Despite markedly improved disease prevention since the introduction of universal infant vaccination, pertussis (whooping cough) is still one of the leading causes of vaccine-preventable deaths. Whereas disease incidence in young children has been declining since the introduction of vaccination in paediatric age groups, the number of reported cases across all other age groups has increased in many European countries during the past decade [Reference Zepp1]. The underlying mechanism of this evolution is not well understood, and is probably multifactorial: waning immunity in adults, probably related to reduced exposure more than 50 years after successful introduction of mass vaccination, increased awareness of physicians coupled to an easier diagnosis by PCR and finally, possible changes in virulence of the circulating pertussis strains. In a 20-year follow-up study, we previously reported that the age distribution in Belgium of cases diagnosed by serology has shifted from children aged <5 years in 1990 to teenagers and adults in 2009 [Reference Vincent2]. Whereas disease symptoms are generally mild in adults, in unvaccinated or not fully vaccinated infants aged <1 year, pertussis has an atypical evolution with little cough but with episodes of apnoea that can be life threatening. These very young infants represent the highest burden in terms of mortality.
As in other industrialized countries, there is evidence for increased circulation of Bordetella pertussis in Belgium. In 2011, the two partner laboratories of the Belgian National Reference Centre for B. pertussis, reported a total of 243 confirmed (PCR or serology) cases of acute infection with B. pertussis. In 2012 this figure doubled to 496 cases, of which 183 were confirmed by PCR/culture and 302 by serodiagnosis (only nine of these cases were confirmed by both assays). Whereas more than half (118, 64%) of the PCR-positive cases were diagnosed in children aged < 9 years, 237 (78%) of the positive cases detected by serology were in adults and the elderly (aged >20 years). Moreover, as pertussis is generally mild in adults, positive cases may have remained undiagnosed and their total number is likely to be underestimated.
To obtain a more complete estimate of the circulation of B. pertussis in a population, seroepidemiology has become a valuable complement in surveillance programmes based on reporting systems. Antibodies to pertussis toxin (anti-PT) have been used as a useful aetiological marker. This antibody is specific for B. pertussis although it correlates poorly with protection. Serosurveys offer the opportunity not only to study waning immunity but also to assess the ‘antigenic pressure’ in the population. After omitting age groups vaccinated within the last 2 years it is possible to estimate the proportions of ‘recent infections' [Reference Hallander3]. For that purpose, cut-off levels above indicative concentrations of anti-PT are chosen. Such estimates are useful on a cohort basis for comparisons between countries and over time but less accurate for individual diagnostic serology. There are no recent seroepidemiological figures for Belgium. The last official report, published in 2003, concerned samples collected in Flanders between April 1993 and February 1994 [Reference Van der Wielen4]. The threshold value for positivity in that study was set at 5 EU/ml, which is completely different from the values used in the present study. In 2006, age-specific seroprevalence of measles, mumps, rubella, diphtheria and tetanus were reported for Belgium, but unfortunately pertussis was not included in this study [Reference Theeten5].
Here, we show the results of a seroprevalence study on 1500 anonymized leftover diagnostic samples collected randomly during the second semester of 2012 by the clinical chemistry laboratories of six participating hospitals, distributed equally between Flanders, Wallonia and Brussels Capital Region. Six laboratories, two from each region collected a total of 250 samples, 125 samples from patients aged 20–29 years and 125 samples from patients aged 30–39 years living in their region. Hence, a total number of 750 samples from each age group was analysed. These age groups were prioritized for the Eupert-Labnet WP6 seroprevalence study, because they were representative of the reproductive age groups, important to monitor as a possible reservoir. For Flanders, samples were collected in the province of East Flanders by UZ Gent (postal codes 9000–9999) and in the province of West Flanders by AZ Sint-Jan (postal codes 8000–8999). For Wallonia, samples for the province of Liège were collected by CHU Liège (postal codes 4000–4999) and for the province of Hainaut by CHU Charleroi (postal codes 6000–6999). For Brussels Capital Region, samples were collected by the laboratory of UZ Brussel (postal codes 1000–1210) and CHU Saint-Pierre (postal codes 1000–1210). A map of the regions and provinces of Belgium can be viewed at http://www.crwflags.com/fotw/flags/be(r.html and http://www.crwflags.com/fotw/flags/be(.html, respectively.
Samples were given consecutive numbers for the study, without reference to the analysis number. Apart from first three digits of the postal code (there were no complete codes to assure anonymity), only date of birth was recorded. Since the sampling was totally anonymous, it was the responsibility of the local laboratory to ensure that the same patients were not sampled twice. As recommended by Eupert-Labnet WP6 and in order to obtain comparable data of anti-PT antibodies from the different member states, sera were analysed using Virion-Serion ELISA (Institut Virion\Serion GmbH, Germany), according to manufacturer's instructions. Briefly sera were diluted 1:100 and tested in duplicate for presence of total anti-PT IgG antibodies. Antibody levels were expressed in international units (IU/ml). Data were analysed using predefined cut-offs of 100 IU/ml and 50 IU/ml as indicators of a recent infection or an infection in the last 2 years, respectively [Reference Hallander3]. This anti-PT ELISA is specific for B. pertussis and has a good linearity down to 3 IU/ml.
