Environmental factors play significant roles in the onset of first-episode major depression during adolescence, although there is no clear-cut indication of which are the most sensitive and specific predictors (Reference Lewinsohn, Roberts and SeeleyLewinsohn et al, 1994; Reference Compas, Grant and EyCompas et al, 1994; Reference RutterRutter, 1994; Reference GoodyerGoodyer, 1995). Psychological risk processes include negative appraisal of social environments, low self-esteem and ruminative coping style (Reference Nolen-HoeksemaNolen-Hoeksema, 1991; Reference HammenHammen, 1992). Variations in steroid levels may also represent risk factors for negative affect and clinical depression in young people (Reference Paikoff, Brooks-Gunn and WarrenPaikoff et al, 1991). Higher dihydroepiandrosterone (DHEA) levels are associated with increased rates of aggression in boys and increased negative emotions in girls (Reference Buchanan, Eccles and BeckerBuchanan et al, 1992; Reference Brooks-Gunn, Petersen and CompasBrooks-Gunn et al, 1995). Basal cortisol hypersecretion predicts an increase in ‘sad’ mood in girls a year later but such mood is more associated with pre-existing depression than pre-existing hormone levels (Reference Susman, Dorn and ChrousosSusman et al, 1991). It is unclear whether changes in the levels of either of these two adrenal steroids precede the onset of clinical depression.
METHOD
Population ascertainment
Three secondary schools in Cambridge were approached and agreed to take part in the study, giving 1451 12- to 16-year-olds available for study. All parents were sent three screening questionnaires: a demographic form; a lifetime exit events questionnaire (covering bereavements and permanent separations); and the EAS temperament questionnaire focused on the adolescent (Reference Goodyer, Ashby and AlthamGoodyer et al, 1993). The EAS measures parent perceptions of general behavioural style and consists of 20 questions, each with a five-point response scale which has three anchor points: 1 - not typical of my child; 3 - fairly typical; 5 - very typical (points 2 and 4 are undefined). There are four sub-scales, ‘emotionality’, ‘activity’, ‘sociability’ and ‘shyness’, of five items each, with the mean scores indicating the degree to which an individual possesses the characteristics subsumed by the dimensions. The higher the score, the greater the level of the dimension (Reference Buss and PlominBuss & Plomin, 1984). Of the 1451 sets of parents, 731 (50%) returned completed screens.
A total of 1409 adolescents completed the life events screening questionnaire during school time, 42 being absent or unwilling; 1394 (99%) also agreed to complete a 38-item check-list of psychological symptoms covering current mood, feelings and anxieties extracted from four questionnaires: the Mood and Feelings Questionnaire (Reference Costello and AngoldCostello & Angold, 1988), the Revised Manifest Anxiety Scale (Reference Reynolds and PagetReynolds & Paget, 1983), the Rosenberg Self-Esteem Questionnaire (Reference RosenbergRosenberg, 1965) and the Leyton Obsessional Inventory (Reference Berg, Rapoport and FlamentBerg et al, 1986).
A comparison was made between the mean symptom check-list scores for adolescents whose parents did and did not return the questionnaires. There was no difference in the mean scores: 21.3 (s.d.=11.4) for returners (n=690) v. 21.6 (s.d.=10.8) for non-returners (n=704) (t=0.4, NS). There was also no difference in the number of life events reported by adolescents whose parents did and did not return forms (two or more events: returners 410/690 (59%) v. non-returners 411/704 (58%)). Since there were no parent data from non-returners it is not known whether there was any ascertainment bias on demographic features or family characteristics.
Definition of high risk
The greatest liability appears to exist for children exposed to the combination of chronic marital difficulties, maternal distress, recent life events and two or more lifetime losses (Reference RutterRutter, 1994; Reference GoodyerGoodyer, 1995). Recent undesirable life events exert their greatest risk when they occur as additive experiences in multiples of more than two over the previous 12 months (Reference Goodyer, Kolvin and GatzanisGoodyer et al, 1987). Although the temperamental trait of high emotionality increases risk for emotional disorders, it is unclear whether this effect operates in association with adverse environments. Since it is not known which combination of risk factors is most likely to result in subsequent onsets, a range of risks was combined in the screen procedure.
A subject at high risk was defined as any adolescent with two or more risk factors from the following list:
-
(a) two or more moderately to severely undesirable life events in the previous 12 months;
-
(b) current marital disharmony or past marital breakdown;
-
(c) two or more lifetime exit events (bereavement and/or permanent separation) of personal significance to the adolescent (i.e. involving a relative or friend);
-
(d) high (>80th percentile) emotionality.
In addition, the presence of the following factor by itself qualified a subject as high risk:
-
(e) a history of parental psychiatric disorder.
A comparison group of low-risk subjects, defined as those with none or only one of the risk elements (except (e)), was ascertained. Subjects with intermediate risk (n=330) were not included; these were individuals with one definite and one partial risk factor - for example, high emotionality plus one recent undesirable life event or one lifetime exit.
