Colorectal cancer (CRC) accounts for a substantial burden of disease and mortality worldwide as the third leading cause of cancer in women and men in the USA and globally(Reference Ferlay, Soerjomataram and Dikshit1). CRC represents a heterogeneous collection of cancers resulting from several genetic and epigenetic changes(Reference Binefa, Rodríguez-Moranta and Teule2). There are at least two different premalignant polyps, adenomatous polyps (AP) and sessile serrated polyps (SSP), with different aetiologies and pathways leading to CRC and, possibly, different risk factors(Reference Fearon and Vogelstein3–Reference Chung, Thiele Orberg and Geis14).
A majority of CRC cases are attributed to modifiable lifestyle factors including diet, obesity, physical activity, alcohol intake and tobacco use(Reference Davenport, Su and Zhao6,Reference Wei, Colditz and Giovannucci13,Reference Platz, Willett and Colditz15–Reference Park, Wilkens and Haiman20) . Dietary behaviour modification represents a potential strategy to prevent CRC. Mounting evidence suggests red and processed meat and saturated fats increase the risk, whereas fibre, fruits and vegetables may protect against CRC(Reference Platz, Willett and Colditz15,Reference Dahm, Keogh and Spencer21,Reference O’Keefe, Li and Lahti22) . Fermentable dairy foods and yogurt specifically may also offer protection against colon cancer, although accumulating evidence is limited and inconclusive.
Yogurt consumption in European countries accounts for up to 32 % of dairy intake(Reference El-Abbadi, Dao and Meydani23). In the USA, the prevalence of yogurt consumption has been increasing particularly as a means for obtaining health benefits(Reference El-Abbadi, Dao and Meydani23,Reference Fisberg and Machado24) . While there is significant variation in commercially available products, yogurt is a source of protein, dietary minerals including Ca, Mg and B vitamins(Reference El-Abbadi, Dao and Meydani23). A growing literature suggests that yogurt consumption and probiotic use may have multiple health benefits including osteoporosis, obesity and metabolic disease, CVD, chronic kidney, mental health disease aside from possible gastrointestinal benefits(Reference El-Abbadi, Dao and Meydani23,Reference Mohammadi, Jazayeri and Khosravi-Darani25–Reference Fernandez, Panahi and Daniel30) .
At the turn of the 20th century, Metchnikoff first proposed that lactic acid-producing bacteria present in yogurt, including Lactobacillus bulgaricus, Streptococcus thermophiles, Lactobacillus acidophilus and Bifidobacterium, might protect against colon cancer by inactivating toxins produced by pathologic bacteria(Reference Liu, Zhuang and Wang18,Reference Meydani and Ha31,Reference Norat and Riboli32) . With better understanding of the interaction between the gut microbiome and colon health, preliminary evidence supports an anti-tumour effect of lactic acid-producing bacteria contained in yogurt and probiotics whereby these bacteria may optimise the environment of the colon(Reference Meydani and Ha31,Reference Vitiñi, Alvarez and Medina33–Reference Perdigón, de Moreno de LeBlanc and Valdez37) .
Few epidemiological studies have evaluated the relationship between yogurt and CRC, and of these, several found an inverse association(Reference Kojima, Wakai and Tamakoshi38–Reference Boutron, Faivre and Marteau42) and the rest were null(Reference Kampman, Goldbohm and van den Brandt43–Reference Senesse, Boutron-Ruault and Faivre50). Lack of associations may be due to a limited statistical power to detect a difference in CRC risk from either a small sample size or a low prevalence of and/or limited variability in yogurt consumption. Fewer studies evaluated the association between yogurt intake and risk of colorectal AP(Reference Boutron, Faivre and Marteau42,Reference Kesse, Boutron-Ruault and Norat45,Reference Karagianni, Merikas and Georgopoulos51,Reference Kampman, Giovannucci and van ’t Veer52) . None has evaluated SSP, recently recognised with the potential for malignant transformation(Reference Snover4), although a recent cohort study found a null association among all serrated polyps, evaluating hyperplastic polyp (HP) and SSP as one entity(Reference Zheng, Wu and Song53). Furthermore, just one small randomised controlled trial performed in Japanese population with prior colorectal tumours evaluated the association between probiotic supplement use and risk of colorectal tumours (adenomas and early CRC), but not SSP. This investigation found an inverse association between probiotic use alone and recurrence of metachronous AP with moderate atypia or higher(Reference Ishikawa, Akedo and Otani54). Thus, we evaluated the association between yogurt consumption and odds of polyps in two colonoscopy-based case–control studies; in one study, probiotic supplement use in relation to odds of polyps was also assessed.
