna25 and one study using HACA Reference Mathews, Jang, Herrera, Lowe, Budman, Erenberg, Naarden, Bruun, Schork, Freimer and Reus24 have shown that the categorical classification of Tourette syndrome using either ICD–10 1 or DSM–IV–TR 2 criteria is likely to consist of several different components comprised of different types of tics and associated behaviours. This finding is supported by the current results. There are notable differences between the previous three studies and the current findings, and also significant similarities.
First, in the two previous principal components factor analysis studies pure tic factors (APF2 and RCF1) were observed that were distinct from the more complex compulsive-like tics that often occur in Tourette syndrome. The pure tic factors included both simple and complex tics. In contrast, in the current report when a promax rotation was implemented, two pure tic factors were observed; one included predominantly simple tics (Factor 3), both motor and vocal/phonic, whereas the other included predominantly complex motor tics (Factor 2). These results suggest that complex motor tics are separate from both simple motor and vocal/phonic tics as well as from complex vocal/phonic tics, a finding that is quite similar to the results reported by Mathews et al; Reference Mathews, Jang, Herrera, Lowe, Budman, Erenberg, Naarden, Bruun, Schork, Freimer and Reus24 in that study, two clusters were identified, one characterised by simple tics, the other by complex tics.
It is also noteworthy that in all three principal components factor analysis studies (the two previous Reference Alsobrook and Pauls23,Reference Robertson and Cavanna25 and the current report) there were two additional factors observed: one characterised by what could be broadly termed ‘aggressive behaviours’, including coprophenomena, spitting, hitting and kicking, and the other characterised by more compulsive-like behaviours (APF1 and RCF2). The current results demonstrate a similar aggressive behaviour factor (Factor 1) and a factor with compulsive-like behaviour (Factor 4). Therefore, even with differences in samples and methodology, there are areas of convergence across these studies. All studies suggest that tics occurring in individuals with Tourette syndrome can be separated into two broad categories: one comprised of simple tics and the other complex tics. Furthermore, the principal components factor analysis studies suggest that these can be further broken down into a ‘pure simple tic’ factor, a ‘pure complex motor tic’ factor, a factor characterised by coprophenomena and aggressive behaviours, and another factor characterised by predominantly compulsive behaviours.
Differences from previous studies
There are a number of reasons why the results of the current study do not agree completely with previous studies. These include the fact that: (a) our study (n=410) is considerably larger than any of the previous three studies; (b) the instruments used were different across the various studies. In the current study, the variables collected by the NHIS were grouped to be consistent with the instrument used in the Alsobrook & Pauls study, Reference Alsobrook and Pauls23 although a few additional variables were included. Robertson & Cavanna Reference Robertson and Cavanna25 also used the NHIS, but augmented the data obtained with another structured interview (Schedule for Affective Disorders and Schizophrenia – Lifetime version) and many self-report scales for obsessionality, mood, and other psychopathology; and (c) the samples were drawn from different populations: one from a clinic in the USA, Reference Alsobrook and Pauls23 another from a single large family ascertained through individuals with Tourette syndrome in the UK, Reference Robertson and Cavanna25,Reference Robertson and Gourdie26 another from two genetic isolates Reference Mathews, Jang, Herrera, Lowe, Budman, Erenberg, Naarden, Bruun, Schork, Freimer and Reus24 and the current one from a dedicated Tourette syndrome tertiary clinic in the UK. Furthermore, Robertson & Cavanna Reference Robertson and Cavanna25 included data about psychopathology in the principal components factor analysis, whereas the three other studies did not. Given these differences it is remarkable that so many of the results are as similar as they are.
Because Factor 1 accounted for the majority of the variance in the sample, it is worth examining more closely. Factor 1 of the current study includes coprophenomena (coprolalia, copropraxia, mental coprolalia), echophenomena (echolalia, echopraxia), paliphenomena (palilalia, palipraxia), random words, as well as forced touching (of other people and/or external objects), hitting, kicking and spitting. This could be argued to be a ‘socially inappropriate factor’, similar to the Alsobrook & Pauls Reference Alsobrook and Pauls23 Factor 1, which included coprolalia, aggressive and self-injurious behaviour, all of which are socially inappropriate. It is important to note that copropraxia, mental coproprolalia and palipraxia were not assessed in the Alsobrook & Pauls Reference Alsobrook and Pauls23 study. There may be some similarity to the Robertson & Cavanna Reference Robertson and Cavanna25 Factor 2, which was predominantly ADHD and aggressive behaviours. Attention-deficit hyperactivity disorder was not included in the factor analysis of the present study, but, interestingly, factor scores on Factor 1 were significantly higher in the subset of the sample with ADHD as well as individuals with a family history of ADHD. The major difference between the current study and the two previous principal components factor analysis studies Reference Alsobrook and Pauls23,Reference Robertson and Cavanna25 is that in the present study, echophenomena (echolalia, echopraxia), paliphenomena and random words also formed part of Factor 1, but were not part of this factor in either the Alsobrook & Pauls Reference Alsobrook and Pauls23 or Robertson & Cavanna Reference Robertson and Cavanna25 studies. Of note and particular historical importance is that Georges Gilles de la Tourette Reference Gilles de la Tourette31 described a syndrome emphasising a triad of multiple motor tics, coprolalia and echolalia, which is very similar to our Factor 1.