Adolescents were excluded from the study if they had a chronic and potentially life-threatening medical illness (including epilepsy, cancer, heart disease) (n=0), learning difficulties that required formal special educational assistance (n=5), or limited understanding of English (n=3).
Study entrants
All potential high-risk (n=250) and low-risk (n=102) subjects were invited to participate in the study itself. Of this sample of 352, 253 (72%) gave informed consent - 188 (75%) high-risk and 65 (64%) low-risk subjects. The proportion of subjects consenting was significantly greater for the high-risk group (Fisher's exact test, P=0.036). Inspection of the individual risk factors showed no differences between those who consented and those who did not for both genders. There were no age or gender differences between adolescents who did and did not agree to enter the study. Of those invited, the proportions agreeing to take part were 71%, 71%, 75% and 61% of school years 8-11, respectively (χ2=4.12, d.f.=3, P=0.248) and 73% of boys compared with 69% of girls (exact test, P=0.344). There was no difference in age distribution between the study sample and the total screen population (the study sample was 29%, 26%, 29% and 16% of school years 8-11, respectively, compared with the total screen sample 25%, 27%, 26%, 22% (χ2=5.5042, d.f.=3, P=0.138)). There was also no difference in the gender distribution (study sample: 46% boys, 54% girls; exact test, P=0.167; total screen sample: 51% boys, 49% girls; exact test, P=0.149).
At entry, all subjects completed a semi-structured interview which consisted of a brief version of a psychopathology assessment, the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS; Reference Kaufman, Birmaher and BrentKaufman et al, 1997) in order to ascertain whether the subject had been free of mental illness over the previous three months. This assessment took place within 4-8 weeks of the screen procedures. Seven of the high-risk group were then excluded on the basis of a recent (within three months of the day of assessment) positive psychiatric history (according to DSM-IV criteria (American Psychiatric Association, 1994) (five major depression with dysthymia, one eating disorder, one attention deficit disorder)). Individuals with depressive symptoms but without social or personal impairment were not excluded.
Pubertal stage was recorded from the entry interview and subjects classified as pre-pubertal (Tanner stage <1) or postpubertal (Reference TannerTanner, 1978). The current medications of all eligible subjects were recorded. Following these procedures, 181 high-risk (108 girls, 73 boys) and 65 low-risk subjects (22 girls, 43 boys) entered the study (see Fig. 1). There were significantly more girls than boys at high risk (exact test, P=0.001), and twice as many boys as girls at low risk. The subjects ranged in age from 12 years 2 months to 16 years 6 months (mean 13 years 9 months).
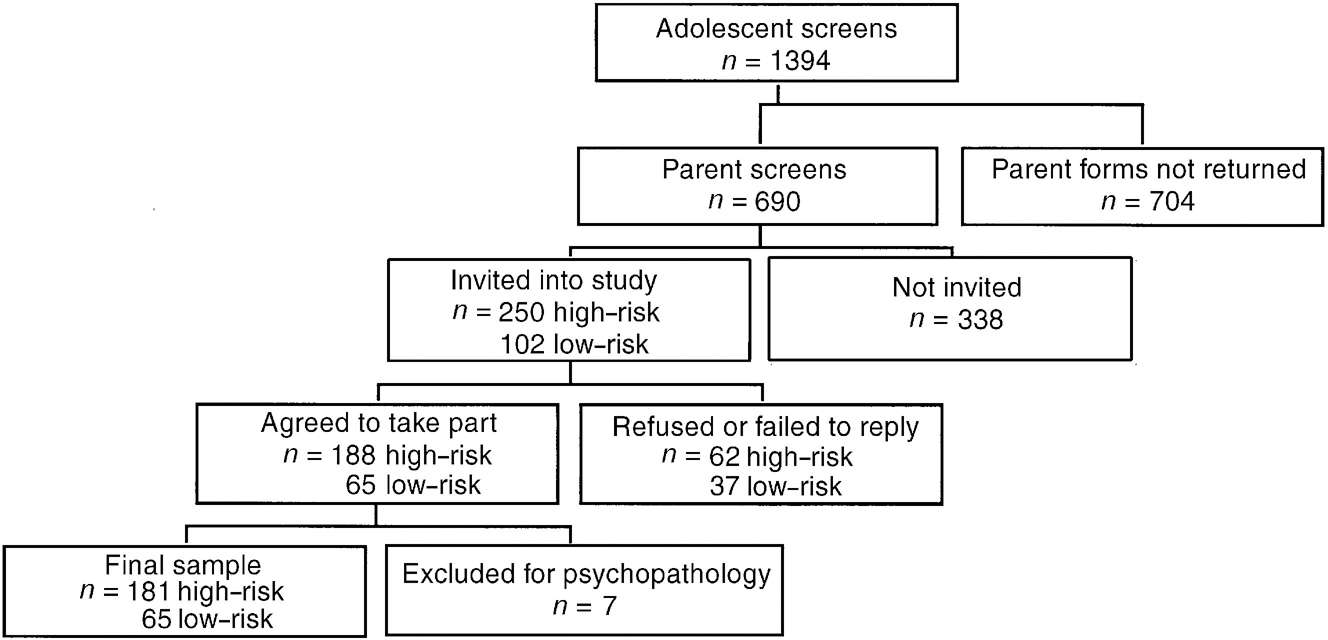
Fig. 1 Sample selection procedure.