Results are shown in Figure 1(a–c) for Flanders, Wallonia and Brussels Capital Region, respectively, and summarized in Table 1.
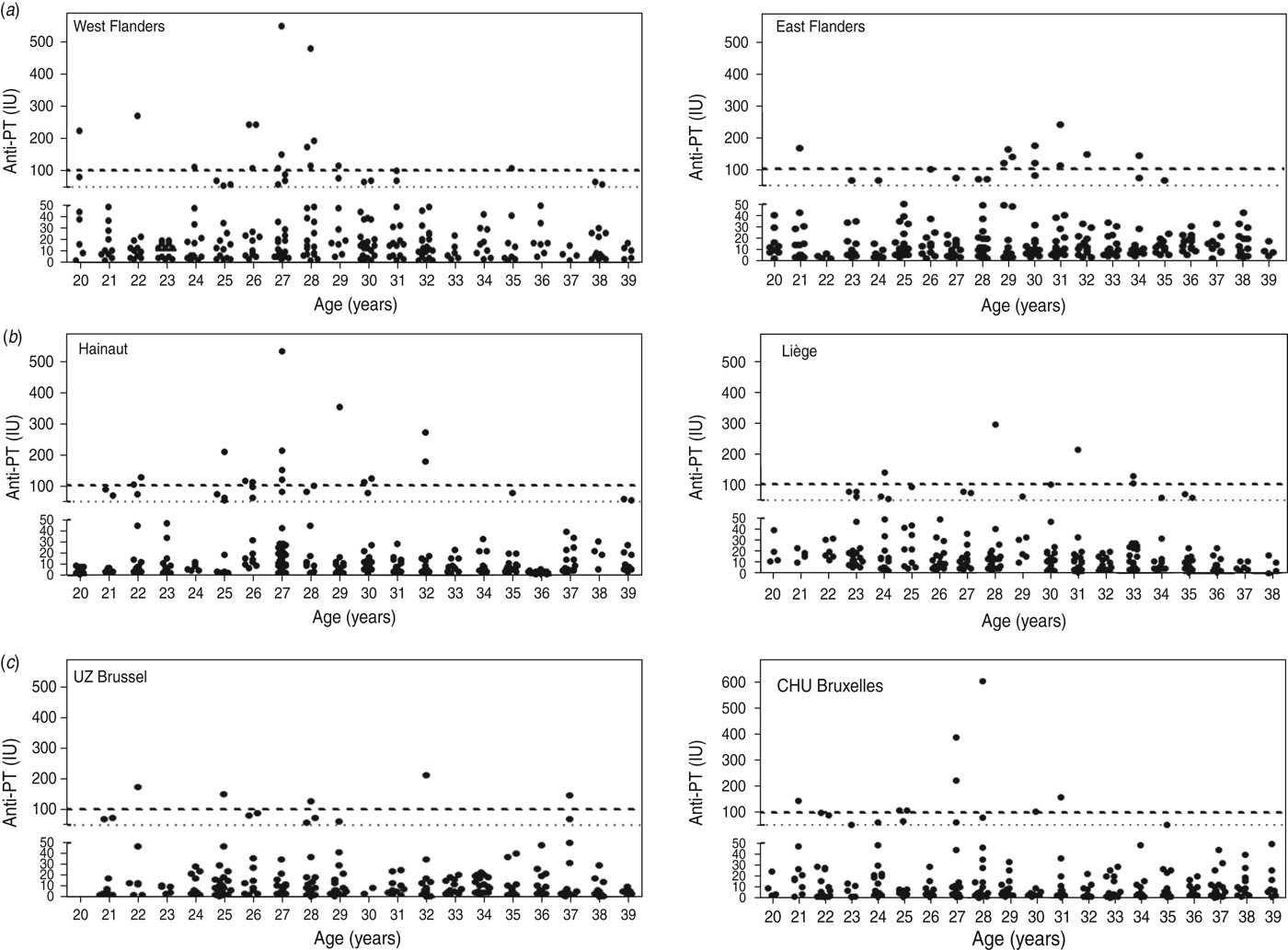
Fig. 1. Anti-PT antibody levels (expressed in IU/ml) detected in adults aged 20–40 years selected from two hospitals each in (a) Flanders, (b) Wallonia and (c) Brussels Capital Region. Cut-off values of 50 IU/ml and 100 IU/ml are indicated by a fine dotted line (····) and a bold dashed line (- - - -), respectively.