Experimental methods
Study populations
Tennessee Colorectal Polyp Study
The Tennessee Colorectal Polyp Study (TCPS) is a colonoscopy-based case–control study conducted from February 2003 to October 2010. Institutional approval for human subjects’ research was granted through the VUMC and VA Institutional Review Boards and the VA Research and Development Committee. The study design has been previously described(Reference Shin, Shrubsole and Ness55). In brief, participants were recruited from those presenting for routine colonoscopy at two medical centres in Nashville, TN, USA. Eligible participants were aged 40–75 years and did not have any of the following: inflammatory bowel disease, a personal or family history of any hereditary CRC syndromes, a prior history of colorectal AP, previous colectomy or a history of cancer other than non-melanoma skin cancer.
In all, 12 585 individuals were approached for participation in the TCPS and 7621 (60·6 %) provided informed consent. This analysis is limited to the 5446 participants diagnosed with HP, SSP, AP or without any polyps who also completed a telephone interview and FFQ with a reported daily consumption of at least 2510 kJ/d and with complete data on yogurt intake.
Participants also completed an interviewer-administered questionnaire which solicited information on the participant’s demographics, medication use, family history and other lifestyle factors and a self-administered FFQ with 108 food items which has been previously described(Reference Signorello, Munro and Buchowski56). Total energy intake (kJ/d) was also derived from the FFQ that asks about dietary patterns over the last 12 months.
Johns Hopkins Biofilm Study
The Biofilm Study recruited patients undergoing colonoscopy for routine care at three endoscopy study sites, Green Spring Station Endoscopy Center in Lutherville, MD, USA, White Marsh Endoscopy Center in Baltimore, MD, USA, and Reading Endoscopy Center in Wyomissing, PA, USA, between August 2016 and April 2018. Prior to colonoscopy, the participant met with the endoscopist and the research coordinator, enrolment was discussed and written informed consent was obtained. A total of 1061 patients were enrolled and had complete data (about 43 % of all eligible). The study was reviewed and approved by the Johns Hopkins Medical Institute Institutional Review Board for human research. The inclusion criteria included adults (aged 40–85 years) with an intact colon. Individuals with inflammatory bowel disease, a history of using blood thinners including warfarin or antiplatelet drugs, individuals with a hemicolectomy and pregnant women were excluded.
Participants completed a questionnaire including socio-demographic information, risk factors for CRC (including detailed questions regarding their medical and surgical history), medication use (including antibiotics, non-steroidal anti-inflammatory drugs (NSAIDS), aspirin, hormone therapy), family history of CRC, patterns of tobacco use, alcohol use and physical activity and history of prior colonoscopy and pertinent findings. Participants were defined as having diabetes mellitus, hypertension or hyperlipidaemia if they self-reported a prior history of those conditions. In addition, they answered basic questions regarding their dietary patterns regarding the frequency of consumption of meat, fish, eggs, cheese, milk and yogurt during the last 12 months. The questionnaire is available in the Appendix.
Yogurt intake and probiotic use
In the TCPS, yogurt intake frequency was defined as never/rarely, monthly but less than weekly (1–3/month), weekly but less than daily (1–6/week) and daily (1+/d). Amount of yogurt intake per d was calculated as the usual portion size (0·25, 0·5 or 1 cup) multiplied by the frequency of intake per d and was categorised into four groups: never/rarely (never or rarely consumed) and tertiles based on the consumption among controls.
In the Biofilm Study, frequency of yogurt intake (1 cup serving size) was collected as never, within the last year, more than once a month and more than once a week. For this analysis and to more closely match the TCPS categories, intake was categorised as never/rarely (never or within the last year), monthly less than weekly (more than once a month) and weekly (more than once a week). Information on daily consumption was not available. Probiotic use was defined as taking a probiotic supplement within the last week.
Case and control definitions
The TCPS process to standardise polyp diagnosis has been previously described in detail(Reference Davenport, Su and Zhao6). In brief, all polyps were systematically reviewed by the study pathologist under the guidance of a senior gastrointestinal clinical and research pathologist to standardise polyp diagnosis. SSP were diagnosed based upon the diagnostic criteria from expert panel standards (at least one distorted, dilated or horizontally branched crypt within the polyp) by joint review of cases(Reference Rex, Ahnen and Baron57). The Biofilm Study abstracted the polyp diagnosis from the medical record to classify study participants. The precise location, size, diagnosis and other characteristics of the colorectal polyps were collected from the colonoscopy and pathology reports. In both studies, cases were classified according to the presence, number and synchronicity of HP, SSP and AP. The HP cases had one or more HP without any synchronous AP or SSP. The AP cases had one or more tubular, tubulovillous or villous AP with or without dysplasia and with or without synchronous HP. The SSP cases had one or more SSP, with or without synchronous HP and AP. Location was defined relative to the splenic flexure with caecum, ascending and transverse categorised as proximal colon and descending, sigmoid and rectum as distal colon. Due to their rarity, traditional serrated adenomas were excluded from this analysis (n 12 for TCPS and n 1 for Biofilm Study). AP were defined as advanced if they were 1 cm or greater or contained villous or dysplastic components. Controls in both studies had a complete colonoscopy with visualisation of the caecum without any evidence of polyps at the present colonoscopy, although some controls in the Biofilm Study, but not the TCPS, may have had a personal history of adenoma (50 % of study participants).