Thirty-nine (17%) of these subjects were taking a steroid inhalant: betamethasone alone (n=10), salbutamol alone (n=12) or both (n=17). The proportions of low- and high-risk subjects taking these medications were not significantly different. Neither betamethasone nor salbutamol had a significant effect on either salivary cortisol or DHEA.
Measures
Subjects next completed a semi-structured psychosocial interview evaluating recent undesirable and desirable life events and the quality of recent friendships. There was good test-retest reliability for the occurrence of events (κ=0.75), significant agreement (κ=0.8) between maternal and adolescent perceptions of occurrence (with the exception of sexual behaviours, which are usually not reported by parents), and high agreement (κ=0.9) between subjective adolescent self-report and objective panel ratings of the degree of negative impact of an undesirable event over the next few weeks (Reference GoodyerGoodyer, 1996; Reference Goodyer, Herbert and TamplinGoodyer et al, 1997b ).
Neither gender nor age of the child influenced reliability and validity. Test-retest reliability (12 months apart) for each subscale was 0.8 or greater for either maternal or paternal report (Reference Wamboldt and ChipuerWamboldt & Chipuer, 1990).
Subjects also completed the following three self-report questionnaires. First, the Mood and Feelings Questionnaire (MFQ; Reference Costello and AngoldCostello & Angold, 1988; Reference Wood, Kroll and MooreWood et al, 1995), a 33-item questionnaire designed to cover the symptom areas specified in DSM-IV for major depressive disorder (MDD). The child form has good test-retest reliability (Pearson's ρ=0.78). Second, the Rosenberg Self-Esteem Questionnaire (Reference RosenbergRosenberg, 1965), a 10-item self-report questionnaire. The items are summed to give one score; higher scores indicate lower self-esteem. Third, the Response Style Questionnaire (Reference Nolen-HoeksemaNolen-Hoeksema, 1991), a 39-item self-report questionnaire, which measures what subjects think, feel or do when they experience low mood (not clinical depression). The scale has high internal consistency (Cronbach's α>0.85) and good evidence for discriminant validity and stability.
Hormone measures
Subjects provided samples of saliva at 08.00 and 20.00 h over four consecutive days within a week of the interview. From the four samples (assayed separately), mean levels of cortisol and DHEA were derived for each time point. Cortisol was measured by enzyme-linked immunosorbent assay (ELISA) on 20 μl samples of saliva without extraction (antibody supplied by Cambio UK). Intra-assay variation was 5.7%; interassay variation was 5.6%. DHEA was measured by validated radio-immunoassay (RIA) on 333 μl samples after extraction into hexane/ether (4:1) (antibody supplied by Bioclin UK). Intra-assay variation was 5.1%, inter-assay variation 7.4%. There is a good correlation between plasma and salivary levels for both steroids (Pearson's ρ =0.6 for cortisol, 0.9 for DHEA (Reference Goodyer, Herbert and AlthamGoodyer et al, 1996)). Salivary levels represented about 5% of those in the blood for both hormones.
Reassessment at 12 months
Subjects were reassessed after 12 months using the same measures. Of the 253 entrants, 234 (95%) - 62 (95%) low-risk and 172 (95%) high-risk - agreed to be reassessed. Interviewers were the same as at entry but were unaware of original risk status. Any positive response on the K-SADS short interview resulted in the full psychiatric interview (n=49) covering all psychopathology syndromes. An episode of major depression was deemed present if at any time over the previous 12 months a subject met DSM-IV criteria for such an episode.
Statistical strategy and procedures
Descriptive statistics were used to determine the prevalence of risk factors for the two groups for each gender. The number and combination of these risks at entry were also computed. The distribution of each risk factor between low- and high-risk groups and between genders was compared by using Fisher's exact tests. Correlations between self-report and hormone measures were calculated. Multivariate analyses of variance (MANOVAs) were used for group comparisons on cross-sectional data. Backwards stepwise logistic regression procedures were used to determine the best-fit model for the dependence of the ‘response’ variable, subsequent onset of MDD, on the self-report and hormone data at entry. Hormone data were transformed by taking the logarithm for multivariate analyses, because the raw data tend to have a very skewed distribution.
RESULTS
Characteristics of risk status
Table 1 shows the distribution of risk factors in low- and high-risk subjects. In the high-risk group the most frequent risk factor was the occurrence of two or more recent undesirable life events. These were reported significantly more often by girls than boys (exact test, P=0.001). In the low-risk group there were significantly more girls than boys with high emotionality (exact test, P=0.035). There were no gender differences in the frequency of other risk factors.
