Introduction
Submitral aneurysm is a relatively uncommon disease characterised by aneurysmal dilation of the left atrioventricular junction resulting from weakness in the posterior annulus of the mitral valve that can lead to heart failure. Reference Manuel, Sousa-Uva and Miguel1–Reference Rajbanshi, Rajbhandari and Pradhan3 Although many reported cases have involved individuals of African ancestry, cases have been reported worldwide. Reference Rajbanshi, Rajbhandari and Pradhan3,Reference Ribeiro, Mendes, Vicente, Menardi and Evora4 Herein, we report the case of a patient with a ruptured submitral aneurysm in the left atrium with severe regurgitation who underwent successful mitral valve repair and aneurysmal correction.
Case report
Written informed consent was obtained from the patient.
A 26-year-old black woman presented with a 1-year history of progressive dyspnoea. Physical examination revealed a grade 3/6 systolic murmur in the mitral valve area. The physical examination and laboratory test results were unremarkable. Chest radiography revealed cardiomegaly with a cardiothoracic index of 0.63. Electrocardiography showed a sinus rhythm with a heart rate of 69 beats/min and signs of left atrial dilatation with no other alterations. A transthoracic two-dimensional echocardiogram revealed a saccular formation arising from the posterior leaflet of the mitral valve (Fig 1A). The aneurysm ruptured into the left atrium (Fig 1B), generating regurgitation in Doppler colour (Figs 1C and 1D). The patient underwent open-heart mitral valve surgery by transatrial approach, and after left atriotomy, a saccular aneurysm ruptured into the left atrium, as demonstrated by echocardiography (Fig 2A). The aneurysm was removed (Fig 2B). An aneurysmal form was observed in the region where the posterior annulus was destroyed; it was closed with a bovine pericardium and a separated suture. Subsequently, the posterior annulus and the leaflet were reconstructed with autologous pericardium fixed in glutaraldehyde solution (Fig 2C). A flexible artificial ring (No. 32; Medtronic Inc., Minneapolis, MN, United States of America) was also implanted (Fig 2D). A saline test was performed, and the results were satisfactory. The cardiopulmonary bypass time was 62 min, and the x-clamping time was 57 min. Intraoperative echocardiography revealed no aneurysms or regurgitations (Fig 2E). Post-operative transthoracic echocardiography showed a transvalvular gradient of 2.7 mmHg with satisfactory left ventricular function. The patient remained asymptomatic after 2 months of follow-up.

Figure 1. Pre-operative transesophageal two-dimensional echocardiogram: a shows the aneurysmal sac in the left atrium (white *). b reveals the ruptured aneurysm into the left atrium (white arrow). c and d show a severe regurgitation primarily caused by the aneurysm rupture. LA = left atrium; LV = left ventricle.
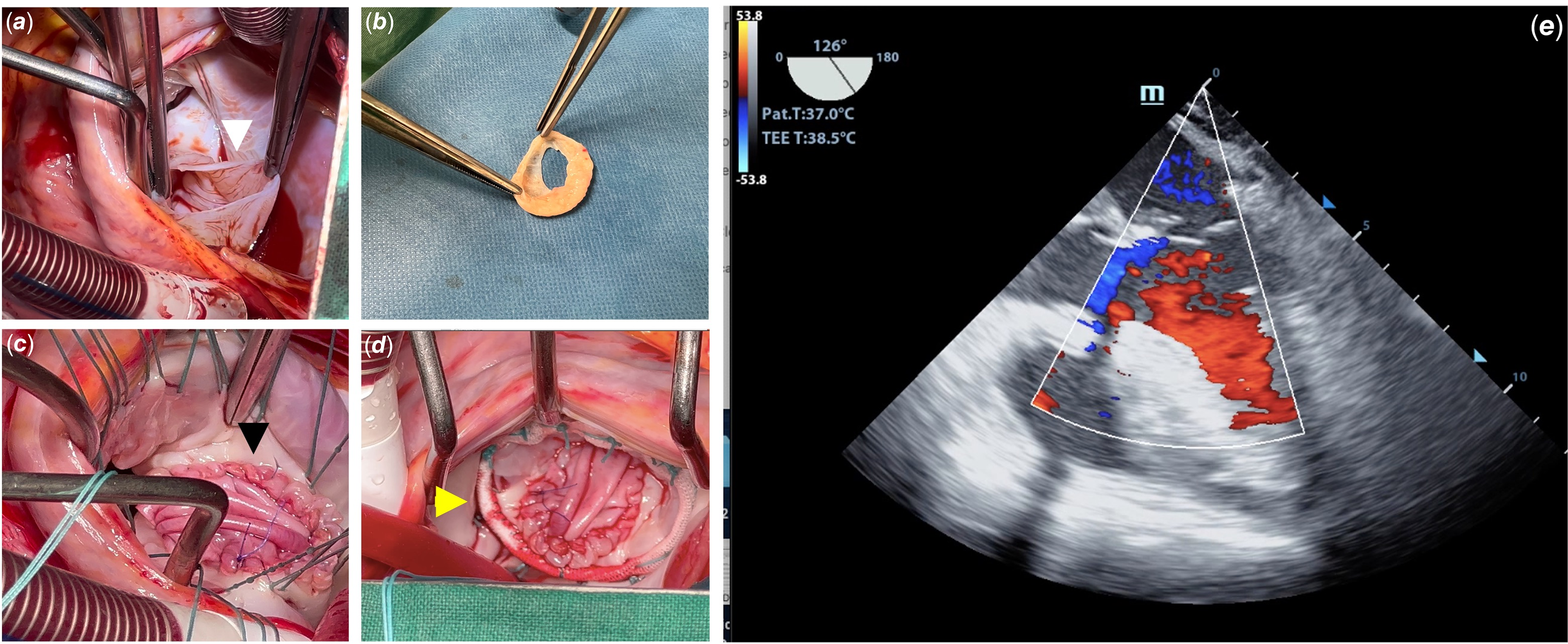