Statistical analysis
Online Supplementary Figs. 1 and 2 show the participant flow charts for the two studies. For both studies, descriptive comparisons between case and control groups were calculated using general linear models (for continuous variables) or Mantel–Haenszel χ 2 testing (for categorical variables) with adjustments for age (5-year age categories from 40 to 75 years) and sex, where appropriate. OR and 95 % CI were derived from multinomial logistic regression models which permitted case–control and case–case comparisons. Potential confounders and established risk factors within the studies were adjusted for in the models. In the TCPS, models were adjusted for sex, age, study site (academic/VA), educational attainment, BMI (kg/m2), physical activity in the past 10 years (yes/no), regular alcohol drinking (current, former, never), cigarette smoking status (current, former, never), NSAIDS use (ever/never), red meat intake (g/d), dietary energy intake (kJ/d) and frequency of non-yogurt dairy intake (never/rarely, monthly less than weekly, weekly less than daily, daily). In the Biofilm Study, risk factors were included in the final model both if they were established risk factors or had a P value ≤ 0·05 in the univariate analysis which included sex, age, cigarette use (current, former, never), overweight (BMI less than or greater than 25 kg/m2), prior colon polyp (yes/no), history of cholecystectomy (yes/no), diabetes mellitus diagnosis (yes/no), hypertension diagnosis (yes/no), hyperlipidaemia diagnosis (yes/no), alcohol use (never/<14 alcoholic drinks/week/>14 alcoholic drinks/week) and moderate or vigorous physical exercise (yes/no). Tests for trend were derived by including the categorical variable as a continuous factor in the model. TCPS statistical analyses were completed using SAS Enterprise 7.15. Biofilm statistical analyses were completed using PC SAS 9.4. P values of ≤0·05 (two-sided probability) were considered statistically significant in all analyses.
We performed power calculations for the TCPS and the Biofilm Study. In TCPS analysis, the minimally detectable OR are 0·69, 0·52 and 0·31 for AP, HP and SSP, respectively, assuming a statistical power of 80 % and a two-sided alpha of 0·05. Assuming the same power and two-sided alpha, the Biofilm Study afforded minimally detectable OR for AP, HP and SSP of 0·68, 0·48 and 0·52, respectively.
Results
Demographic characteristics for each study by case–control status are shown in Table 1. A limited number of demographics were collected between both studies (age, sex, race, smoking, BMI, alcohol and physical activity). Among these features, sex, smoking, alcohol use, physical activity and history of colonic polyps differed the most between studies, whereas the patients in both studies were of similar age and most were Caucasian. In both studies, polyp cases were more likely to have a personal history of smoking. Within the TCPS, polyp cases were