Table 1 Proportion of subjects in the study sample with each risk factor
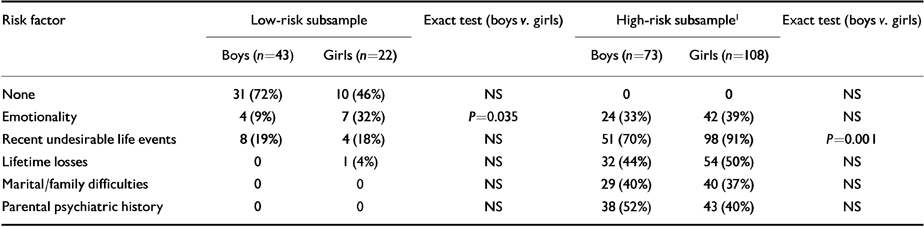
| Risk factor | Low-risk subsample | Exact test (boys v. girls) | High-risk subsample1 | Exact test (boys v. girls) | ||
|---|---|---|---|---|---|---|
| Boys (n=43) | Girls (n=22) | Boys (n=73) | Girls (n=108) | |||
| None | 31 (72%) | 10 (46%) | NS | 0 | 0 | NS |
| Emotionality | 4 (9%) | 7 (32%) | P=0.035 | 24 (33%) | 42 (39%) | NS |
| Recent undesirable life events | 8 (19%) | 4 (18%) | NS | 51 (70%) | 98 (91%) | P=0.001 |
| Lifetime losses | 0 | 1 (4%) | NS | 32 (44%) | 54 (50%) | NS |
| Marital/family difficulties | 0 | 0 | NS | 29 (40%) | 40 (37%) | NS |
| Parental psychiatric history | 0 | 0 | NS | 38 (52%) | 43 (40%) | NS |
There were no significant differences between the risk groups in parental age, social class, maternal age at birth of first child, parental educational attainments, or current employment hours outside the home for adults within the household. Parents of high-risk subjects reported more financial problems (exact test, P=0.001). There were no significant differences in maternal reports of developmental problems in the subject's early childhood (speech/language delay, motor control, sleeping habit, enuresis/encopresis). Seven subjects, all boys, were rated as pre-pubertal. Pubertal classification was not a significant factor in any of the ensuing analyses.
In the high-risk group the number of risks ranged from one to five. Five (3%) subjects had only one risk element (i.e. parental psychiatric risk); of the remainder, 71 (39%) had two, 61 (34%) had three, 33 (18%) had four and 11 (6%) had five risk elements. The median number was three for both genders.
Associations between affective-cognitive and hormonal measures
Among all 246 subjects, there were relatively large associations between the three cognitive measures (mood and feelings, self-esteem, ruminations: Pearson's ρ=0.5-0.7, P<0.001 in all cases). There were smaller associations between the four endocrine measures (cortisol at 08.00 h and 20.00 h and DHEA at 08.00 h and 20.00 h), particularly between 08.00 h and 20.00 h values of the same hormone (Pearson's ρ=0.2-0.7, P<0.01). By contrast, there were no significant associations between cognitive and endocrine measures (Pearson's ρ=0.03-0.16, P>0.05 in all cases).
Associations with risk status
Self-reports on mood and feelings, self-esteem and ruminative coping style
The mean scores on the three self-report scales for low-risk and high-risk samples and for both genders are shown in Table 2.
Table 2 Mean (s.d.) self-report scores at entry
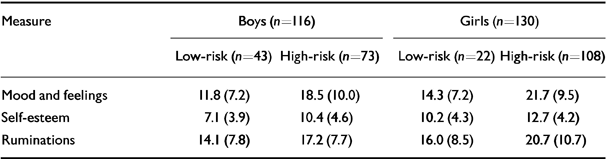
| Measure | Boys (n=116) | Girls (n=130) | ||
|---|---|---|---|---|
| Low-risk (n=43) | High-risk (n=73) | Low-risk (n=22) | High-risk (n=108) | |
| Mood and feelings | 11.8 (7.2) | 18.5 (10.0) | 14.3 (7.2) | 21.7 (9.5) |
| Self-esteem | 7.1 (3.9) | 10.4 (4.6) | 10.2 (4.3) | 12.7 (4.2) |
| Ruminations | 14.1 (7.8) | 17.2 (7.7) | 16.0 (8.5) | 20.7 (10.7) |
A full factorial MANOVA with scores on MFQ, self-esteem and ruminations as the ‘dependent variables’ showed no significant multivariate effect for the interaction of risk by gender (Pillai's exact F=0.768, d.f.=3,238, P=0.513). There was a significant multivariate difference between girls and boys (Pillai's exact F=5.96, d.f.=3,238, P=0.001), with main effects on MFQ score (F=4.28; d.f.=1,240, P=0.041) and self-esteem (F=16.48, d.f.=1,240, P=0.001) (both girls greater than boys), but only a trend for ruminations (F=3.48, d.f.=1,240, P=0.063).
