Sarcopenia is one of the hallmarks of ageing. This term defines age-associated loss of muscle mass, strength and function( Reference Evans and Campbell 1 ). Sarcopenia is an important health problem among the elderly. It has been determined that approximately 11–50 % of people aged above 80 years suffer from sarcopenia, especially those living in nursing homes( Reference Landi, Liperoti and Fusco 2 ). Maintenance of muscle functionality is important to avoid frailty and to increase the independence and quality of life during ageing. It seems clear that for daily life activity, and hence a good quality of life, not only strength but also endurance is needed. Apart from the maintenance of a series of basic exercises, several nutritional bioactive compounds have been proposed to increase muscle function during ageing and to avoid sarcopenia( Reference Sanchis-Gomar, Pareja-Galeano and Mayero 3 ). These compounds act by affecting mitochondrial activity and turnover through modulating PPAR γ co-activator 1 α (PGC-1α) and other modulators such as sirtuins( Reference Sanchis-Gomar, Pareja-Galeano and Mayero 3 ).
Resveratrol (RSV) is a polyphenol found in grapes, red wine, walnuts, peanuts and berries. The potential benefits of RSV have been demonstrated as a mimicker of energy intake restriction (CR)( Reference Lopez-Lluch and Navas 4 ), modulator of postmenopausal-dependent osteopenia( Reference Zhao, Li and Li 5 ), regulator of circadian clocks in high-fat diets( Reference Miranda, Portillo and Madrid 6 ) or as a positive factor in motor behaviour and neuronal function during ageing( Reference Shukitt-Hale, Bielinski and Lau 7 ) among many other different effects. An enormous body of evidence indicates that RSV modulates the activity of the sirtuin-5' AMP-activated protein kinase-PCG-1α (SIRT–AMPK–PGC-1α) pathway and then stimulates mitochondrial turnover and biogenesis( Reference Kulkarni and Canto 8 – Reference Baur, Pearson and Price 11 ). This pathway is also stimulated by exercise and is responsible for a higher physical capacity and mitochondrial activity( Reference Hood 12 – Reference Winder, Holmes and Rubink 14 ). Many studies performed in rodents have demonstrated the positive influence of RSV in increasing the effect of exercise on muscle( Reference Dolinsky, Jones and Sidhu 15 – Reference Murase, Haramizu and Ota 17 ), affecting this same regulatory pathway( Reference Hart, Sarga and Csende 18 ). However, in humans, different studies carried out to determine the effect of RSV on exercise and muscle capacity have produced controversial results( Reference Craig, Ashcroft and Belew 19 ). Dose, time and age of the individuals used in human studies can explain the differences found in the relationship with the positive effect of RSV found in rodents. In fact, the few studies performed in humans have been based on young and healthy individuals or in old individuals taking low doses of RSV( Reference Gliemann, Schmidt and Olesen 20 – Reference Olesen, Gliemann and Bienso 22 ). In this study, the age reached in mice would be about 85 years in humans and the dose used in our study would represent a dairy intake amount of 1200 mg/individual (approximately 75 kg) in humans, but many of the doses used in human studies are not higher than 500–600 mg/d( Reference Gliemann, Schmidt and Olesen 20 – Reference Olesen, Gliemann and Bienso 22 ).
Improving physical capacity during ageing is essential in order to avoid frailty and increase individual independence. The design of physical exercises adapted for elderly people is very important. The incapacity of muscle to respond to physical stimuli makes the use of priming compounds important to reach a higher physical capacity and muscle performance in elderly people. The aim of this study was to determine whether RSV by itself is able to increase physical capacity or whether RSV-supplemented diets can improve the effect of training in physical performance in old organisms.
Materials and methods
Animals and feeding regimens
Male C57BL/6J mice (Charles River) were used in this study. After a week of adaptation, we started supplementation with RSV at three different ages: 2 months (young group), 12 months (mature group) and 18 months (old group) (n 16 animals/group). Animals were fed Teklad Global Diet Chow 2014S (Harlan Teklad) and housed in enriched environmental conditions in groups of 4 animals/polycarbonate cage in a colony room under a 12 h light–12 h dark cycle (00.00–12.00 hours) under controlled temperature (22±3°C) and humidity. Animals were maintained according to a protocol approved by the Ethics Committee of the University Pablo de Olavide and following the international rules for animal research.
Animals of each age group (2, 12, 18 months) were randomly divided into two groups: control and RSV. The control groups received water-containing ethanol as vehicle (0·02 % ethanol in water), whereas the RSV groups received a dose of 0·01 % RSV (Cayman Chemicals) in opaque bottles to avoid light-dependent decomposition as indicated previously( Reference Tung, Rodriguez-Bies and Ballesteros-Simarro 23 ). RSV stability was controlled in our housing conditions, and drinking water was changed twice a week for all the groups. Animals drank approximately 4–5 ml of water/d, and their weight was approximately 30 g during the experiments; the calculated dose of RSV was approximately 500 μg/animal per d (a calculated dose of approximately 16–17 mg/kg per d).
Animals were maintained for 4·5 months under these conditions. Subsequently, mice of each group were randomly divided again into two new groups: non-trained (sedentary) and trained (T) animals. Trained animals were adapted to exercise for 1 week, and after that a training protocol was carried out using a rodent treadmill (Treadmill Columbus 1055M-E50; Cibertec SA) with 8 % inclination for 20 min/d, 5 d/week for 6 weeks. The training protocol comprised a 3-min warm-up, followed by a training bout in which the running speed was gradually increased to 20 m/min.
At the end of the experimental procedures, ages of the animals were 8 (young group), 18 (mature group) and 24 (old group) months. After 3 d of the last training session or strenuous test procedure, animals were killed by cervical dislocation and the gastrocnemius muscle was quickly removed, immediately frozen in liquid N2 and stored at −80°C until processing and analysis.
Physical capacity determinations
Motor activity and coordination tests were carried out on rotarod. Animals were placed on the rod until reaching 45 rpm, and the time until animals fell down was determined. Muscular force tests were performed using a Grip Strength Columbus for mice (Cibertec SA). Extenuating activity test was performed on a treadmill (Treadmill Columbus 1055M-E50; Cibertec SA) with 8 % inclination starting at 10 m/min and increasing the speed by 5 m/min every 5 min until reaching 25 m/min. We established the end of the experiment as the moment the animal stopped for more than 5 s under electric stimuli without trying to move back to the treadmill.