slightly older, and more likely to be male and overweight, to have lower educational attainment, to consume more red meat, and less likely to exercise, use NSAIDS, and to consume dairy products in comparison with controls. In the Biofilm Study, cases with AP or SSP were more likely to have had a cholecystectomy and a history of colon polyps and less likely to have had gastrointestinal surgery in comparison with controls. In the Biofilm Study, AP cases were older and more likely to be male and overweight, whereas SSP cases were less likely to be overweight and heavily use alcohol and HP cases were more likely to be male and less likely to use aspirin than polyp-free controls.
Table 1. Characteristics of the study participants in the Tennessee Colorectal Polyp Study and Biofilm Study
(Numbers and percentages; least square means)
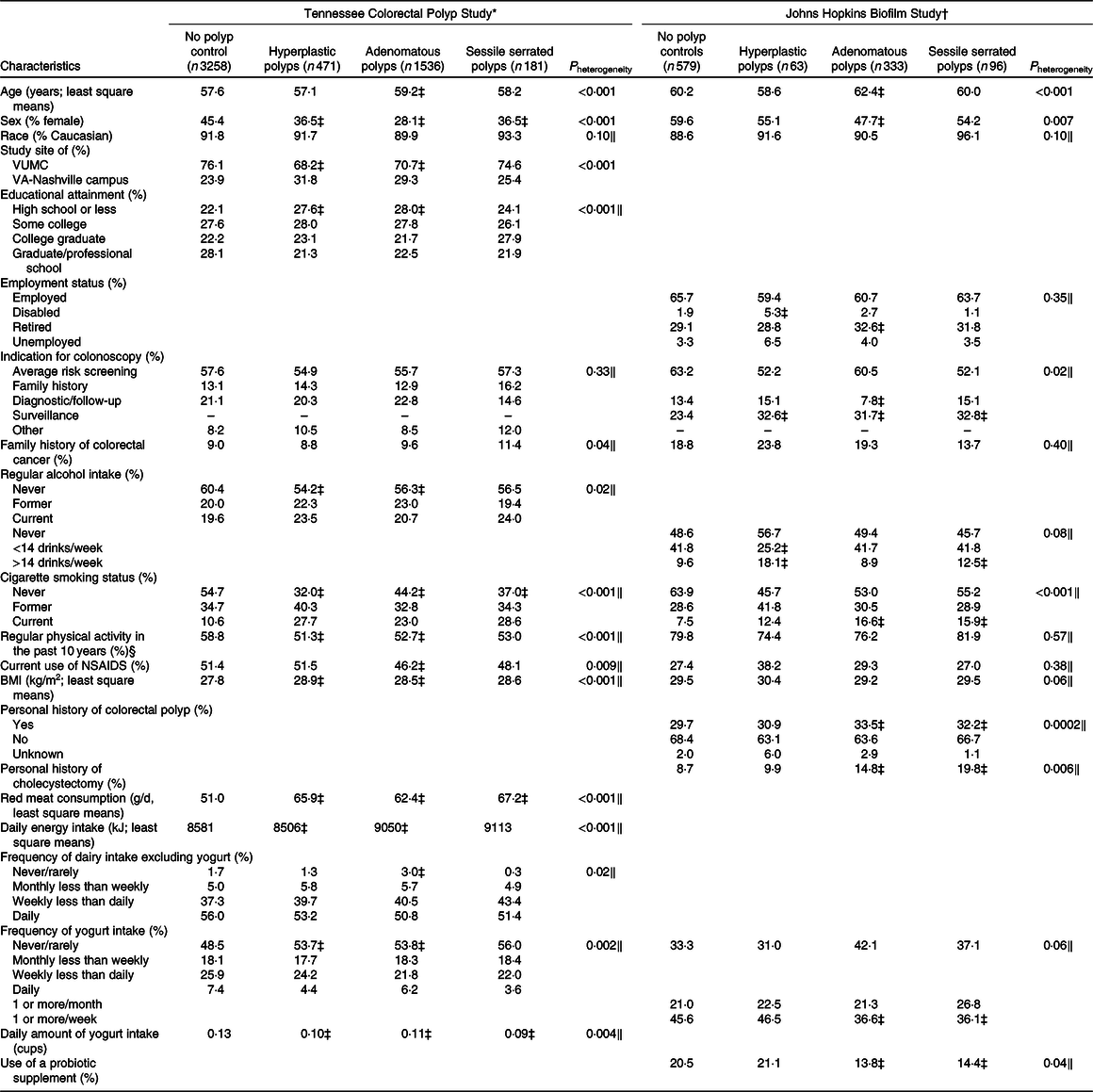
VUMC, Vanderbilt University Medical Center; NSAIDS, non-steroidal anti-inflammatory drugs.
* Data presented as least square means of log-transformed data (continuous) or frequencies standardised to age (5-year categories) and sex distribution of controls with the exception of age, sex and study site, which are presented as n and percentages for categorical data and mean values and standard deviations for continuous data.
† Data presented as least square means of log-transformed data (continuous) or frequencies standardised to age (5-year categories) and sex distribution of controls with the exception of age and sex, which are presented as n and percentages for categorical data and mean values and standard deviations for continuous data.
‡ Case group least square mean or frequencies are significantly different from the control group.
§ Current moderate/vigorous physical activity in the Johns Hopkins Biofilm Study (%).
‖ P values adjusted for age (5-year categories) and sex.
The associations between yogurt intake and odds of polyp type are presented in Table 2 and online Supplementary Tables. In the TCPS, frequency was inversely associated with odds of serrated polyps (SP; HP and SSP). In comparison with those who did not consume yogurt, daily intake was associated with a 50 % decreased odds of HP (OR 0·54; 95 % CI 0·31, 0·95) and a similar, but non-significant reduced odds of SSP (OR 0·49; 95 % CI 0·19, 1·24). The association with HP was even stronger among males (OR 0·28; 95 % CI 0·09, 0·91). Daily intake of yogurt was inversely associated with odds of SP without synchronous AP and, particularly, with decreased odds of SP and AP (online Supplementary Table S1) overall and separately among men and women. Frequency and amount of yogurt intake were not associated with overall odds of AP, although weekly intake of yogurt was significantly associated with a reduced odds of AP among women (OR 0·73; 95 % CI 0·55, 0·98). The association with daily use was also reduced, but no longer significant with fewer numbers and reduced power (OR 0·68; 95 % CI 0·44, 1·06).