There was also a significant multivariate difference between low- and high-risk groups (Pillai's exact F=9.07, d.f.=3,238, P=0.0001), with main effects on all three variables. MFQ score (F=26.01, d.f.=1,240, P=0.0001), self-esteem (F=19.34, d.f.=1,240, P=0.0001) and ruminations (F=7.74, d.f.=1,240, P=0.004) (all high-risk greater than low-risk). Age was not a significant covariate in any of these analyses (Pillai's exact F=1.53, d.f.=3,238, P=0.207).
Cortisol and DHEA levels at 08.00 h and 20.00 h
Table 3 shows mean levels of salivary cortisol and DHEA in the low-risk and high-risk groups for both genders. With the ‘dependent variable’ as the log of the four hormone measurements, there was no significant multivariate effect for the interaction of risk by gender (Pillai's exact F=1,288, d.f.=4,226, P=0.275). There was a significant multivariate difference between girls and boys (Pillai's exact F=4.86, d.f.=4,226, P=0.001), with main effects on all four hormone measurements: cortisol at 08.00 h (F=9.68, d.f.=1,229, P=0.002), cortisol at 20.00 h (F=9.01, d.f.=1,229, P=0.003), DHEA at 08.00 h (F=4.03, d.f.=1,229, P=0.046), DHEA at 20.00 h (F=9.85, d.f.=1,229, P=0.002) (all girls greater than boys).
Table 3 Cortisol and dihydroepiandrosterone (DHEA) concentration broken down by risk group and gender
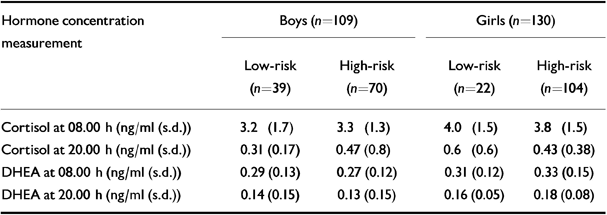
| Hormone concentration measurement | Boys (n=109) | Girls (n=130) | ||
|---|---|---|---|---|
| Low-risk (n=39) | High-risk (n=70) | Low-risk (n=22) | High-risk (n=104) | |
| Cortisol at 08.00 h (ng/ml (s.d.)) | 3.2 (1.7) | 3.3 (1.3) | 4.0 (1.5) | 3.8 (1.5) |
| Cortisol at 20.00 h (ng/ml (s.d.)) | 0.31 (0.17) | 0.47 (0.8) | 0.6 (0.6) | 0.43 (0.38) |
| DHEA at 08.00 h (ng/ml (s.d.)) | 0.29 (0.13) | 0.27 (0.12) | 0.31 (0.12) | 0.33 (0.15) |
| DHEA at 20.00 h (ng/ml (s.d.)) | 0.14 (0.15) | 0.13 (0.15) | 0.16 (0.05) | 0.18 (0.08) |
By contrast, there was no significant multivariate difference between the low-and high-risk groups (Pillai's exact F=0.118, d.f.=4,226, P=0.976). This was investigated further, treating the number of risk factors (0-5) as a covariate (instead of grouping risk into a two-level factor); the resultant MANOVA, allowing for age and gender, showed no significant dependence of the hormone data on the number of risks.
There was, however, a significant multivariate effect of age as a covariate (Pillai's exact F=8.82, d.f.=4,226, P=0.0001), with main effects for cortisol at 20.00 h (F=11.38, d.f.=1,229, P=0.001), DHEA at 08.00 h (F=23.36, d.f.=1,229, P=0.0001) and DHEA at 20.00 h (F=21.91, d.f.=1,229, P=0.001), with a trend for cortisol at 08.00 h (F=3.47, d.f.=1,229, P=0.064) (all older greater than younger).
Onsets of major depression by 12-month follow-up
The proportions of subjects with an episode of MDD at follow-up from low-and high-risk groups at entry, broken down by gender, are shown in Table 4.
Table 4 Onsets of major depression between baseline and 12-month follow-up

| Major depression | Low-risk (n=62) | High-risk (n=172) | ||
|---|---|---|---|---|
| Boys (n=41) | Girls (n=21) | Boys (n=68) | Girls (n=104) | |
| Absent (n=203) | 41 (100%) | 20 (95%) | 60 (88%) | 82 (79%) |
| Present (n=31) | 0 (0%) | 1 (5%) | 8 (12%) | 22 (21%) |
The findings show that of the 172 high-risk subjects, 30 (17%) had an onset of MDD during the subsequent 12 months compared with only one of the 62 subjects (2%) in the low-risk group (exact test, P=0.001). Within the high-risk group there was no significant gender difference in the proportion who became cases (girls: 22/104 (21%); boys: 8/68 (12%); exact test, P=0.15).
Fourteen subjects (five boys, nine girls) (45%) met MDD criteria at the time of the interview, while 17 (three boys, 14 girls) (55%) had onsets in the intervening 12 months and recovered (i.e. had fewer than two depressive symptoms at reassessment) by the time of interview. Nine (29%) of the MDD group were comorbid for one or more non-depressive diagnoses. By definition, all cases had clinically significant levels of personal impairment (equivalent to a children's global assessment rating from the K-SADS of less than 61).