Tissue homogenisation
For enzymatic and Western blot analyses, frozen gastrocnemius was homogenised in nine volumes of ice-cold, tissue-lysis buffer containing 2 mm-Tris-HCl, 20 mm-hepes, 1 mm-EDTA, 70 mm–sucrose, 220 mm-mannitol and 1 mm-PMSF with protease inhibitors (Roche). Homogenates were centrifuged at 1000 g for 10 min at 4°C. Pellets containing unlysed cells and cellular debris were discarded. For lipid peroxidation procedures, frozen tissue was homogenised in nine volumes of ice-cold tissue-lysis buffer containing 20 mm-Tris-HCl. Protein concentration was determined by Bradford’s method.
Oxidative damage assays
Lipid peroxidation assay was performed by determining the reaction of malondialdehyde with two molecules of 1-methyl-2-phenylindole at 45°C as indicated previously( Reference Tung, Rodriguez-Bies and Ballesteros-Simarro 23 ). Peroxidised lipids are shown as µmol malondialdehyde equivalents/mg protein.
Antioxidant activities determination
For total superoxide dismutase (SOD) activity, the SOD determination kit (Sigma) was used as indicated by the manufacturer. Catalase (CAT) activity was measured in triplicate according to the method of Aebi( Reference Aebi 24 ) by monitoring the disappearance of H2O2 at 240 nm; activity was determined as nmol of hydrogen peroxide converted/min per mg total protein. Glutathione peroxidase (GPx) activity was measured using a coupled enzyme assay( Reference Flohé and Günzler 25 ). NAD(P)H quinone dehydrogenase 1 (NQO1) activity was determined spectrophotometrically by monitoring the reduction of the standard electron acceptor, 2,6-dichlorophenol-indophenol (DCPIP) at 600 nm( Reference Benson, Hunkeler and Talalay 26 ) in the absence or presence of dicoumarol. The dicoumarol-inhibitable part of DCPIP’s reduction was calculated as NQO1 activity using the extinction coefficient of 21·0 mm −1 cm−1 and expressed as nmol of DCPIP reduced/min per mg protein. Total cytochrome B5 reductase (CytB5Rase) activity was assayed by measuring the rate of potassium ferricyanide reduction spectrophotometrically. The enzyme activity was calculated using the extinction coefficient of 6·22 mm −1 cm−1 for NADH and expressed as nmol/min per mg protein.
Western blot analysis
Equal amounts of protein homogenates were separated on PAGE-SDS gel and transferred onto a nitrocellulose membrane. Ponceau S (Sigma) staining was recorded using ChemiDoc™ XRS+System and compiled using Image Lab™ 4.0.1 software (Bio-Rad Laboratories) to monitor transfer efficiency and quantification of whole protein loading. Primary antibodies anti-CAT (219010; Calbiochem-Merck Millipore), anti-Cu, Zn-SOD (574597; Calbiochem-Merck Millipore), anti-CytB5Rase (rabbit polyclonal antibody kindly provided by Dr J. M. Villalba, Universidad de Córdoba, Spain)( Reference Bello, Alcain and Gomez-Diaz 27 ), anti-cytochrome C (556433; BD Pharmingen), anti-NQO1 (C-19 sc-16464; Santa Cruz), anti-nuclear respiratory factor-1 (NRF1) (H-300 sc-33771; Santa Cruz) and anti-mitochondrial transcription factor A (mtTFAM) (A-17 sc-23588; Santa Cruz) were used. After washing, HRP-conjugated secondary antibodies were used (Calbiochem), and finally blots were developed using an enhanced chemiluminescence detection substrate Immobilon™ Western Chemiluminescent HRP Substrate (Merck Millipore). Protein levels were visualised using ChemiDoc™ XRS+System and compiled using Image Lab™ 4.0.1 software for quantification. Protein expression levels were corrected for whole protein loading determined by staining the membrane with Ponceau red S and further quantification( Reference Romero-Calvo, Ocon and Martinez-Moya 28 ).
Statistical analysis
SigmaStat 3.5 program was used for the statistical analysis and figures were obtained using SigmaPlot 10.0 program (Systat Software Inc.). All data are expressed as mean values with their standard errors. The information obtained from each group was statistically processed according to the most suitable technique for each case. Student’s t test and one-way or two-way ANOVA followed by post hoc pairwise multiple comparison procedures (Bonferroni’s t test) were performed. The critical significance level α was=0·05, and statistical significance was defined as P<0·05.
Results
Resveratrol increases physical capacity in trained mature and old mice
To determine whether animals fed RSV or/and trained showed different mass at the end of the experiment, we weighted them every 15 d. Only the young group showed a significant increase during the 6 months of the procedure, whereas neither mature nor old mice showed any modification along the experiment. In each age group, trained and/or RSV-supplemented animals showed the same average weight at the end of the experiment, when the physical tests were performed, indicating no influence of mass differences between groups on their respective physical capacity (Table 1).
Table 1 Weight (g) of mice at the beginning and after the treatment with resveratrol (RSV) and/or exerciseFootnote * (Mean values and standard deviations of the body mass (g))
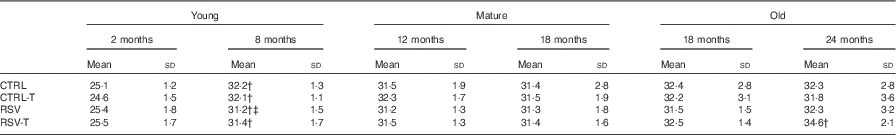
* Young animals started RSV supplementation at 2 months and finished at 8 months. Mature animals started at 12 months and finished at 18 months and old animals started at 18 months and finished at 24 months.
† Significant differences v. body mass at the beginning of the procedure, P<0·05, by using paired t test analysis.
‡ Significant differences v. body mass in respective age control group, P<0·05, by using two-way ANOVA test.