Table 2. Associations between yogurt consumption and probiotic use with risk of colorectal polyps
(Numbers; odds ratios and 95 % confidence intervals)
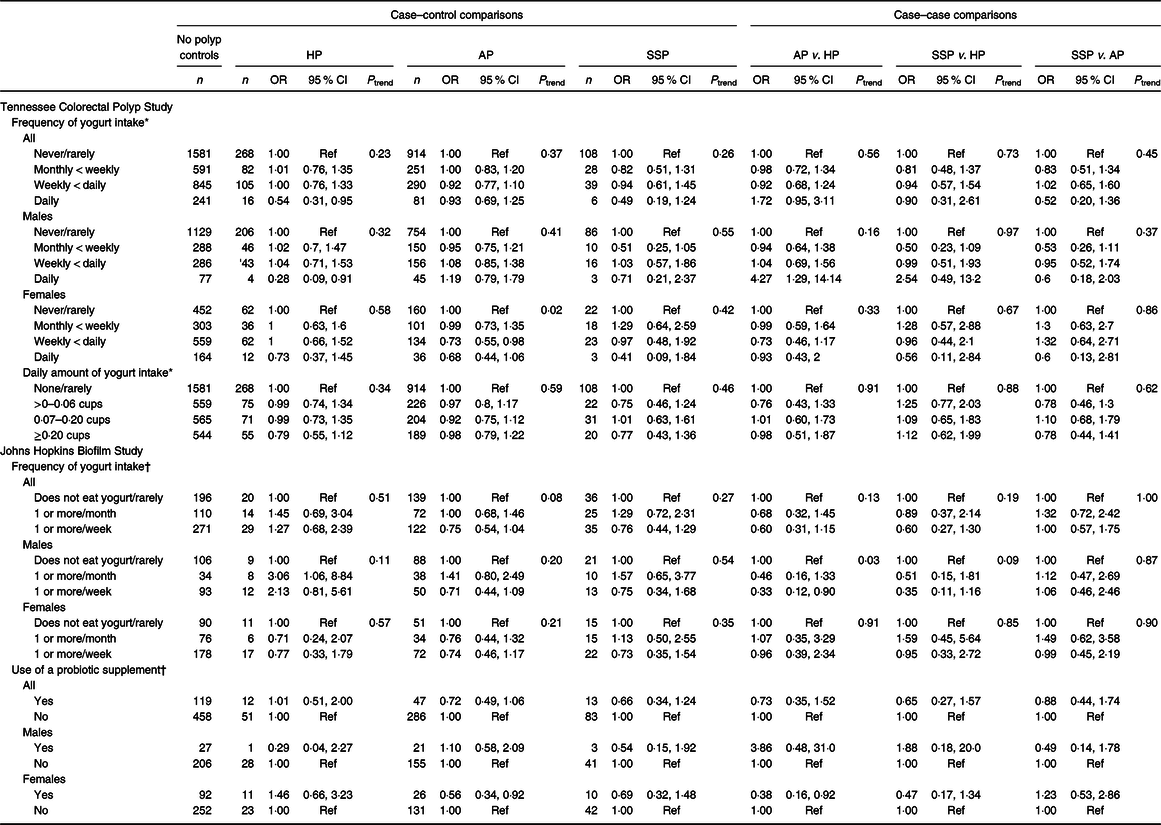
HP, hyperplastic polyp; AP, adenomatous polyp; SSP, sessile serrated polyp.
* Adjusted for sex, study location, age, regular alcohol drinking status, BMI, smoking status, physical activity in the past 10 years, educational attainment, non-steroidal anti-inflammatory drug use, red meat intake, dietary energy intake and frequency of non-yogurt dairy intake.
† Adjusted for sex, age, cigarette use (current/former/never), overweight (BMI less than or greater than 25 kg/m2), prior colon polyp (yes/no), history of cholecystectomy (yes/no), diabetes mellitus diagnosis (yes/no), hypertension diagnosis (yes/no), hyperlipidaemia diagnosis (yes/no), physical activity (yes/no) and >10 alcohol drinks/week (yes/no).
The Biofilm Study also demonstrated a non-significant reduction in odds of SSP for regular yogurt consumption (OR 0·75; 95 % CI 0·44, 1·28 for weekly intake v. no/rare intake) with similar magnitude for both men and women. However, unlike the TCPS, yogurt intake was not associated with a reduced odds of HP (OR 1·12; 95 % CI 0·62, 2·02) but was associated with a non-significant reduction in overall AP odds (OR 0·75; 95 % CI 0·54, 1·04) that also did not vary by sex. A similar non-significant reduction in odds of AP was also observed for probiotic use (OR 0·72; 95 % CI 0·49, 1·06), which was more apparent among women than among men. Use of probiotics was reported by 24 and 11 % of women and men, respectively. To evaluate whether the differences between the TCPS and the Biofilm Study were due to the inclusion of individuals with a history of polyps in the Biofilm Study, we performed a sensitivity analysis in which we restricted the Biofilm Study analysis to people without a prior polyp (data not shown). This sensitivity analysis eliminated approximately 50 % of the study population, as 55 % of women and 44 % of men did not have a history of polyps. Among those without a history of polyps, the association between weekly yogurt intake and AP odds became significant (OR 0·54; 95 % CI 0·33, 0·89) particularly among women, the association between probiotic use and AP became stronger but not significant (OR 0·56; 95 % CI 0·30, 1·04), although the association with SSP odds was similar.