None of the cases reported psychotic symptoms; one-third met specifiers for mild disorder while two-thirds met specifiers for moderate disorder and required further assessment by a psychiatrist (I.M.G.).
Risk factors at entry and subsequent MDD
The distribution of risk factors in the high-risk group between those who did and did not become depressed is shown in Fig. 2. There were no differences in the proportions of subjects exposed to any of the measured risk factors (all exact tests, P>0.1). No particular combination of two or more risks was significantly associated with MDD (all exact tests, P>0.1).
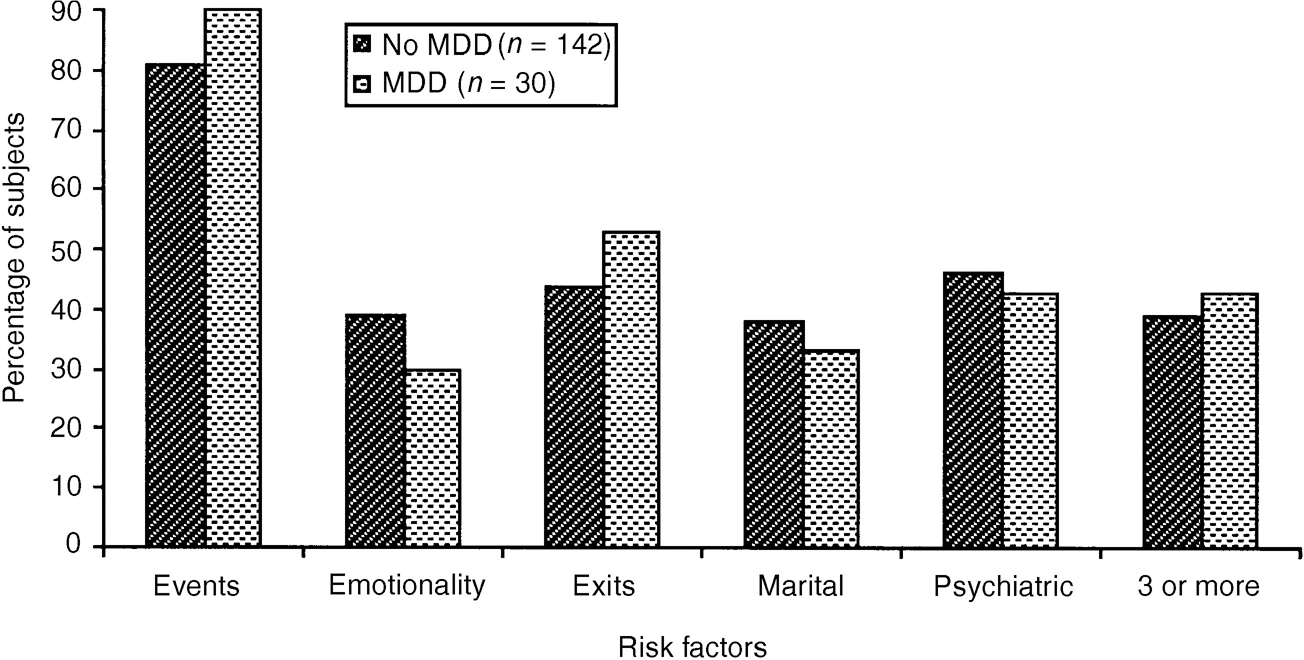
Fig. 2 Risk factors at entry for high-risk subjects with and without major depressive disorder (MDD) by follow-up.
Relative contributions of affective-cognitive and hormone factors at entry to subsequent onset of MDD
The presence or absence of MDD at 12 months was taken as the response variable and a backward stepwise logistic regression was carried out in order to determine the best fit of all the affective-cognitive and hormone variables at entry, together with age and gender, in the high-risk group. Factors were retained in the model if P<0.05. The findings, together with the raw scores for affective-cognitive and hormone data, and a summary of the best-fit logistic model, are shown in Tables 5,6,7.