Treadmill tests demonstrated an age-dependent decrease in the capacity of animals to run until exhaustion, with old animals showing lower capacity to cover lesser distances, P<0·001, and running for less time P<0·001 (Fig. 1). Mature animals also showed lower performance compared with young animals affecting both distance (P=0·008) and time running until exhaustion (P=0·025). When we considered the effects of RSV and/or training in each age group, neither RSV nor training induced any improvement in the young group. However, in mature animals, RSV by itself showed a small, although not significant, increase in the distance covered, whereas training significantly increased both distance (P=0·003) and time (P=0·010). Interestingly, RSV primed the effect of training, as trained animals supplemented with RSV ran until exhaustion for more time (P=0·003) and covered more distance (P=0·029). This priming effect was maintained in old animals as RSV-treated and trained animals showed the highest performance in this age group, whereas training or RSV alone improved their capacity to a lower degree. Old animals supplemented with RSV and/or trained reached similar performance as mature and young non-supplemented and non-trained animals running for the same time and covering the same distance (Fig. 1).
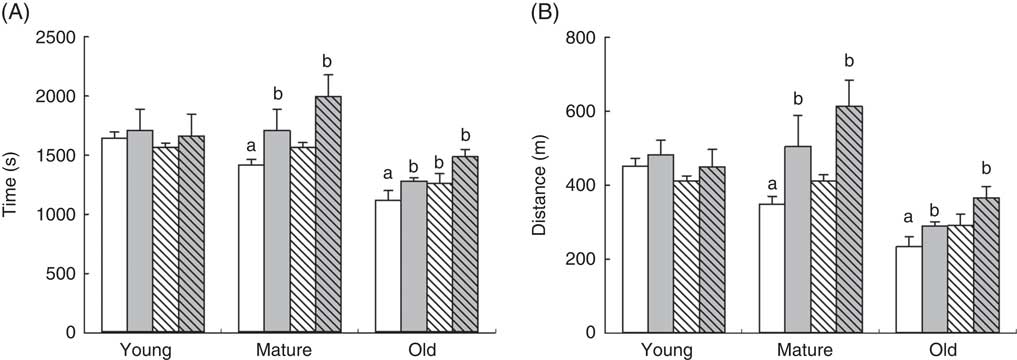
Fig. 1 Endurance performance of mice at different ages in an extenuating activity on treadmill. Endurance capacity of the animals is indicated as time after reaching extenuation (A) and the distance covered by the animals (B). Data from control animals are indicated in plain columns (![]() ,
, ![]() ). Data from resveratrol (RSV)-treated animals are indicated with dashed columns (
). Data from resveratrol (RSV)-treated animals are indicated with dashed columns (![]() ,
, ![]() ). Non-trained animals are shown as white columns (
). Non-trained animals are shown as white columns (![]() ,
, ![]() ), and trained animals are indicated as grey columns (
), and trained animals are indicated as grey columns (![]() ,
, ![]() ). Values are means and standard deviations of the time in seconds consumed until extenuation and distance in metres covered by the animals until reaching extenuation are represented by vertical bars. a Significant difference v. young control group is indicated, P<0·05, by using one-way ANOVA with Bonferroni’s post hoc test; b significant differences v. control group in each age group, P<0·05, by using two-way ANOVA test.
). Values are means and standard deviations of the time in seconds consumed until extenuation and distance in metres covered by the animals until reaching extenuation are represented by vertical bars. a Significant difference v. young control group is indicated, P<0·05, by using one-way ANOVA with Bonferroni’s post hoc test; b significant differences v. control group in each age group, P<0·05, by using two-way ANOVA test.
Rotarod experiments also demonstrated an age-dependent effect of RSV and training. Clearly, the latency to fall in the rotarod test decreased with age, already affecting mature animals. Older animals were those that maintained less time on the rod (P=0·001) in comparison with control young animals (Fig. 2(a)). In this case, only old animals showed a significant increase in the latency to fall after training, P=0·040, whereas young and mature animals did not show any improvement, although a non-significant trend to increase was found in RSV-supplemented and trained animals. Interestingly, only training improved the performance in old animals in both trained and RSV-supplemented and trained animals, P=0·011, and no priming effect of RSV was found in this case (Fig. 2(a)).
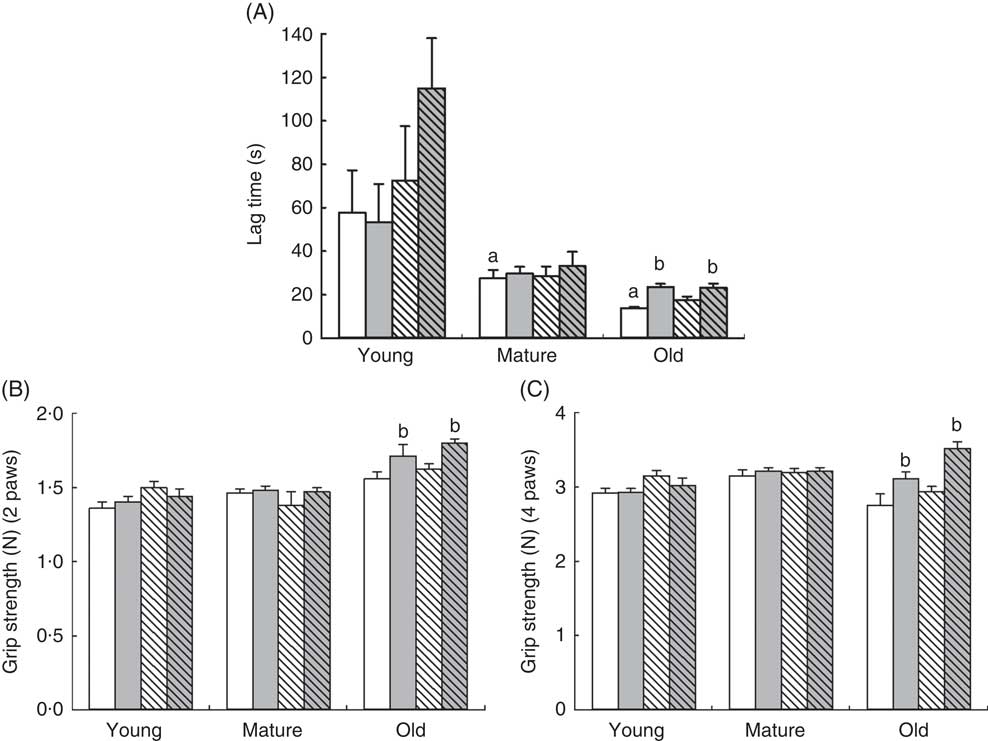
Fig. 2 Determination of coordination on rotarod (A) and grip strength (B, C) of mice at different ages. (A) Lag time (s) to fall from the rotarod and standard deviation of young, mature and old animals fed resveratrol (RSV) and/or trained. a Significant difference v. young control group, P<0·05, by using one-way ANOVA with Bonferroni’s post hoc test; b significant differences v. control group in each age group, P<0·05, by using two-way ANOVA test. (B) Grip strength of the forelimbs (two paws) (B) and fore/hind limbs (four paws) (C) determined in Newtons (N) and standard deviations. b Significant differences v. control group in each age group, P<0·05, by using two-way ANOVA test. ![]() , Control-NT;
, Control-NT; ![]() , control-T;
, control-T; ![]() , RSV-NT;
, RSV-NT; ![]() , RSV-T.