To evaluate whether the associations between polyp odds and yogurt and probiotic intake varied by region of the colorectum, we evaluated the associations comparing polyp-free controls, left-sided polyps, right-sided polyps and synchronous right- and left-sided polyps (online Supplementary Table S2). The studies varied in their association by region. In the TCPS, daily yogurt intake was inversely associated with left-sided polyps (OR 0·56; 95 % CI 0·38, 0·83) in comparison with no intake and was most apparent among women. In the Biofilm Study, yogurt intake at least weekly was non-significantly inversely associated with odds of polyps only on the right side (OR 0·70; 95 % CI 0·48, 1·04). Probiotic use was associated with a non-significant reduced odds of right-sided-only polyps (OR 0·69; 95 % CI 0·43, 1·11), although this was limited to women (OR 0·67; 95 % CI 0·38, 1·18). There was no relationship between yogurt intake and odds of advanced adenomas (online Supplementary Table S3).
Discussion
We found in two colonoscopy-based case–control studies that frequency of yogurt consumption was associated with a trend towards decreased odds of colorectal polyps. While both studies found an inverse association between yogurt and colorectal polyps and the Biofilm Study found an inverse association between probiotics and colorectal polyps, the findings differed between the two studies in terms of polyp type, polyp location and statistical significance. In the TCPS, daily yogurt intake was associated with a decreased odds of SP, particularly HP. Weekly, but not daily yogurt intake, was associated with decreased odds of AP among women, whereas in the Biofilm Study, weekly consumption or more of yogurt was associated with a non-significant decreased odds of overall AP. Daily yogurt intake was associated with a decreased odds of left-sided lesions particularly among women in the TCPS and decreased odds of right-sided polyps in the Biofilm Study, respectively. Probiotic use was not associated with a statistically significant polyp risk reduction overall, although it was associated with a borderline reduced odds of AP and right-sided polyps among women.
Lactic acid-producing bacteria are present in probiotic supplements and in fermented milk products such as yogurt. There are several proposed mechanisms by which these bacteria may prevent colon carcinogenesis. Lactic acid bacteria may decrease the risk of colon polyp formation by stimulating the mucosal immune system, increasing cytokine production, modulating T cell function and/or increasing natural killer cells and IgA-secreting lymphocytes that then may modify microbiome function(Reference Vitiñi, Alvarez and Medina33–Reference Perdigón, de Moreno de LeBlanc and Valdez37,Reference Palm, de Zoete and Cullen58) . In addition, these bacteria may also act to decrease CRC risk by decreasing inflammation. In a randomised controlled trial of paediatric patients with active ulcerative colitis, use of probiotics led to resolution of endoscopic and mucosal inflammation 2·5 times more frequently than in controls(Reference Ohkawara, Furuya and Nagashima34,Reference Geier, Butler and Howarth36,Reference Perdigón, de Moreno de LeBlanc and Valdez37,Reference Miele, Pascarella and Giannetti59) . Lactic acid bacteria may also reduce the concentration of secondary bile acids and dietary carcinogenic metabolites produced by meat ingestion including N-nitroso compounds and heterocyclic aromatic amines by binding to and inactivating them and reducing their bioavailability(Reference Perdigón, Maldonado Galdeano and Valdez35,Reference Rafter60,Reference de Moreno de LeBlanc and Perdigón61) . Further, certain bacterial strains may reduce bacterial enzyme activities present in the colon such as β-glucuronidase and nitroreductase, which hydrolyse and activate carcinogenic molecules contained in burnt and processed meat products(Reference Meydani and Ha31,Reference Pool-Zobel, Selvaraju and Sauer62) . Finally, lactic acid-producing bacteria secrete SCFA, including butyrate, which is the primary colonocyte energy source and proposed to possess antitumourigenic properties. Butyrate inhibits histone deacetylase and thereby decreases cell proliferation and promotes apoptosis(Reference Young, Hu and Le Leu63–Reference Wu, Yang and Zhang65). Decreases in butyrate-producing bacteria and enrichment of pathogenic bacteria are a common finding in studies comparing differences between CRC cases and controls(Reference Sobhani, Tap and Roudot-Thoraval66–Reference Zackular, Baxter and Iverson69).
Our finding of a possible inverse association between yogurt and probiotic consumption and colorectal neoplasia risk is consistent with prior studies. In the only randomised trial of probiotic use that assessed the effect on AP, Ishikawa et al.(Reference Ishikawa, Akedo and Otani54) randomised individuals with recent colorectal tumours (AP or early cancers) to one of the four arms: diet instruction, Lactobacillus casei, wheat bran or both L. casei and wheat bran. At the end of 4 years, individuals who took L. casei had a lower prevalence of metachronous AP with moderate or greater atypia. Although this trial included only a single probiotic bacterium, it provides initial evidence of a possible preventive role for probiotic bacteria in colorectal carcinogenesis. In our analysis, we also observed a decreased odds of AP associated with probiotics consumption.
