Introduction
Less than 15 per cent of older adults 60 years of age and older meet recommendations of at least 150 minutes/week of moderate-to-vigorous physical activity (MVPA) (Colley et al., Reference Colley, Garriguet, Janssen, Craig, Clarke and Tremblay2011; Troiano et al., Reference Troiano, Berrigan, Dodd, Masse, Tilert and McDowell2008) and as few as 5 per cent regularly perform 2 days/week of both strength and balance training (Bennie et al., Reference Bennie, Pedisic, van Uffelen, Gale, Banting and Vergeer2016; Merom et al., Reference Merom, Pye, Macniven, van der Ploeg, Milat and Sherrington2012). Physical inactivity is a leading modifiable risk factor for cardiovascular and other chronic diseases and fall-related injuries, and contributes substantially to the increasing global health care costs (Janssen, Reference Janssen2012; Warburton, Nicol, & Bredin, Reference Warburton, Nicol and Bredin2006). Previous studies have demonstrated the efficacy of physical therapist (PT) or other health promoter-led exercise interventions to improve health outcomes and reduce fall risk in older adults (Clemson et al., Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012; Pahor et al., Reference Pahor, Guralnik, Ambrosius, Blair, Bonds and Church2014; Sherrington et al., Reference Sherrington, Michaleff, Fairhall, Paul, Tiedemann and Whitney2016). However, a feasible and cost-effective model of delivery for exercise prescription in a real-world setting remains unknown (Katz, Lambert-Lanning, Miller, Kaminsky, & Enns, Reference Katz, Lambert-Lanning, Miller, Kaminsky and Enns2012). Integration of exercise into lifestyle activities combined with behaviour change counselling can enhance exercise adoption and adherence (Weber et al., Reference Weber, Belala, Clemson, Boulton, Hawley-Hague, Becker and Schwenk2018) and overcome common barriers to exercise (e.g., time, access to facilities, aversion to structured exercise) (Costello, Kafchinski, Vrazel, & Sullivan, Reference Costello, Kafchinski, Vrazel and Sullivan2011). Therefore, lifestyle-based functional exercise may represent a generalizable and sustainable strategy to increase physical activity (PA) levels (Clemson et al., Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012; Dunn et al., Reference Dunn, Marcus, Kampert, Garcia, Kohl and Blair1999), prevent falls (Clemson et al., Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012) and manage chronic disease in older adults (Clemson et al., Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012; Dunn et al., Reference Dunn, Marcus, Kampert, Garcia, Kohl and Blair1999). Yet, there is limited evidence on how to effectively implement lifestyle exercise interventions into practice, especially for sedentary older adults with multiple chronic conditions.
Primary care represents a real-world setting for identifying older adults in need of PA intervention. However, recent primary care-based exercise referral or counseling trials have not been associated with any major or statistically significant increases in PA in community-dwelling middle-aged and older adults (Fitzsimons et al., Reference Fitzsimons, Baker, Gray, Nimmo and Mutrie2012; Fortier et al., Reference Fortier, Hogg, O’Sullivan, Blanchard, Sigal and Reid2011; Knight & Petrella, Reference Knight and Petrella2014; Lawton et al., Reference Lawton, Rose, Elley, Dowell, Fenton and Moyes2008). Additionally, there is limited evidence on their effectiveness in improving longer-term objectively-measured MVPA levels in inactive older adults (≥75 years of age) (Pavey et al., Reference Pavey, Taylor, Fox, Hillsdon, Anokye and Campbell2011). Therefore, we need to identify evidence-based PA programs for older adults with the potential for real-world implementation, including effectiveness in improving PA, health, and functional outcomes. Clemson et al. (Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012) found that teaching older adults how to integrate functional strength and balance exercises into daily life activities (known as the Lifestyle-Integrated Functional Exercise (LiFE) program) was associated with an increase in self-reported PA, a reduction in fall rate, and improvements in balance and lower limb strength, as compared with controls. The LiFE program is unique in that it includes theory-driven behaviour change strategies (e.g., action planning, habit reforming) (Clemson & Munro, Reference Clemson, Munro and Pachana2017) as well as exercise training elements (e.g., balance, muscle strengthening) (Clemson et al., Reference Clemson, Singh, Bundy, Cumming, Weissel and Munro2010). LiFE also demonstrates acceptable adherence (64% completed balance and strength activities ≥3 days/week) and retention rates (76% completed follow-up assessments) in older adults with a high risk of falling (Clemson et al., Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012). Few clinical trials of exercise move beyond establishing efficacy to test implementation in primary care or other health care settings. Thus, a group-based version of the LiFE program (referred to herein as Mi-LiFE) was evaluated as a more generalizable and resource-effective strategy for implementation in an interprofessional primary care context.