, RSV-T.
When muscle strength was determined by grip strength tests, the effect of RSV or exercise was found again only in old animals affecting neither young nor mature animals. We did not find significant differences in strength because of the age of animals in control groups. Training alone improved strength in both upper (P=0·009) and all limbs strength (P=0·015). RSV-treated and trained animals showed greater increase in strength affecting both upper limbs and forelimbs, P=0·001 in both cases (Fig. 2(b)).
In comparison with previous studies in mice( Reference Lagouge, Argmann and Gerhart-Hines 10 , Reference Baur, Pearson and Price 11 ), our results demonstrate that low amounts of RSV show low effects on muscle performance but are able to prime the effect of exercise and affect mature and old animals more than young animals.
Resveratrol reduces lipid peroxidation in old gastrocnemius muscle
In order to study the mechanisms involved in this priming effect, we determined the degree of oxidative damage in the gastrocnemius muscle in old animals. The presence of oxidised lipids was the highest in old muscle, P=0·001 (Fig 3). A small decrease was found in the mature group in comparison with young animals but without reaching statistical significance. Neither exercise nor RSV produced any effect in young or mature animals. However, in the old group, RSV reduced the degree of lipid peroxidation in the gastrocnemius muscle significantly, P=0·005 (Fig. 3). On the other hand, training tended to increase lipid peroxidation levels in both control and RSV-supplemented animals. This tendency can be due to the longer strenuous activity performed by these animals in comparison with sedentary or non-trained animals (Fig. 1). In any case, RSV-supplemented animals showed the lowest peroxidation levels in the old group (Fig. 3).
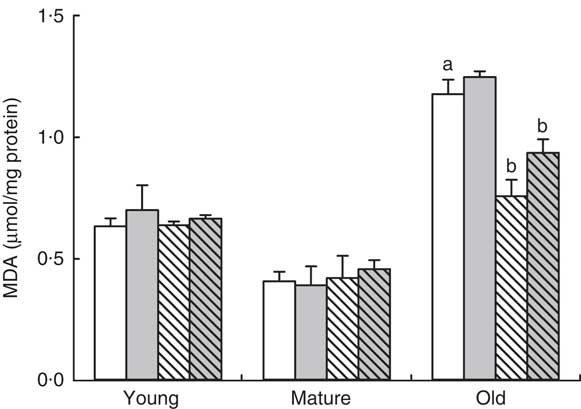
Fig. 3 Lipid peroxidation levels in different age groups fed resveratrol and/or trained. Data represent malondialdehyde (MDA) levels in muscle in µmol/mg protein with their standard errors in young, mature and old animals fed resveratrol (RSV) and/or trained are represented by vertical bars. a Significant difference v. young control group is indicated, P<0·05, by using one-way ANOVA with Bonferroni’s post hoc test. b Significant differences v. control group in each age group, P<0·05, by using two-way ANOVA test. ![]() , Control-NT;
, Control-NT; ![]() , control-T;
, control-T; ![]() , RSV-NT;
, RSV-NT; ![]() , RSV-T.
, RSV-T.
Antioxidant activities are affected by ageing and modulated by resveratrol and physical activity
We also determined the levels of main antioxidant activities involved in general reactive oxygen species scavenging (CAT, GPx and SOD) and membrane peroxidation protection (CytB5Rase and NQO1) in muscle with ageing (Fig. 4). Interestingly, CAT activity decreased during ageing in muscle, P=0·020. Neither SOD nor GPx activities were affected. CytB5Rase and NQO1 showed a different behaviour, with CytB5Rase showing higher levels in old muscle, P=0·034, and NQO1 showing lower levels, P=0·039 (Fig. 4).