There are a limited number of epidemiological studies evaluating the relationship between yogurt and CRC risk, and their results are inconclusive. In case–control and cohort studies, there have been reports of inverse(Reference Kojima, Wakai and Tamakoshi38–Reference Boutron, Faivre and Marteau42) associations with CRC risk, although most have been null(Reference Kampman, Goldbohm and van den Brandt43–Reference Senesse, Boutron-Ruault and Faivre50). Two cohorts out of eight observed an inverse association and three case–control studies out of five reported an inverse association(Reference Kojima, Wakai and Tamakoshi38–Reference Senesse, Boutron-Ruault and Faivre50). When an inverse association has been observed, it has been reported with rectal cancer (38), colon cancer(Reference Pala, Sieri and Berrino39–Reference Peters, Pike and Garabrant41), Japanese men(Reference Kojima, Wakai and Tamakoshi38) and among Italians(Reference Pala, Sieri and Berrino39). A pooled analysis of ten cohort studies examined 5734 CRC cases and observed a weak inverse association between consumption of yogurt with CRC risk that was of borderline significance(Reference Cho, Smith-Warner and Spiegelman70). Conversely, previous epidemiological studies were more consistent regarding the relationship between yogurt intake with AP risk, although there are no studies evaluating risk for SSP. Three(Reference Boutron, Faivre and Marteau42,Reference Senesse, Boutron-Ruault and Faivre50,Reference Karagianni, Merikas and Georgopoulos51) previous European case–control studies observed an inverse association between colorectal AP and yogurt intake, but two European cohorts found no association(Reference Kesse, Boutron-Ruault and Norat45,Reference Kampman, Giovannucci and van ’t Veer52) . The observed relationships were modest and limited to large or advanced adenomas. One recent report from two large US cohort studies found an inverse association only among men who consumed yogurt and risk of AP, but no associations were found for SP risk or for polyp risk among women(Reference Zheng, Wu and Song53). Instead, we found a possible weak association with overall AP odds in the Biofilm Study and a significant association with AP odds among women and a strong association with HP in the TCPS. The heterogeneity in the design of these studies may contribute to the differences including variation in exposure definition (several assessed broader categories including fermented dairy products)(Reference Kojima, Wakai and Tamakoshi38–Reference Kampman, Goldbohm and van den Brandt43), extreme heterogeneity of available probiotics and yogurt products (including both the types and quantities of lactic acid-producing bacteria strains contained in each), the underlying population and diet and analytic methods including controlling for confounders(Reference Boutron, Faivre and Marteau42,Reference Kampman, van ’t Veer and Hiddink44–Reference Zheng, Wu and Song53) . Another possible explanation for the inconsistent findings may be misclassification of polyp status in many of the previous studies given the recent understanding of enhanced risk with SSP. Finally, the studies with small sample sizes may be inadequately powered to detect an association.
Our study is strengthened by the use and comparison of two study populations to evaluate the association between yogurt consumption and colorectal polyps, despite some differences between the studies and their findings. Differences may be due to variations in amount of yogurt ingestion and bacterial strains. These two studies were conducted during different eras of yogurt consumption. Yogurt has been growing in popularity in the US population due to companies marketing its health benefits. The prevalence of yogurt consumption in the American diet has increased from 4 to 9 % of adults reporting weekly intake from 2004 to 2012(Reference Fisberg and Machado24). Using National Health and Nutrition Examination Survey data from 2005 to 2014, we also found the intake of yogurt increased over time from 6·1 to 9·2 % and the amount of yogurt consumed increased from 10·0 to 17·9 g/d (unpublished results). Among controls, weekly or more frequent consumption of yogurt was slightly higher in the Biofilm Study (45·5 %) than in the TCPS (40·7 %). Unlike in the TCPS, daily yogurt intake was not able to be evaluated in the Biofilm Study. Thus, the observed association for weekly consumption in the Biofilm Study may reflect daily intake or may also reflect a dilution of the true association for daily users. Moreover, the types of yogurt available and sold in stores have also evolved during the time period between the two studies. In 2010 when TCPS enrolment was ending, Greek yogurt (a more concentrated yogurt with higher protein and reduced sugar content and higher bacterial count) began replacing regular yogurt intake in the US population(Reference German71). In addition, with increasing publicity regarding yogurt health benefits, yogurt companies began modifying yogurt products to include additional bacterial strains (yogurt and probiotic products) with advertisements regarding the health benefits including symptomatic relief from GI symptoms(Reference German71). It is possible that the observed differences between the two studies, and with previous studies, are a result of increased frequency of use or differences in yogurt types or strains.
The Biofilm Study also included participants who had prior polyps and therefore represents a higher risk population. Yogurt use might act differently in these two populations because of a dissimilar underlying risk of forming colorectal polyps. However, when the analysis was restricted to the participants in the Biofilm Study without a history of colorectal polyps, the association was stronger and significant for AP and unchanged for SSP and HP. In contrast, polyp-free controls with a prior history of polyps are predisposed to form polyps, but predisposed individuals may not have had enough time to form polyps between their last colonoscopy and the current colonoscopy. Finally, the two studies employed two different methods to diagnose SSP and HP. As the TCPS was conducted prior to the distinction between HP and SSP, this study performed a thorough review of all serrated polyps to update the diagnoses. The colonoscopies performed during the Biofilm Study were done after the change in clinical practice, and therefore, the HP or SSP diagnosis could be audited directly from the medical records. Within the TCPS, the use of one pathologist to diagnose the outcome might have standardised the diagnosis and review of difficult cases with a senior gastrointestinal pathologist might have improved the accuracy of diagnosis.
