INTRODUCTION
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is a technique for managing non-compressible hemorrhagic shock. A recent report combined the experience of 11 hospitals performing REBOA; however, none of the sites were located in Canada.Reference Brenner, Inaba and Aiolfi1 At our institution, while we have used REBOA within the operating room (OR), herein, we describe the first deployment of this technology in a Canadian emergency department (ED).Reference Rice, Ahmed and Rezende-Neto2
CASE REPORT
A previously healthy, 52-year-old male in hemorrhagic shock, from a high-speed, side-impact motor vehicle collision at 06:50, underwent initial resuscitation at a non-trauma centre, including intubation and a massive blood-based resuscitation. Upon arrival, the patient was profoundly hypotensive (blood pressure [BP] 55/30) and Glasgow Coma Scale (GCS) of 8 (eyes 1, verbal 2, and motor 4). Basic investigations included a chest X-ray and pelvis X-ray, demonstrating bilateral pneumothoraces and an unstable open-book pelvis fracture (anteroposterior compression II, Young Burgess Classification). Management prior to transfer included insertion of bilateral chest tubes, application of a pelvic binder, and administration of 1 gram of tranexamic acid (TXA). He received 4 litres of 0.9% normal saline, 10 units of packed red blood cells (pRBCs), and 1 unit of fresh frozen plasma (FFP). Despite this massive resuscitation, an epinephrine infusion (1 mcg/kg/min) was initiated prior to transfer to our trauma centre because of persistent hypotension (BP 60/40).
The patient was received at our trauma centre at 09:50, under the care of a Royal College emergency medicine (FRCP-EM)-trained trauma team leader (TTL) in charge of a multi-disciplinary trauma team including anesthesia and orthopedic residents, a trauma surgery resident and fellow, a staff trauma surgeon, three trauma nurses, and two respiratory therapists. On repeat assessment, the airway remained secure, and an X-ray demonstrated bilateral lung re-expansion. A FAST exam was positive in the abdominal views, and the pelvis, previously bound, was not re-examined. During transfer, the patient had required the addition of a norepinephrine infusion; both norepinephrine and epinephrine infusions were dosed at 1 mcg/kg/min, with the systolic blood pressure (sBP) remaining between 45 and 60 mm Hg, heart rate of 102 bpm, and temperature of 33.2°C (91.8°F). Initial investigations included a pH of <6.8 (normal 7.35–7.42), lactate of >15.0 mmol/L (normal 0.5–2.3), fibrinogen 0.5 g/L (normal 1.8–4.0), hemoglobin of 143 g/L (normal 130–170), and platelets of 106 × 109/L (normal 140–400).
Our massive transfusion protocol was activated, and the patient received six additional units of pRBCs, three units of FFP, one additional gram of TXA, and one pool of platelets upon arrival at our centre. His sBP did not increase above 60 mm Hg; however, he continued to demonstrate neurologic function with reactive pupils and a GCS of 7T (E2VTM5), localizing the endotracheal tube with both hands.
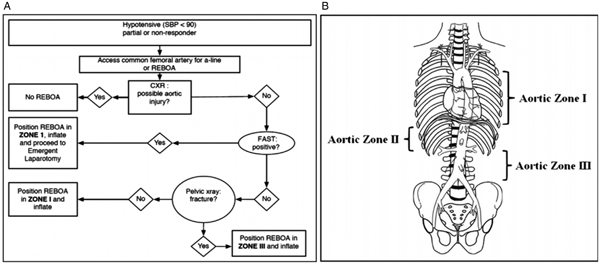
The TTL and trauma surgeon considered options for this patient, including an urgent transfer to the OR, resuscitative thoracotomy, and/or REBOA. While this patient, who was in profound hemorrhagic shock with a positive FAST exam, had indications for immediate surgery, transfer to the OR at our institution requires approximately 10 minutes because of the distance and travel by elevator. Given the persistent shock state (sBP < 60 mm Hg) and concern for cardiac arrest despite substantial resuscitative efforts, the transfer was deemed unsafe. Approximately one hour after the patient arrived at our institution (10:55), as an alternative to resuscitative thoracotomy with aortic cross-clamping, the surgeon-TTL team collaboratively elected to place a Zone 1 REBOA device as a temporizing measure to facilitate transfer to the OR (Figure 1).