The objectives of our pilot study were to evaluate the feasibility, potential effectiveness, and implementation of the Mi-LiFE program in a primary care-based family health team (FHT) practice for older adults 75 years of age and older. The primary objectives were related to feasibility outcomes (recruitment, retention, and adherence) and aimed to determine over the 6-month implementation period: (1) the number of participants that we could recruit, (2) retention at follow-up, and (3) adherence to the Mi-LiFE program. It was hypothesized that the Mi-LiFE program would be considered feasible without modifications if 30 participants were recruited (Lee et al., Reference Lee, Patel, Costa, Bryce, Hillier and Slonim2017); 75 per cent of participants were retained (Clemson et al., Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012); and 50 per cent of participants completed the LiFE strength and balance activities ≥ 3 days/week (Clemson et al., Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012). Secondary objectives of this pilot study evaluated the potential effectiveness of the Mi-LiFE program in improving participants’ PA levels, physical performance, and quality of life. We also examined process outcomes to inform future trials and implementation. A detailed description of the study objectives and hypotheses has been published elsewhere (Gibbs et al., Reference Gibbs, McArthur, Milligan, Clemson, Lee and Boscart2015).
Materials and Methods
Study Overview
We conducted a pre-post pilot study to evaluate the feasibility, potential effectiveness, and implementation of the Mi-LiFE program delivered by a PT in a primary care-based FHT practice (ClinicalTrials.gov: NCTO2266225). In Ontario, Canada, FHTs consist of groups of health professionals (family physicians, nurses, pharmacists, and other interdisciplinary health care providers) working together to provide primary care using a patient-centred approach (Rosser, Colwill, Kasperski, & Wilson, Reference Rosser, Colwill, Kasperski and Wilson2010). In the present study, the FHT setting comprised 18 family physician practices with a combined population of 27,997 patients (Lee et al., Reference Lee, Patel, Costa, Bryce, Hillier and Slonim2017).
The Reach Effectiveness Adoption Implementation Maintenance (RE-AIM) framework was used to guide the selection, evaluation, and reporting of implementation outcomes (Glasgow, Vogt, & Boles, Reference Glasgow, Vogt and Boles1999), which was described in detail elsewhere (Gibbs et al., Reference Gibbs, McArthur, Milligan, Clemson, Lee and Boscart2015). The Mi-LiFE program involved one individual session and four group sessions with a PT, which occurred every 1–2 weeks, and two follow-up phone calls. Participants completed assessments at baseline and after 6 months. All assessments and Mi-LiFE program sessions took place at the same site affiliated with the FHT practice. The study protocol was published according to the Consolidated Standards of Reporting Trials (CONSORT) extension for pilot and feasibility trials (Eldridge et al., Reference Eldridge, Chan, Campbell, Bond, Hopewell and Thabane2016) and Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statements (Chan et al., Reference Chan, Tetzlaff, Altman, Laupacis, Gotzsche and Krleza-Jeric2013). Research ethics approval was received at the University of Waterloo and Hamilton Integrated Research Ethics Boards.
Participants
We planned to recruit a minimum of 30 individuals or 15 per cent of potentially eligible individuals based on data collected from the FHT geriatric screening program (Lee et al., Reference Lee, Patel, Costa, Bryce, Hillier and Slonim2017). The FHT geriatric screening was a part of a larger, comprehensive program to identify older adults who are frail (including physically inactive) and might be at risk for co-morbid conditions underlying their frailty. We used two recruitment modes: (1) the FHT geriatric screening program participant pool, which was established between April 2013 and June 2014 (prior to the program start date) and (2) in-clinic physician/nurse referral from the FHT geriatric screening program, which took place prospectively over a 6 month period (July – December 2015). Before regularly scheduled medical appointments, all patients aged 75 years and older without an acute illness were screened for PA levels through the FHT geriatric screening program (Gibbs et al., Reference Gibbs, McArthur, Milligan, Clemson, Lee and Boscart2015) and asked to choose which statement best described their activity status (Topolski et al., Reference Topolski, LoGerfo, Patrick, Williams, Walwick and Patrick2006):
1. Not physically active beyond moving around or walking during activities of daily living;
2. Physically active occasionally or during certain seasons more than others;
3. Physically active and participating in ≥ 30 minutes of moderate-intensity physical activity ≥ 5 days/week.
Patients who were physically inactive or only occasionally active were approached at the clinic by their physician or nurse who would ask them whether they were interested in receiving notification about future exercise programs offered at the FHT practice (recruitment mode 1) or specific participation in the Mi-LiFE program (recruitment mode 2). If interested, the research coordinator called the potential participants to describe the Mi-LiFE program and invite them to enroll. Physicians of interested participants assessed whether their patient met the eligibility criteria, and ruled out exercise contraindications (American College of Sports Medicine, 2013).