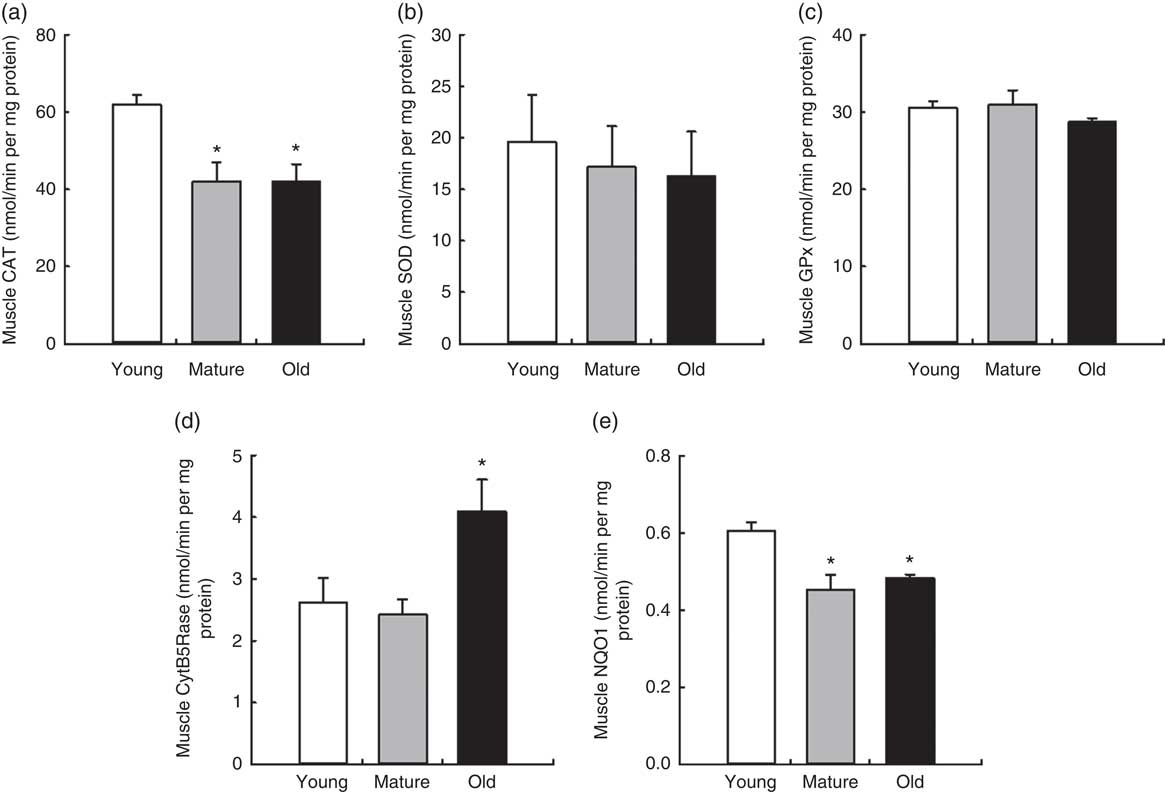
Fig. 4 Antioxidant activities in mice muscle during ageing. Values are means with their standard errors of different endogenous antioxidant activities in muscle in young, mature and old control animals are represented by vertical bars. (a) Catalase (CAT), (b) superoxide dismutase (SOD), (c) glutathione peroxidise (GPx), (d) cytochrome B5 reductase (CytB5Rase) and (e) NAD(P)H quinone dehydrogenase 1 (NQO1) activities measured in nmol/min per mg protein. * Significant differences v. young group, P<0·05 by using one-way ANOVA with Bonferroni’s post hoc test.
In old animals, training and RSV induced different responses in these activities. Both training and RSV showed a trend for increased CAT and SOD activities, although not reaching statistical significance. However, SOD activity was higher in animals fed RSV and trained (Fig. 5). GPx activity was not affected. In the case of CytB5Rase, training showed a trend to increase the activity in both control and RSV-treated animals, whereas NQO1 did not respond to these effectors.
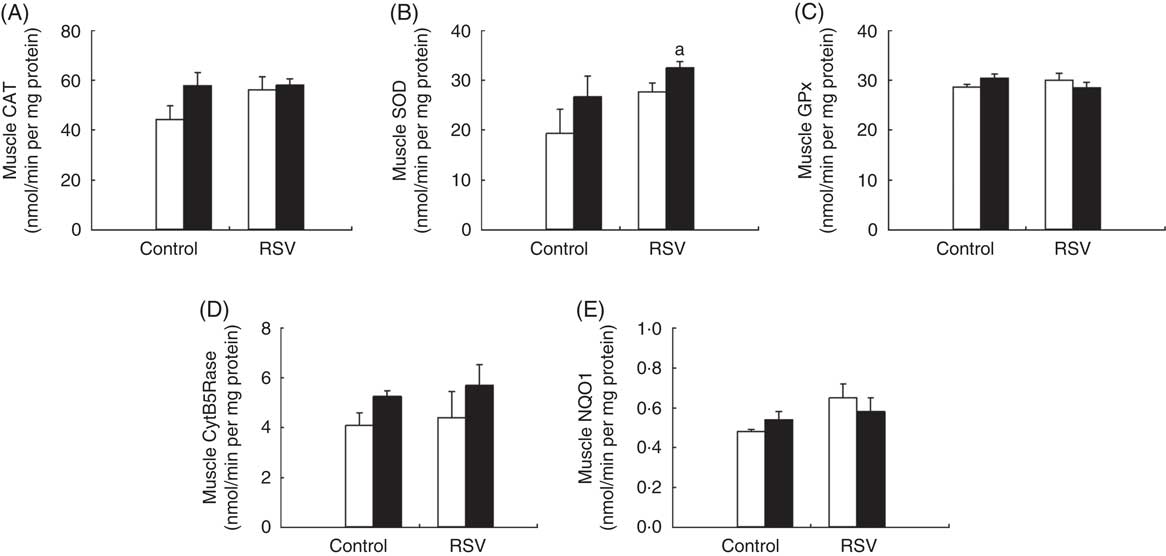
Fig. 5 Effect of resveratrol (RSV) and/or training on antioxidant activities of the gastrocnemius muscle in old mice. (A) Catalase (CAT), (B) superoxide dismutase (SOD), (C) glutathione peroxidise (GPx), (D) cytochrome B5 reductase (CytB5Rase) and (E) NAD(P)H quinone dehydrogenase 1 (NQO1) activities measured in nmol/min per mg protein. Values are means with their standard errors of different endogenous antioxidant activities in old animals. ![]() , Non-trained animals (sedentary); ■, trained animals. a Significant difference v. control and non-trained group, P<0·05, by using two-way ANOVA test.
, Non-trained animals (sedentary); ■, trained animals. a Significant difference v. control and non-trained group, P<0·05, by using two-way ANOVA test.
Interestingly, these low modifications in the activities were accompanied by significant changes at the protein level (Fig. 6). CAT protein levels were increased by training, RSV and their combination, P=0·007, P=0·019, P=0·032, respectively. Similar response was found with Cu/Zn SOD with significant increases, P=0·026, P=0·001, P=0·001, respectively. In the case of CAT, the effect of RSV was similar to that of training but higher in the case of SOD. No priming effect of RSV was found in these cases. No changes in the levels of CytB5Rase protein were found, whereas NQO1 was induced only by 37 % by RSV, P=0·037, but, interestingly, this induction was inhibited by training.