As with prior studies, power remains an issue in the two present studies especially in subgroup analyses by polyp type. While the overall sample sizes of the two studies were adequate based on power calculations (see Methods), after performing subgroup analyses by polyp type, the samples sizes and power were reduced, especially for SSP given the relative rarity of these polyps. With the collective SSP between both studies, our power to detect a 30 % decrease in odds among people who consumed yogurt at least weekly compared with never/rarely was only 18 %. Our power to detect a 30 % decrease in odds in AP was 67 %. Finally, residual confounding may also explain differences between the two studies or with previous findings. In the Biofilm Study, we did not collect overall energy intake, which is a known confounder when assessing for effects of nutrients on colon polyps(Reference Kanazawa, Negishi and Kishi72). The effects of probiotics may be stronger when consumed with prebiotics, such as indigestible fibre that lactic acid-producing bacteria consume and which is proposed to enhance the benefits of probiotic ingestion(Reference Kumar, Singh and Sinha73). Prebiotic use was not collected in the Biofilm Study. Total fibre intake was collected in the TCPS; however, adjustment for fibre did not substantially alter the associations between yogurt and polyps.
The collection of probiotic supplementation in the Biofilm Study is a strength as there are limited data available regarding the effect on colon cancer in epidemiological studies. However, it is important to note we only collected information regarding use in the week prior to colonoscopy, and no data regarding frequency of probiotic use or duration of use were collected. This may lead to misclassification of exposure if there were significant differences in intensity and duration of probiotic use among this population.
Overall, using two colonoscopy studies, we were able to observe that both yogurt and probiotics, two different products containing lactic acid-producing bacteria, have independent inverse associations with colorectal polyp odds that were either statistically significant or of borderline significance. We observed a reduced odds of AP in the Biofilm Study and reduced odds of AP among women and reduced odds of SP, particularly HP, in the TCPS, associated with yogurt intake. We observed a non-significant reduced odds of AP associated with probiotic use in the Biofilm Study. Our collective results raise the possibility of a protective effect of lactic acid bacteria, but are limited due to differences in study design, lack of clear dose–response relationships and small number of cases to draw inferences, especially in the smaller Biofilm Study and in subgroup analyses. Future, rigorous studies to assess the effect of bacterial strains and yogurt types on polyp types and the dose and duration of yogurt intake and probiotic use needed for prevention are warranted, particularly in light of recent results challenging the positive benefit of probiotic products(Reference Freedman, Williamson-Urquhart and Farion74–Reference Suez, Zmora and Zilberman-Schapira77). Further research might prove that interventions with yogurt and probiotics may be potential low-cost strategies for CRC prevention, particularly considering the global surge in CRC and among individuals under 50 years of age(Reference Ferlay, Soerjomataram and Dikshit1,Reference Siegel, Medhanie and Fedewa78) .
Acknowledgements
This publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by grant number UL1 TR 001079 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research, and the Johns Hopkins Clinical Research Network (JHCRN). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, JHCRN, NCATS or NIH.
This study was supported by grants P50CA950103 (W. Z.), R01CA97386 (W. Z.), K07CA122451 (M. J. S.), U2CCA233291 (M. J. S., C. L. S.), R01CA196845 (C. L. S., F. M. G.), Bloomberg Philanthropies and the Johns Hopkins Cancer Center Support Grant, NCI P30CA006973. Surveys and sample collection and processing for this study were conducted by the Survey and Biospecimen Shared Resource, which is supported in part by P30CA68485 (Vanderbilt). All data from R01CA196845 (Johns Hopkins) were stored in Research Electronic Data Capture (REDCap) which, at Vanderbilt, is supported in part by the Vanderbilt Institute for Clinical and Translational Research (UL1TR000445). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. A portion of the participants were studied as the result of resources and the use of facilities at the VA Tennessee Valley Healthcare System.
TCPS: M. J. S., W. Z. and H. J. M. designed and implemented the study. R. N., W. E. S., M. J. S. and X. Z. participated in data collection. M. J. S. and X. Z. provided the overall statistical analysis and oversight. M. J. S. wrote the manuscript. All authors provided the critical review of the manuscript and approved the final manuscript. Biofilm Study: C. L. S. and F. M. G. designed and implemented the study and reviewed all data. E. H. S., G. E. M., D. K., L. L., J. L. D., J. J. G. and L. M. H. participated in data collection. S. B. R. analysed the data and performed statistical analysis. S. B. R., C. L. S. and F. M. G. wrote the manuscript. All authors provided the critical review of the manuscript and approved the final manuscript.
C. L. S. is supported, in part, by research grants from Bristol Myer Squibb and Janssen; C. L. S. was a consultant to Merck in 2019; no other authors have any conflicts of interest to report.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520000550