Figure 1. REBOA placement algorithm (A). Aortic zones of occlusion (B), adapted from Stannard, Eliason, and Rasmussen.Reference Stannard, Eliason and Rasmussen9 Zone 1 between the left subclavian artery and celiac artery is the recommended location for suspected intra-abdominal hemorrhage; Zone 2 between the celiac arteries and renal arteries is not a recommended placement location; and Zone 3, from the lowest renal artery to the aortic bifurcation, is the recommended location for suspected pelvic hemorrhage. Following the decision to proceed with REBOA placement, the clinician must determine where to position the occlusive balloon (Figure 1A). In this case, the patient had a positive FAST, and following the algorithm in Figure 1A, we placed the device in Zone 1.
While the TTL continued to guide resuscitation, the surgery team obtained central venous access in the right subclavian vein for blood product administration. The REBOA procedure began at 10:58 with ultrasound-guided puncture of the left common femoral artery, followed by the advancement of a 0.035 inch, 260 cm guidewire using a modified Seldinger technique. Serial dilation allowed insertion of a 14 Fr vascular access sheath. A Coda 46 mm balloon catheter (Cook Incorporated, Bloomington, IN, USA) was inserted into the distal thoracic aorta, followed by guidewire removal. The balloon was inflated with sterile saline until the sBP suddenly increased from 60 mm Hg to 105 mm Hg; a total of 7 cc of saline was used for inflation. Shortly after that, the epinephrine infusion was stopped, and the norepinephrine dose was decreased to 0.5 mcg/kg/min. Seven minutes elapsed from decision to REBOA and successful aorta occlusion.
The patient was transferred urgently to the OR and underwent a splenectomy, a small bowel resection, and pre-peritoneal pelvic packing. The largest volume of blood and most likely source of persistent hemodynamic instability was felt to be the pelvic injuries. After surgical hemostasis, but while still in the OR, the Coda balloon was deflated by the surgical team, with no change in mean arterial pressure (60–65 mm Hg). There was resistance upon attempting to remove the device that may have occurred because the balloon did not fully retract into the introducer sheath. To ensure complete deflation of the balloon and facilitate removal of the device, the catheter was transected, severing the lumen communicating with the balloon, and was then successfully removed without resistance.
A temporary abdominal closure was performed because of persistent coagulopathy (international normalised ratio [INR] of 2.25–3.7), followed by immediate pelvic angiography. There was no evidence of injury or REBOA complication in any Zone 1 or 2 vessels. Bleeding from bilateral internal iliac artery injuries required proximal embolization. The left external iliac artery demonstrated absent flow with clinical signs of ischemia including a pulseless and pale left foot two hours after removal of the REBOA catheter. Seven hours after arrival at our institution (and 12 hours post-injury), he was returned to the OR for vascular sheath removal, arteriorrhaphy, left femoral thrombectomy, and four-compartment fasciotomies of the left leg. The patient demonstrated increasing hemodynamic instability and worsening multi-organ dysfunction, prompting a repeat laparotomy. Several unnamed mesenteric vessels at the site of a prior bowel resection were ligated; however, the patient continued to bleed because of profound coagulopathy despite laboratory-guided blood product administration. Unfortunately, the patient died of his injuries 18 hours after his arrival at our institution. This report was approved by St. Michael's Hospital Research Ethics Board (18-081).
DISCUSSION
Numerous trauma centres worldwide now include ED-REBOA within their algorithm for the management of hemorrhagic shock; however, to our knowledge, this is the first report of a REBOA performed in a Canadian ED.Reference Moore, Martin, Harvin, Wade and Holcomb3, Reference Sato, Kuriyama and Takaesu4 As part of our local quality improvement process, we reviewed this case and identified a number of important lessons related to the clinical logistics and implementation of ED-REBOA that may be valuable for other institutions with little experience applying this technology (Table 1).