Figure 2. Surgical findings, approach, and post-operative: a shows the aneurysmal sac ruptured into the left atrium (white arrow). b shows the aneurysmal sac with the exit hole for the left atrium. c demonstrates the aspect after the annulus reconstruction and posterior leaflet enlargement with autologous pericardium (black arrow). d shows the prosthetic ring used to reinforce the ring (yellow arrow). e shows a post-operative transesophageal two-dimensional echocardiogram with no residual aneurysm or mitral valve regurgitation. Ao = aorta; LA = left atrium.
Discussion
Submitral aneurysm is defined as an aneurysmal dilatation of the left atrioventricular junction resulting from weakness in the posterior annulus of the mitral valve. Reference Manuel, Sousa-Uva and Miguel1,Reference Manuel5 Submitral aneurysms are most commonly reported in young black adults with acquired diseases such as tuberculosis or previous mitral valve surgery, but no acquired aetiology has been proven. Reference Manuel, Sousa-Uva and Miguel1,Reference Morais, Manuel and Costa2,Reference Manuel5 However, reports of submitral aneurysms in children suggest a potential congenital aetiology, although this remains unproven. Reference Manuel, Sousa-Uva and Miguel1,Reference Méot, Bajolle and Bonnet6 Our patient was a black woman without a history of infectious diseases or previous cardiac surgery; therefore, CHD appears to be a plausible aetiology in our patient. Clinically, patients present with heart failure, cardiac murmur, and cardiothoracic index enlargement based on the size and whether the aneurysm has ruptured. Reference Morais, Manuel and Costa2,Reference Morais8 Our patient presented with all these clinical findings. Two-dimensional transthoracic echocardiography is the gold standard diagnostic tool; however, chest CT and MRI can be useful for surgical planning. Reference Manuel5,Reference Morais, Sousa-Uva, Martins, Manuel and Costa7,Reference Morais8 In our case, no other imaging modalities were required. Surgery is the treatment of choice and involves closing the aneurysmal neck. When the aneurysm is large, the procedure must be performed with bovine pericardium, and whenever possible, with valve-sparing, although mitral valve replacement may be required and has been performed in many cases. Reference Manuel, Sousa-Uva and Miguel1,Reference Méot, Bajolle and Bonnet6,Reference Purushotham, Manohar, Sivasubramaniam and Neelakandhan9 Repairs are preferable to replacements. In our case, we obtained a favourable outcome with no complications or adverse events. The patient remained asymptomatic during the follow-up period.
Data availability statement
The data underlying this article will be shared by the corresponding authors upon reasonable request.
Acknowledgements
None.
Author contribution
Valdano Manuel performed the surgery and idealised, wrote, and revised the manuscript.
Marília da Costa performed the surgery and acquired the images.
Joseney Carvalho performed the surgery and care for the patient in the post-operative period.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
The authors declare none.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the institutional committee.