Table 5 Contribution of affective-cognitive factors to the onset of major depressive disorder (MDD)

| Measure | Boys (n=68) | Girls (n=104) | ||
|---|---|---|---|---|
| Non-cases (n=60) | MDD (n=8) | Non-cases (n=82) | MDD (n=22) | |
| Mood and feelings (mean (s.d.)) | 17.4 (9.2) | 20.9 (12.3) | 20.6 (9.3) | 27.3 (8.2) |
| Self-esteem (mean (s.d.)) | 10.4 (4.6) | 9.8 (6.1) | 12.3 (4.2) | 15.1 (2.9) |
| Ruminatins (mean (s.d.)) | 16.6 (7.6) | 22.9 (6.2) | 20.3 (10.9) | 24.1 (9.3) |
Table 6 Contribution of endocrine factors to the onset of major depressive disorder (MDD)
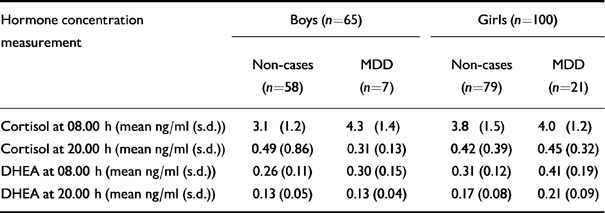
| Hormone concentration measurement | Boys (n=65) | Girls (n=100) | ||
|---|---|---|---|---|
| Non-cases (n=58) | MDD (n=7) | Non-cases (n=79) | MDD (n=21) | |
| Cortisol at 08.00 h (mean ng/ml (s.d.)) | 3.1 (1.2) | 4.3 (1.4) | 3.8 (1.5) | 4.0 (1.2) |
| Cortisol at 20.00 h (mean ng/ml (s.d.)) | 0.49 (0.86) | 0.31 (0.13) | 0.42 (0.39) | 0.45 (0.32) |
| DHEA at 08.00 h (mean ng/ml (s.d.)) | 0.26 (0.11) | 0.30 (0.15) | 0.31 (0.12) | 0.41 (0.19) |
| DHEA at 20.00 h (mean ng/ml (s.d.)) | 0.13 (0.05) | 0.13 (0.04) | 0.17 (0.08) | 0.21 (0.09) |
Table 7 Best-fit logistic model (see Table 6; further details available from the first author upon request)
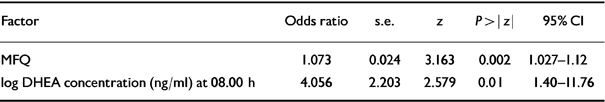
| Factor | Odds ratio | s.e. | z | P>|z| | 95% CI |
|---|---|---|---|---|---|
| MFQ | 1.073 | 0.024 | 3.163 | 0.002 | 1.027-1.12 |
| log DHEA concentration (ng/ml) at 08.00 h | 4.056 | 2.203 | 2.579 | 0.01 | 1.40-11.76 |
The backwards stepwise analysis discarded from the model all variables except MFQ scores and log DHEA concentration at 08.00 h. Table 7 shows that a unit increase in log DHEA at 08.00 results in an increment of 4.05 in the odds of being a subsequent MDD case, whereas for MFQ score the corresponding increment is 1.07.
DISCUSSION
Sample recruitment and risk definition
The procedure used in this study for the assessment of adolescents at risk proved acceptable to the cohort; refusals to complete the questionnaires in the classroom were minimal (<5%). There was a high non-cooperation rate (50%) from parents at the initial screen. In addition, the composition of the final sample indicates that more high-risk than low-risk families agreed to enter the study. Of the subjects who were chosen to be in the study, there were no differences on any risk factor between those who agreed to take part and those who did not. In addition, there was no difference in mean scores on the adolescent symptom check-list at screen between those whose families agreed to take part and those whose families did not.
Low risk as defined here is unusual for this population of adolescents. It is possible that a randomly ascertained non-high-risk comparison group would have given less striking differences on the cognitive measures. This issue was indirectly addressed by comparing two subsamples of the low-risk group - those with one risk factor (n=24) and those with none (n=41). No differences were found on MFQ score at entry (13.4 (s.d.=6.0) v. 12.2 (s.d.=8.0), F=0.4, d.f.=1,64, P=0.52), but both subgroups were significantly different from the high-risk group. This suggests that one risk may be insufficient to elevate concurrent self-reported depressive symptoms.
Reporting of recent life events
Significantly more girls than boys were detected as being at high risk, while, conversely, significantly more boys were at low risk, owing to higher rates of recent undesirable life events reported by girls. This finding is in accord with previous studies showing a gender-related bias in self-reported information, suggesting that girls are more likely than boys to appraise similar proximal environments as personally threatening (Reference Brooks-GunnBrooks-Gunn, 1991).
Affective-cognitive and endocrine correlates of risk
Cognitive self-reports
Girls reported higher levels of depressive symptoms and lower levels of self-esteem than boys regardless of risk, a finding that agrees with previous work on self-esteem in adolescents (Reference Block and RobinsBlock & Robins, 1993). Our finding of a trend for a more ruminative style in females is also in accord with previous research on young adults (Reference Nolen-Hoeksema and GirgusNolen-Hoeksema & Girgus, 1994).
Those at high risk had significantly higher scores on the MFQ, consistent with previous research on adolescents (Reference Lewinsohn, Hops and RobertsLewinsohn et al, 1993). Lower self-esteem and more ruminations were also associated with high risk. These findings suggest that there is a risk-associated set of negative cognitions and cognitive coping style for both genders. The three affective-cognitive measures are highly correlated. This may be because they measure either the same construct or different components of a complex affective-cognitive process.
Endocrine correlates
Salivary cortisol and DHEA levels at both time points were higher for girls than boys. These gender differences in salivary cortisol were unexpected but robust and novel. This striking finding needs further investigation in the context of the gender-related differences in cognitive appraisal of life events and rates of MDD, since salivary and cerebrospinal fluid levels seem similar (Reference Guazzo, Kirkpatrick and GoodyerGuazzo et al, 1996) and corticoid levels are known to influence mood.