Participants were eligible for the program if they were: (1) ≥75 years of age and (2) able to understand instructions in English. Exclusion criteria were: (1) current participation in lower-extremity strength and balance training ≥ 3 days/week, (2) a known diagnosis of dementia, (3) any significant lung disease or moderate-to-severe chronic obstructive pulmonary disease (COPD), and (4) exercise contraindications as determined by their FHT physician. A two stage screening process was used to determine final clearance for capacity to consent (Gibbs et al., Reference Gibbs, McArthur, Milligan, Clemson, Lee and Boscart2015). If participants had cognitive impairment, they were required to attend the program with a caregiver. Regardless of cognitive status, all participants were encouraged to attend the program with a spouse/partner, family member, or caregiver. If the spouse/partner, family member, or caregiver was over 65 years of age, they were invited to complete all assessments. All participants provided written informed consent.
Mi-LiFE Program
The theoretical basis of the original LiFE program and the details pertaining to the strength and balance training exercises have been previously described (Clemson et al., Reference Clemson, Singh, Bundy, Cumming, Weissel and Munro2010; Clemson et al., Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012). Strength LiFE activities were tied to specific strength training principles that were taught in the program (e.g., move slowly—bend your knees or squat; increase the number of times that you use a muscle—sit-to-stands). Balance LiFE activities were tied to specific balance training principles (e.g., reduce your base of support—tandem stand; shifting weight to the limits of stability—lean from side-to-side). The Mi-LiFE program was delivered by a PT over one individual and four group sessions, and two follow-up phone calls after completion of the final group session (approximately week 6) and 1 month later (approximately week 10) (Clemson et al., Reference Clemson, Singh, Bundy, Cumming, Weissel and Munro2010; Clemson et al., Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012). The PT received training on the delivery of the LiFE program through the review of the LiFE Trainer’s Manual (Clemson & Munro, Reference Clemson and Munro2014b) and instruction from Dr. Clemson (creator of LiFE program) via teleconference. A pre-pilot test of the program with four older adults 76–85 years of age was conducted to test the format and delivery of the program sessions and evaluate the PT’s fidelity. The pre-pilot study findings were published elsewhere (Gibbs et al., Reference Gibbs, McArthur, Milligan, Clemson, Lee and Boscart2015). In brief, we learned that the LiFE Trainer’s Manual is an adequate resource for deliverer training; LiFE can be adapted as a group format; and participants are highly satisfied with the group program aside from some challenges with planning the activities and preference for more demonstration.
In the individual session, the PT administered: (1) a 10 item LiFE functional balance and strength assessment tool and (2) a daily routine chart (Clemson & Munro, Reference Clemson and Munro2014b; Clemson et al., Reference Clemson, Singh, Bundy, Cumming, Weissel and Munro2010). All participants received a participant’s manual (Clemson & Munro, Reference Clemson and Munro2014a). Participants were taught the LiFE strength and balance training principles and how to document their plans, and execution of the activities using an activity planner (Clemson & Munro, Reference Clemson and Munro2014b). In the group sessions, the PT worked with the participants to determine how and where they could embed the strength and balance activities into their daily routine; increasing the intensity and upgrading the activities in small groups formed by convenience (five or fewer people). The prescribed frequency was daily or “as often as you can” with the activities ideally done multiple times throughout the day. The PT and participants discussed ways to increase the number of times they performed each activity safely and effectively (e.g., where and when to embed each activity) and how to move more (e.g., park further away, take the stairs instead of the elevator).
The PT used the LiFE model of behaviour change to encourage self-efficacy and adherence to the program, which included positive reinforcement, habit reforming, discussing benefits, and self-monitoring (Clemson & Munro, Reference Clemson, Munro and Pachana2017; Clemson et al., Reference Clemson, Singh, Bundy, Cumming, Weissel and Munro2010; Clemson et al., Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012). During the group sessions, activities were planned as a group and ideas for how, when, and where to perform the activities were shared among participants and recorded using the activity planner. Participants demonstrated the activities in the group setting, which represented peer-to-peer learning opportunities among participants (Burke, Carron, Eys, Ntoumanis, & Estabrooks, Reference Burke, Carron, Eys, Ntoumanis and Estabrooks2006). All participants were encouraged by the PT to continue with the program on their own following the final group session. In the follow-up phone calls, the PT discussed the progression of the LiFE activities, strategies to increase PA, and any successes and challenges related to the program, which lasted 5–10 minutes in length.