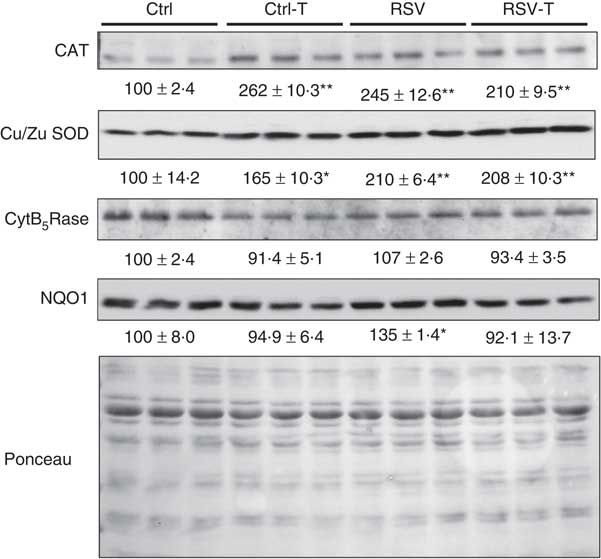
Fig. 6 Antioxidant protein levels in the gastrocnemius muscle of old animals fed resveratrol (RSV) and/or trained. Blots of catalase (CAT), Cu/Zn superoxide dismutase (SOD), cytochrome B5 reductase (CytB5Rase) and NAD(P)H quinone dehydrogenase 1 (NQO1) proteins determined by Western blotting. Ponceau, used as control loading, is also indicated. Levels indicated as the percentage of signal v. levels found in control group. Values are means with their standard errors of each group. * Significant difference v. control group, P<0·05; ** significant differences v. control group, P<0·01 by using one-way ANOVA test.
These results indicate that the lower oxidative damage found in the gastrocnemius muscle in old animals supplemented with RSV can be due, at least in part, by the induction of endogenous antioxidant defences.
Mitochondrial biogenesis is enhanced by both, resveratrol and exercise
It is known that CR and exercise induce mitochondrial biogenesis and then maintain higher muscle capacity( Reference Rodriguez-Bies, Santa-Cruz Calvo and Fontan-Lozano 29 ). In order to determine whether higher mitochondria biogenesis can be related to the higher capacity found in old animals fed RSV and trained, three different markers of mitochondrial amount were determined by Western blotting. Interestingly, cytochrome C levels were significantly higher in both RSV, P=0·03, and trained animals, P=0·006. The highest levels were found in trained animals supplemented with RSV, P<0·001 (Fig. 7(a)). mtTFAM is another marker of mitochondrial levels, and in this case RSV showed the highest effect reaching three times the levels found in control animals, whereas training doubled these levels, P=0·002 and P=0·049, respectively. The combination of RSV and exercise did not increase the levels already reached with RSV, P=0·003 (Fig. 7(b)). Finally, the mitochondria regulator NRF1 was only induced when both RSV and exercise were combined, P=0·008 (Fig. 7(c)). These results indicate that the combination of RSV and training induced mitochondrial biogenesis in the gastrocnemius muscle of old animals.
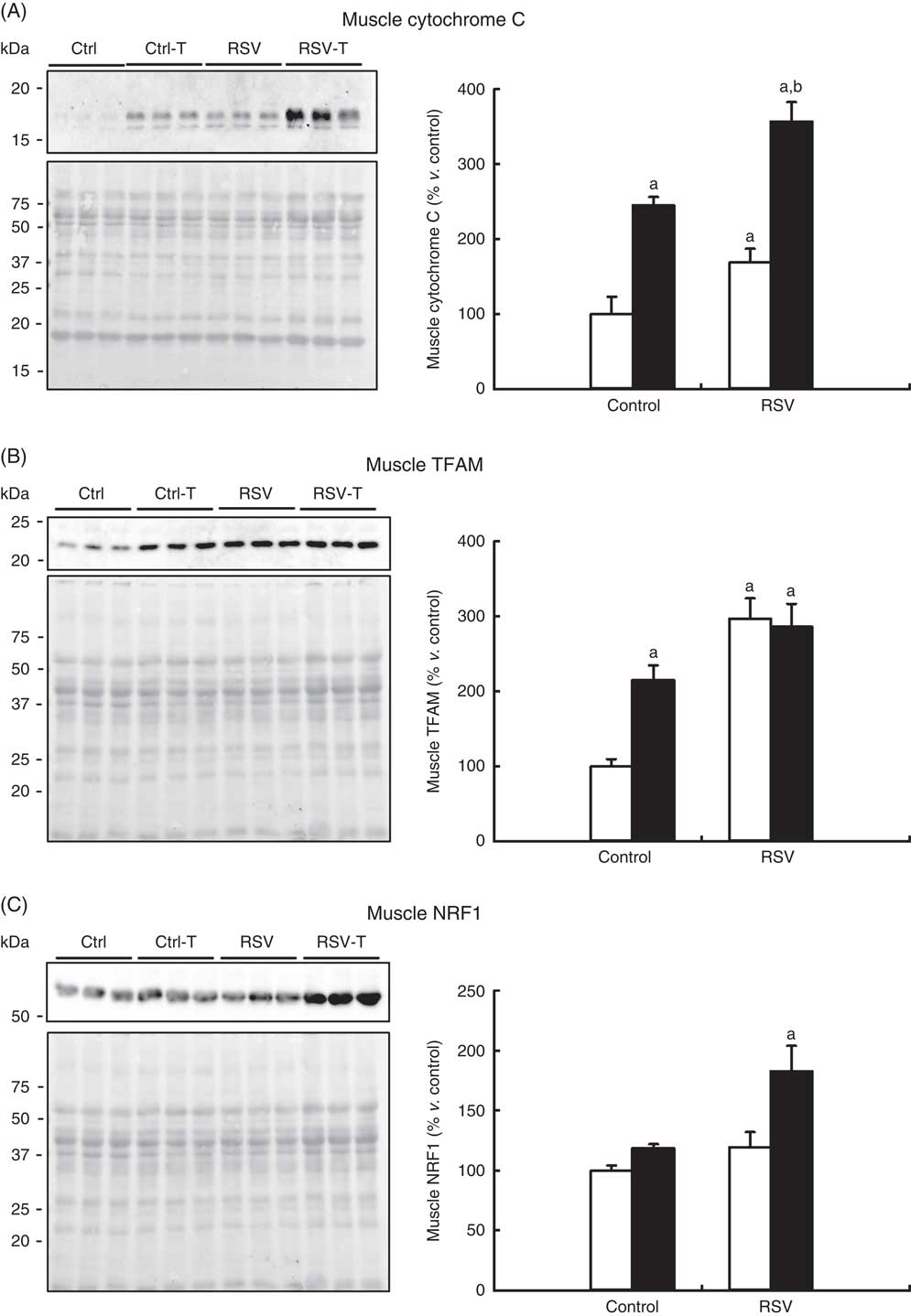
Fig. 7 Determination of mitochondrial mass markers in the gastrocnemius muscle of old mice. Western blotting of cytochrome C (A), TFAM (B), anti-nuclear respiratory factor-1 (NRF1) (C) and respective Ponceau S staining and densitometry quantification of blots. Quantification is represented as the percentage v. control group in mean values and standard deviations. a Significant difference v. control group, P<0·05; b significant differences v. resveratrol (RSV)-treated and non-trained group, P<0·05, by using two-way ANOVA test. ![]() , Sedentary; ■, trained.