Self-injury and Tourette syndrome
Georges Gilles de la Tourette also considered self-injurious behaviour as an important component, as has recent research. Reference Robertson, Trimble and Lees32,Reference Mathews, Waller, Glidden, Lowe, Herrera, Budman, Erenberg, Naarden, Bruun, Freimer and Reus33 In the current cluster analyses and those of Mathews et al Reference Mathews, Jang, Herrera, Lowe, Budman, Erenberg, Naarden, Bruun, Schork, Freimer and Reus24 and Alsobrook & Pauls, Reference Alsobrook and Pauls23 self-injurious behaviour resides in the same broad cluster as other complex symptoms such as copro- pali- and echophenomena. It is also an important symptom encountered in individuals with mild Tourette syndrome such as those previously undiagnosed and not under medical care for their symptoms, Reference Robertson and Gourdie26 and has been shown to be intimately and significantly related to both obsessionality Reference Robertson, Trimble and Lees32 and impulsivity. Reference Mathews, Waller, Glidden, Lowe, Herrera, Budman, Erenberg, Naarden, Bruun, Freimer and Reus33 It is therefore possible, indeed probable, that there are different types of self-injurious behaviour, some more aggressive and others more compulsive, which could account for the differential loading in the different studies as well as the fact that self-injurious behaviour is in both Factor 1 and Factor 4 in the current results. Furthermore, it is also possible that the manner in which self-injurious behaviour is perceived or stressed in the interviews may have been different or there may be cultural differences across the samples in their interpretation or expression of this behaviour. Nevertheless, it is consistent with a certain ‘face validity’ of self-injurious behaviour in Tourette syndrome that it be variably grouped as either aggressive or compulsive, as it appears to be both.
That the socially inappropriate behaviours and compulsive-like tics were observed to be associated with different co-occurring psychopathology raises the hope that there may be consistent, dissociable and potentially genetically informative phenotypes within Tourette syndrome which may enhance genotypic examinations. Moreover, as both genetic and environmental factors have been demonstrated in aggressive Reference van Beijsterveldt, Bartels, Hudziak and Boomsma34 and compulsive-like Reference Hudziak, Van Beijsterveldt, Althoff, Stanger, Rettew, Nelson, Todd, Bartels and Boomsma35 phenomena, these data also raise the possibility of enhancing the yield of exploration into gene v. environment interactions for Tourette syndrome as these environmental factors, important for aggressive and compulsive phenomena, become better characterised.
Summary of conclusions and limitations
The results of the current research and those of the previously published studies, although not identical, suggest that the phenotype of Tourette syndrome is complex. Thus, one distinct component encountered in Tourette syndrome includes tics alone which may be either simple or complex. A second component appears to consist of behaviours that may best be classified as ‘socially inappropriate’ (which in some cases could be argued to be aggressive), including coprolalia, mental coprolalia, copropraxia, spitting, hitting and kicking and, in the current study, the more complex vocal/phonic tics such as echolalia and palilalia. Put together, these two components are what were originally described by Georges Gilles de la Tourette in 1885. Reference Gilles de la Tourette31 A statistically separate component appears to consist of behaviours that are best categorised as compulsive-like, including forced touching and repetitive looking at objects (e.g. checking their whereabouts) and other ritualised behaviours.
It is also clear from all studies that there is additional phenotypic variance that is not accounted for by the factors described in the analyses. Nevertheless, the similarity between all studies suggests that there are certain clusters of tics that may occur together more often than expected by chance. Furthermore, some of these factors may be uniquely heritable. Reference Alsobrook and Pauls23
Limitations of our study include the fact that it was not identical to any previous study with regard to data or methods used. For example, the NHIS did not collect identical information or measures used in the Alsobrook & Pauls Reference Alsobrook and Pauls23 study (with similar methods), so a direct comparison between the studies was not possible. The data collected were, however, similar to Robertson & Cavanna, Reference Robertson and Cavanna25 but the principal components factor analysis methods were different and their study was performed on a larger extended pedigree, whereas our study was on people in the clinic setting.
Acknowledgements
We thank Rachel Carter and Elizabeth Cowan for entering data. We also acknowledge the helpful critiques of two anonymous reviewers. Their suggestions significantly improved the manuscript. The work was funded by NIH Grants NS16648 and NS40024 (D.L.P.) and The National Hospital for Neurology and Neurosurgery Development Foundation (M.M.R.).








eLetters
No eLetters have been published for this article.