Table 1. Practical considerations for REBOA implementation

REBOA = resuscitative endovascular balloon occlusion of the aorta.
REBOA indications and complications
The established indications for REBOA insertion remain institution specific and may be influenced by local factors of timeliness of access to definitive care. Our institutional policy is slightly more restrictive than published criteria (Table 2), as we perform REBOA only for blunt trauma patients with either pelvic ring fractures or a positive FAST with an sBP of <90 mm Hg that is refractory to damage control resuscitation.Reference Sato, Kuriyama and Takaesu4 REBOA contraindications are informed by a combination of expert opinion and clinical experience (Table 2).Reference Moore, Martin, Harvin, Wade and Holcomb3
Table 2. REBOA indications and contraindications summarized from published literature

Vascular complications remain a significant concern in REBOA application. The requisite large-bore arterial puncture and occlusion, coupled with a frequent need for vascular cut-down, if the Seldinger-technique access fails, may result in substantial complications including aortoiliac dissection, rupture, thrombosis, pseudoaneurysm, and distal thromboembolism.Reference Brenner, Inaba and Aiolfi1 Some authors report as high as a 40% amputation rate among survivors of REBOA using large-calibre sheaths.Reference Saito, Matsumoto and Yagi5 In our patient, unilateral lower extremity ischemia would have represented a significant source of morbidity had the patient survived. A newly developed REBOA balloon device (Prytime Medical, Lakewood, CO, USA) that is introduced through a 7 Fr sheath, instead of a 12–14 Fr sheath, may decrease these risks, though this remains an outstanding concern of the technique. While a smaller diameter device would likely decrease some complications, we are reminded that REBOA is an invasive technique, with significant risks and potential morbidity.
Equipment and logistical considerations
The REBOA kit, which has been stocked in our trauma bay for three years, consists of three mini bundles within a single package assembled at our institution (Supplementary Table 1). This intuitive all-in-one packaging system facilitated rapid deployment by our team, who had never placed the device previously. Importantly, we achieved aortic occlusion within seven minutes that was only marginally slower than the reported time-to-occlusion in a large case series (3.5–6 minutes) at centres where REBOA is an established practice.Reference Brenner, Inaba and Aiolfi1, Reference Brenner, Moore and DuBose6 Use of a well-designed equipment bundle may reduce unnecessary delays related to missing or poorly organized equipment.
Despite the clear visibility of our REBOA kit within our trauma bay, many team members are unfamiliar with REBOA indications and management principles. We identified a clear need for team-based education related to equipment application, physiologic monitoring, and post-inflation transfer. As Canadian trauma surgeons typically do not manage peripheral vascular injuries, an institutional protocol including vascular surgery stakeholders may simplify post-insertion management plans, and this is the guideline recommended.Reference Brenner, Inaba and Aiolfi1 Infrequent use of REBOA requires multi-disciplinary team training to optimize outcomes.Reference Davidson, Russo and Reva7 We estimate, based on a review of our trauma registry, that one to two patients a month would receive REBOA. This case represented the first REBOA application by the trauma surgeon; however, he had extensive experience with the device in animal model research studies.
CONCLUSIONS
We report the first case of a REBOA placed in a Canadian ED. The application of this endovascular technology produced a dramatic hemodynamic response that facilitated transport from our trauma bay to definitive hemorrhage control, though ultimately the patient did succumb to his injuries and traumatic coagulopathy. The decision to proceed with REBOA warrants careful consideration including local expertise and access to definitive therapies, given the potential for serious complications. Future efforts should focus on identifying patients who would and would not benefit from this invasive procedure.
Acknowledgements
The authors would like to thank Melissa McGowan and Amanda McFarlane for their logistical expertise and the members of the trauma team involved in this case.
Financial support
None.
Competing interests
None.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cem.2018.476.