, Sedentary; ■, trained.
Discussion
Our results demonstrate that a low daily intake of RSV primes the effect of exercise, increasing the performance in mature and particularly in old animals whereas no effects are shown in young animals. Importantly, the effect of RSV was obtained even when the animals started the supplementation with RSV at an advanced age. Thus, no lifelong treatment with RSV is needed to obtain a priming effect. Physical activity improvement was higher in old animals probably because of the higher protection against oxidative damage and the increase in mitochondrial mass in muscle found in these animals.
Our results are in agreement with a recent study performed in rats supplemented with a similar dose of RSV and exercised through swimming, where the combination of RSV and exercise increased the effect on the phosphatidylinositol-4,5-bisphosphate 3-kinase/protein kinase B/forkhead box O (PI3K/AKT/FOXO) pathway, on the reduction of TNF-α and apoptotic markers in the hearts of 18-month-old rats( Reference Lin, Lin and Ting 30 ). The age-dependent effect agrees with previous studies performed in mice fed under every-other-day CR model, which showed the same age-dependent effect in relationship with the antioxidant protection( Reference Rodriguez-Bies, Navas and Lopez-Lluch 31 ). This age-dependent effect has also been found in mice and rat fed under classical CR with higher effect in old than in young animals( Reference Lopez-Lluch, Rios and Lane 32 , Reference De Cabo, Cabello and Rios 33 ). Old animals suffered an increase in lipid peroxidation in comparison with young and mature animals and RSV was able to decrease these levels. This suggests that in conditions where oxidative stress occurs, RSV produces benefits probably by increasing the activity of endogenous antioxidants. Interestingly, we found the same result in previous studies using CR as a prolongevity effector( Reference Lopez-Lluch, Rios and Lane 32 , Reference De Cabo, Cabello and Rios 33 ). Old mice and rats under CR showed changes in antioxidant protection in liver plasma membrane, whereas young animals did not respond by modulating CytB5Rase and NQO1 activities and levels( Reference Lopez-Lluch, Rios and Lane 32 , Reference De Cabo, Cabello and Rios 33 ). The same effect was also found in the brain( Reference Hyun, Emerson and Jo 34 ). Interestingly, in humans, we have also found an age-dependent response to exercise in the protection against lipid peroxidation in blood plasma. Young individuals show low levels of lipid peroxidation in plasma in comparison with old people. Exercise did not produce changes in young people, whereas we found a direct relationship between the level of physical activity and coenzyme Q10 levels in plasma that is involved in the protection against oxidative damage of lipoproteins. In fact, higher physical activity only in old people increased coenzyme Q10 levels in plasma and decreased lipid peroxidation and LDL oxidation( Reference Del Pozo-Cruz, Rodriguez-Bies and Ballesteros-Simarro 35 , Reference Del Pozo-Cruz, Rodriguez-Bies and Navas-Enamorado 36 ). It seems that age is an important factor in the induction of antioxidant response by RSV, CR or exercise probably because of more delicate antioxidant equilibrium during ageing.
Several studies have indicated that polyphenols are considered antioxidants, but the clearest effects are that they modulate endogenous mechanisms against oxidative stress instead of acting directly as antioxidants( Reference Halliwell, Rafter and Jenner 37 ). This action is exerted by increasing the activity of endogenous antioxidant enzymes such as SOD, CAT, GPx and others and also by decreasing the activity of enzymes that produce reactive oxygen species in cells such as NADPH-oxidase or hypoxanthine/xanthine oxidase( Reference Carrizzo, Forte and Damato 38 ). In agreement with these studies, we have also recently found that RSV modulates liver antioxidant activities in old animals( Reference Tung, Rodriguez-Bies and Ballesteros-Simarro 23 ), in an effect that can be related with lower inflammation in this organ( Reference Tung, Rodriguez-Bies and Talero 39 ). Interestingly, the effect of RSV and also physical activity was organ dependent, being more clear in the liver, muscle and heart but less effective in the kidney and brain( Reference Tung, Rodriguez-Bies and Thanh 40 ).
Bioactive compounds such as polyphenols, CR and exercise also induce molecular mechanisms involved in mitochondrial biogenesis and turnover, avoiding the accumulation of damaged mitochondria in tissues, the increase in reactive oxygen species levels and accumulation of dysfunctional mitochondria( Reference Lopez-Lluch, Santos-Ocana and Sanchez-Alcazar 41 ). In fact, the accumulation of abnormalities in the mitochondrial electron transport chain has been associated with muscle fibre loss and sarcopenia in rats( Reference Bua, McKiernan and Wanagat 42 ). Recently, in an accelerated model of ageing, it has been shown that sarcopenia is affected by dysregulation of the control of mitochondrial quality( Reference Joseph, Adhihetty and Wawrzyniak 43 ). Exercise has been proposed to modulate the activity of the mitochondrial components affecting the development of sarcopenia, supporting the mitochondrial theory of ageing( Reference Johnston, De Lisio and Parise 44 ). Regulators of mitochondrial physiology and biogenesis such as PGC-1α that are induced by exercise or RSV have been associated with the prevention of loss of skeletal muscle mass and strength during ageing( Reference Wenz, Rossi and Rotundo 45 ). Taking these evidences into consideration, we can believe that bioactive compounds, CR and exercise can contribute to maintain a higher activity of organs and tissues during ageing and, in the case of skeletal muscle, to delay the reduction of physical capacity and age-related sarcopenia. Our results indicate that RSV in combination with exercise increases the amount of mitochondria and probably its capacity. The higher performance found in trained animals supplemented with RSV can be due to a higher capacity to increase fat oxidation as has been demonstrated with CR or RSV in high-fat-fed animals( Reference Lagouge, Argmann and Gerhart-Hines 10 , Reference Baur, Pearson and Price 11 , Reference Lopez-Lluch, Irusta and Navas 46 ). It is known that RSV increases fat metabolism in muscle( Reference Baur, Pearson and Price 11 , Reference Lopez-Lluch, Irusta and Navas 46 ), enhancing fatty acid oxidation and improving mitochondrial efficiency( Reference Chen, Zhang and Zheng 47 ). Moreover, RSV could be considered as an ergogenic compound improving muscle response to exercise and probably protecting muscle against weakness and sarcopenia during ageing.