Table 1. Number of sera with anti-PT IgG titres indicative of recent or acute pertussis infection
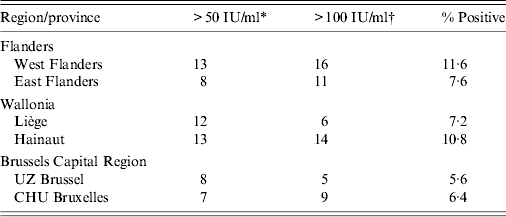
* Anti-PT IgG titre reflecting probable pertussis infection during the past 2 years.
† Anti-PT IgG titre reflecting probable acute pertussis infection.
Although positive cases were detected in all ages, in Flanders 74·1% (20/27) of serum samples indicative of acute infection were found in the 26–32 years age group. Overall, there was a higher seroprevalence in West Flanders (29 cases, 11·6%) than in East Flanders (19 cases, 7·6%). In Wallonia, positive cases were detected in all ages, but 65% (13/20) of serum samples indicative of acute infection were found in the 26–32 years age group, with higher seroprevalence in the province of Hainaut (27 cases, 10·8%) than in the province of Liège (18 cases, 7·2%). Finally, overall seroprevalence in Brussels Capital region was somewhat lower than in Flanders or Wallonia and comparable for the two collecting centres, i.e. 5·6% for UZ Brussels and 6·4% for CHU Bruxelles. Positive cases were detected in all age groups, although the three sera with highest IgG levels were in two individuals aged 27 years (221 IU/ml, 340 IU/ml) and one aged 28 years (606 IU/ml).
Although we have not covered the whole Belgian territory in our study, our results are strongly suggestive of a B. pertussis reservoir in the adult Belgian population. Universal vaccination against pertussis (coupled to tetanus and diphtheria) was started in Belgium in 1959. Polio is the only mandatory vaccine in Belgium, but the diphtheria-tetanus-pertussis (DTP) vaccine is included in the recommended schedule of childhood vaccinations. In 2001, the cellular pertussis component in DTwP vaccine based on whole bacteria was replaced by the acellular component in DTaP. The subjects in our study were aged 20–39 years (born between 1972 and 1992) and those that were vaccinated, received the whole cell-based pertussis vaccine, reported to induce lower levels of anti-PT antibodies than acellular vaccines [Reference Olin6, Reference Hendrikx7]. Positive cases were detected in all age groups, but perhaps somewhat unexpectedly, the majority of the cases were not detected in the oldest subjects (in whom waning of immune response would probably be the strongest), but rather in the 26–32 years age group. As serum samples were anonymized, it is not possible to know whether the higher titres in this age group were the result of booster vaccinations administered in the context of a cocoon vaccination or the actual reflection of a higher prevalence of B. pertussis in this age group, which are mostly young parents. Although cocoon vaccination is offered to future or young parents in Belgium, the procedures are tedious and so far the strategy is not implemented on a large scale in Belgium. A recent study by Wiley et al. reported on a meta-analysis of sources of pertussis infection in young children and concluded that most identified sources were indeed from the household, of which 39% were mothers, 16% fathers and 5% grandparents [Reference Wiley8].
Neither vaccination nor infection induces a lifelong protection against pertussis and waning is thought to occur 4–12 years after the last booster dose or 7–20 years after an episode of illness [Reference Wendelboe9]. As discussed by de Greeff and colleagues [Reference de Greeff10], investing in expensive recurring vaccination programmes for adults to boost their immunity can be questioned, as pertussis symptoms are relatively mild in adults (as confirmed by 4% of cases of acute infection we detected in apparently healthy adults), and natural boosting of their immunity may result in only a few cases of severe disease [Reference Aguas, Goncalves and Gomes11]. It is clear that a further reduction in severe cases of infant pertussis will require serious commitment and new vaccination approaches beyond infancy, such as vaccination of healthcare workers or ‘cocoon’ vaccination of mothers of newborns and of household contacts of infants and toddlers [Reference DeMaria and Lett12, Reference Forsyth13]. Booster vaccination of women during pregnancy is an alternative that has recently been recommended in the USA [Reference Munoz14]. However, the development of improved vaccines that induce long-lasting immunity and do not require regular boosting may be needed to ultimately control this infectious disease.
ACKNOWLEDGEMENTS
We thank Professor H. Hallander (Swedish Institute for Infectious Diseases) for initiating the European Eupert-Labnet WP6 serosurveillance study. We are grateful to Dr J. M. Collard (WIV-ISP) for critically reading the manuscript.
The protocol of this study was approved by the Ethics Committee of the Universitair Ziekenhuis Brussel (B.U.N. 143201214680).
DECLARATION OF INTEREST
None.