Feasibility Outcomes
The primary outcomes were related to feasibility, including the number of participants who were recruited and retained over 6 months (recruitment and retention), and the number of days/week that the exercises were completed (adherence). Retention was defined as completing or partially completing (questionnaires only) the 6 month follow-up assessment. Adherence was self-reported daily on calendar-style diaries and as attendance rates for the five sessions. Adherence was 100 per cent if a participant completed strength and balance activities ≥ 3 days/week. For participants who had missing data or chose to discontinue diary completion, adherence was retrospectively self-reported by phone or in person during follow-up. We analyzed adherence data wherein the withdrawals were included and considered non-adherent or the withdrawals were excluded from the analysis to examine the sensitivity of the withdrawals on the adherence results. We also reported on the combined adherence to the program sessions (four or more sessions) and the home exercise participation (≥ 3 days/week over 6 months) as a post-hoc analysis of the program maintenance at the participant level. Participants who withdrew prior to the program (n = 4) were not included in the adherence analyses.
Based on the results of the present pilot feasibility study, the program will be either (Eldridge et al., Reference Eldridge, Chan, Campbell, Bond, Hopewell and Thabane2016; Thabane et al., Reference Thabane, Ma, Chu, Cheng, Ismaila and Rios2010): (1) not feasible, (2) feasible with modifications, (3) feasible with close monitoring, or (4) feasible without modifications.
Change in PA, Physical Performance, and Quality of Life
Participants wore a commercially available accelerometer (ActiGraph GT3x, ActiGraph, Pensacola, FL 32502) over the hip for 7 days following baseline assessment, and then again for the 7 days after follow-up as an objective measure of PA levels. Acquisition and analysis protocols for the accelerometer data were previously reported (Gibbs et al., Reference Gibbs, McArthur, Milligan, Clemson, Lee and Boscart2015). Cut-points were applied to objectively determine sedentary time (hours/day) (≤ 100 counts/minute), light PA (100–1,952 counts/minute) and MVPA (minutes/week) (≥1,952 counts/minute) (Freedson, Melanson, & Sirard, Reference Freedson, Melanson and Sirard1998). Tri-axial data were analyzed in 60 second epochs. Non-wear time was excluded if ≥ 60 minutes of continuous zeros (Troiano et al., Reference Troiano, Berrigan, Dodd, Masse, Tilert and McDowell2008). Only participants who wore the accelerometer for at least 4 days and 10 hours/day were analyzed (Chase, Lockhart, Ashe, & Madden, Reference Chase, Lockhart, Ashe and Madden2014).
Changes in self-reported MVPA (minutes/week), walking (minutes/week), and sedentary time (hours/day) were measured using the International Physical Activity Questionnaire (IPAQ) at baseline and follow-up. Additional non-validated questions were added to ask participants about the amount of time that they had engaged in strength and balance activity (minutes/week) during the past 7 days. Reliability and validity data of the IPAQ have been reported (Craig et al., Reference Craig, Marshall, Sjostrom, Bauman, Booth and Ainsworth2003). Both accelerometer- and IPAQ-measured PA levels were compared with the initial self-reported PA classification to determine representativeness of the participants (Topolski et al., Reference Topolski, LoGerfo, Patrick, Williams, Walwick and Patrick2006). Physical performance was measured using the validated Short Physical Performance Battery (SPPB) (4 m walk test, five times sit-to-stand test, and timed stance balance tests) at baseline and 6 months (Guralnik et al., Reference Guralnik, Simonsick, Ferrucci, Glynn, Berkman and Blazer1994). A score on the SPPB of 9 or lower is indicative of physical limitations and higher risk of major mobility disability. Health-related quality of life was assessed using the EuroQol Five-Dimensional Questionnaire with 3 Levels (EQ5D-3L) at baseline and 6 months. The EQ5D visual analogue scale (VAS) self-perceived health rating (0–100) and mobility, self-care, usual activities, pain/discomfort, and anxiety/depression subscale results were reported. The mean (standard deviation [SD]) EQ5D VAS rating in a population-based survey of adults 70–79 years of age was 75 (19) (Kind, Dolan, Gudex, & Williams, Reference Kind, Dolan, Gudex and Williams1998). Evidence of construct validity and good test–retest reliability for the EQ5D-3L has been shown (Brazier, Jones, & Kind, Reference Brazier, Jones and Kind1993).