Interestingly, it seems that RSV mimics the effects we have found with CR in muscle. In a previous study, we found that CR was able to increase physical capacity in young mice( Reference Rodriguez-Bies, Santa-Cruz Calvo and Fontan-Lozano 29 ). This effect has been related to a higher mitochondrial capacity in the gastrocnemius muscle determined by the increase in fat-consuming type I fibres and a better architecture of interfibrillar mitochondria, increasing the number and modifying their shape in this muscle( Reference Rodriguez-Bies, Santa-Cruz Calvo and Fontan-Lozano 29 ). The same effect has been found in old animals in which the combination of CR and exercise increased the performance of animals on the treadmill (Rodriguez-Bies, unpublished results). Muscle of old mice under CR and exercise also showed a higher number of type I fibres. This type of fibres was also increased by RSV in mice fed high-fat diets( Reference Lagouge, Argmann and Gerhart-Hines 10 ).
The most controversial problem with the effect of bioactive compounds is to determine whether the positive effects found in preclinical studies performed in model animals produce the same response in humans. Regarding the effect of different polyphenols on physical capacity in humans, different clinical trials carried out to date have been unsuccessful or show controversial results and further studies are needed. The amount of polyphenols, the duration of the supplementation and the type of exercise are important factors that affect the results. Taking into consideration the low dose used in our study, in humans, a dairy intake amount of 1200 mg/person (approximately 75 kg) would be needed. However, many of the doses used in humans are not higher than 500–600 mg/d.
A recent study suggested a RSV-dependent impairment in the effect of exercise in old men( Reference Gliemann, Schmidt and Olesen 20 ). These authors showed that the use of RSV in old men blunted the increase in maximal oxygen uptake found after training. On the other hand, another study of the same group showed that RSV inhibited the effect of exercise on protein carbonylation and TNF-α mRNA decrease in old skeletal muscle( Reference Olesen, Gliemann and Bienso 22 ). However, in these studies, the changes induced by exercise were minor and affected only a few variables of the total, and, in many of the cases, RSV and placebo showed the same effect as has been highlighted by other authors( Reference Smoliga and Blanchard 48 , Reference Buford and Anton 49 ). In another recent study, a low dose of RSV (100 mg/d) during 90 d in young military firefighters did not produce significant effects on antioxidant capacity against strenuous exercise but, contrary to the findings in old people( Reference Olesen, Gliemann and Bienso 22 ), RSV did reduce the levels of proinflammatory cytokines( Reference Macedo, Vieira and Marin 21 ). Furthermore, it has been shown that combination of RSV and quercetin in young athletes reduces the oxidation of lipids after exercise by mechanisms that are not related with their molecular antioxidant capacity( Reference McAnulty, Miller and Hosick 50 ). Another recent study performed with a moderate dose of RSV (500 mg) combined with 10 mg of piperine, an alkaloid found in black and long pepper, found improvement in mitochondrial capacity after light intense exercise in healthy young individuals( Reference Polley, Jenkins and O’Connor 51 ). Furthermore, a RSV dose of 600 mg/d for 7 d did not produce deleterious effects or positive effects in mature marathon runners (40–55 years), although a light trend to decrease inflammatory markers in plasma such as C-reactive protein and muscle soreness was reported( Reference Laupheimer, Perry and Benton 52 ).
As has been previously indicated, the different results found in studies regarding the use of different nutraceuticals in sport performance in humans indicate the need for more research on the effect of these compounds in humans( Reference Braakhuis and Hopkins 53 ). Probably human studies need to fix the correct dose of RSV and other polyphenols and the time of supplementation to determine the positive or negative effects of these compounds and the effect of different combinations of different polyphenols on human physiology. Our study suggests that the age of the organism is another important factor and indicates that the effect of these compounds can be more relevant in aged than in mature or young organisms. Whether this age-dependent effect can be extrapolated to humans remains to be determined. Probably higher doses of RSV or combinations with other polyphenols will produce better results. RSV can be a promising dietary supplement, which can enhance the physical capacity of elderly people and avoid to some degree age-dependent sarcopenia, permitting a more active and healthy ageing. Probably, a higher consumption of fresh and polyphenol-rich vegetables and other bioactive compounds that modulate mitochondrial activity in muscle could increase the efficiency of physical exercise even in the elderly, thereby increasing healthspan and delaying frailty.
Acknowledgements
The authors thank Rosario Rodríguez Griñolo for her advice in statistics.
The research group is financed by the Andalusian Government as BIO177 group through FEDER funds (European Commission). Research has been financed by the Spanish Government grant DEP2012-39985 (Spanish Ministry of Science and Innovation). T. B. T. received a fellowship from the AECID program (Spanish Ministry of Foreign Affairs). E. R.-B., P. N. and G. L.-L. are also members of the Centro de Investigación Biomédica en Red de Enfermedades Raras, Instituto Carlos III.
G. L.-L.: design, conduct, analysis and writing of the article; E. R.-B. and T. B. T.: development of the research and analysis of data; P. N.: analysis of the results and writing of the article.
The authors declare that there are no conflicts of interest.