The endocrine data showed no association with high-risk status for either gender. The absence of any correlate with the measured risk factors or self-reports suggests that the variation in levels of hormones may arise from more distal origins than recent life events and current ongoing difficulties.
Correlates of major depression
The 12-month prevalence rate of major depression in female adolescents in the Cambridge area has been estimated at 6% (Reference Cooper and GoodyerCooper & Goodyer, 1993). The present study found rates over three times greater (21%) for high-risk girls, providing evidence that the risk criteria were valid. However, it is possible that the rates of major depression were somewhat inflated by interviewer bias. The same interviewers conducted both initial and follow-up interviews, and although they were unaware of risk status at reassessment it was not always possible to remain blind during the interview. However, using the same interviewers had tangible advantages in achieving high compliance rates at follow-up.
No particular combination or number of risk factors was associated with subsequent cases of MDD. Although 90% of cases were preceded by two or more undesirable life events in the 12 months before entry, two or more such events also occurred in 80% of the high-risk group who did not become depressed. Since 82% of the high-risk group did not report an episode of major depression at follow-up, there are likely to be other factors (e.g. intervening life events) that contributed to the transition from high risk to clinical disorder.
Depressive feelings, self-esteem, ruminative style and MDD
None of the cases met criteria for any depressive disorder, including dysthymia, in the three months prior to entry. Lifetime psychiatric histories were not taken, so it is not possible to state that the episodes reported arose in previously well subjects. Previous findings from both community and clinical studies suggest that around half of the cases may have met criteria for a non-depressive episode in childhood, but less than 5% will have had a major depressive disorder (Reference KovacsKovacs, 1997). Higher scores on the MFQ were significantly associated with the subsequent onset of major depression. Among these cases, MFQ scores at entry ranged from 7 to 48, with 50% scoring 25 or below. A score of 27 or greater gives a sensitivity and specificity of 0.8 for current clinical depression (Reference Wood, Kroll and MooreWood et al, 1995); subjects with such high scores may have had incipient major depression at the time of entry. However, of 54 high-risk subjects with scores of more than 26 (70th percentile and above in this sample) only 15 (28%) had an onset during the follow-up period. Incipient depression sufferers might be expected to reach onset earlier in the follow-up period, but there were no differences in MFQ scores between those with onsets in the six months closest to entry (25.7 (s.d.=8.8)) and those with onsets in the six months furthest (25.4 (s.d.=11.0)) from entry.
Overall, the findings suggest that some type of dysfunctional affective-cognitive process precedes and contributes to the onset of first-episode major depression in adolescents. Further investigation should include more detailed and experimental measures of current mood and cognitions in order to complement the use of self-reports (Reference Ingram, Miranda and SegalIngram et al, 1998).
Salivary cortisol and DHEA concentration at entry and subsequent MDD
Higher DHEA concentration at 08.00 h was associated with subsequent MDD. This finding held even after controlling for age. Whether cortisol is also associated with subsequent disorder remains unclear, partly because the behavioural roles of cortisol and DHEA at this stage in the life-cycle remain relatively unexplored (Reference Brooks-Gunn, Petersen and CompasBrooks-Gunn et al, 1995). The implications of these findings require further investigation before any firm conclusions can be reached.
The pattern of salivary steroids at entry associated with subsequent MDD is unlike that found during MDD itself. Current depression is associated with high evening cortisol and low morning DHEA concentration in about 40% of this age group (Reference Goodyer, Herbert and AlthamGoodyer et al, 1996). Cortisol to DHEA ratios in depressed children have been found to predict subsequent adverse social experiences and the duration of illness (Reference Goodyer, Herbert and AlthamGoodyer et al, 1997a ). These differences suggest that the contribution of these steroids to the onset of disorder may be different from that associated with persistent depression.
Overall, the findings support the notion that variations in adrenal steroid function are not simply a consequence of MDD but indicate a potential contribution of the steroid milieu to the onset of first-episode disorder in adolescents.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Girls appraise recent life events as more personally threatening than boys.
-
▪ Environmental risks and higher self-reported depression scores are necessary but insufficient factors to explain subsequent depressive onsets.
-
▪ Recognising undetected major depression in adolescents at secondary schools should be encouraged and supported by National Health Service trusts.
LIMITATIONS
-
▪ The non-cooperation rate may limit the generalisability of the findings.
-
▪ Self-report measures of depressive mood thoughts and behavioural style are not sufficiently sensitive to access cognitive structure and process.
-
▪ Sample size limits the power of the statistical analysis of gender differences in the cortisol and dihydroepiandrosterone (DHEA) profiles.
ACKNOWLEDGEMENTS
We thank Sandi Secher for organising data collection and entry, Carol Stott and Deborah Jones for help with interviewing and Helen Shiers and Sarah Cleary for carrying out the hormone assays. This study was supported by a project grant from the Wellcome Trust.












eLetters
No eLetters have been published for this article.