Implementation Outcomes
Implementation outcomes, including barriers and facilitators, participant feedback on the program, and fidelity, were measured according to the RE-AIM framework to summarize lessons learned and provide a guide for adaptation in future trials (Glasgow et al., Reference Glasgow, Vogt and Boles1999; Harden et al., Reference Harden, Smith, Ory, Smith-Ray, Estabrooks and Glasgow2018). Barriers and facilitators to implementation from the perspectives of the PT, research staff, and participants (and caregivers if necessary) were identified using a variety of methods including a success/challenge tracker, journaling, and in-person or teleconference semi-structured interviews (Gibbs et al., Reference Gibbs, McArthur, Milligan, Clemson, Lee and Boscart2015). A success/challenge tracker was filled out by the research coordinator throughout the 6 month implementation period. PTs also kept a journal of notes detailing their experience delivering the program and follow-up phone calls. Semi-structured interviews were conducted in person with the research coordinator, PT, and all participants at the 6 month follow-up. The interviews with the research coordinator and PT included open-ended questions about barriers and facilitators to implementation and program delivery (successes/challenges, lessons learned, available resources, group format modifications, feasibility for future larger-scale implementation). The interviews with the participants included open-ended questions to understand their experience and level of satisfaction with the program (reasons for joining the program, observed benefits, areas for improvement, what they liked/disliked, general strategies for PA).
Two members of the research staff coded data from these sources (research team’s success/challenge tracker, PT journal notes, interviews transcribed verbatim) for major categories of information using descriptive content analysis and identified the emerging themes (Graneheim & Lundman, Reference Graneheim and Lundman2004). Coding and final themes were developed using a process of collaboration and consensus amongst the reviewers via memoranda and analysis meetings. Using the behaviour change wheel (BCW) framework, final themes related to barriers and facilitators were categorized into capability, opportunity, and motivation (COM-B model) (Michie, West, Campbell, Brown, & Gainforth, Reference Michie, West, Campbell, Brown and Gainforth2014). Trustworthiness of the data was established using a combination of techniques including memorandum writing and peer debriefings (Eakin & Mykhalovskiy, Reference Eakin and Mykhalovskiy2003). Feedback on the Mi-LiFE program was described via in-person and teleconference semi-structured interviews with the participants (or caregivers if necessary) at the program completion (∼week 6) and after 6 months.
Program fidelity by the PT was evaluated through an audit of videotaped individual and group sessions for the first and last five participants enrolled in the program. Two members of the research staff reviewed the videotaped sessions. The video reviewers received at least one training session from the research coordinator on the intervention and fidelity evaluation procedures. They also pilot tested the fidelity evaluation form on one video session prior to review of the other videos. Fidelity rating forms were filled out with 34 program criteria for the individual session (e.g., purpose and aims of LiFE program explained), and 17 program criteria for group sessions (e.g., PT demonstrated the activity to the group and identified situations to embed the activity). Each program criterion was scored out of 2 (0 = not done at all, 1 = done but could be better, 2 = done well) with any disagreement resolved via a third party. Written consent was obtained from all participants involved in the videotaped sessions assessed for fidelity.
Additional process outcomes were collected to inform future trials or implementation, including number of individuals eligible/ineligible, number of eligible individuals interested in participation, descriptive characteristics of those who declined participation (e.g., age, sex), reasons for declining participation, number of physician/nurse referrals from the 13 local FHT individual practices and the 5 non-local FHT individual practices, and number of falls per participant.
Descriptive Data, Falls, and Adverse Events (AEs)
A medical history and health status questionnaire was used to collect information on demographic characteristics, past and present health conditions, and fall history. Height was measured without shoes using a measuring tape mounted on a wall to the nearest 0.1 cm. Weight was measured without shoes or any heavy clothing using a calibrated electronic scale (Hometrends EB9313H) to the nearest 0.1 kg.
Via calendar-style diaries, participants were instructed to report whether a fall occurred throughout the 6 month program, and to report AEs or injuries by phone and at program sessions. When a fall occurred, a fall incident form was completed by the research coordinator. When an AE occurred, an AE reporting form was completed by the research coordinator and reviewed by the primary investigator and a physician affiliated with the trial. Three types of AEs were reported: (1) serious AEs (SAEs, Health Canada definition—events that result in death, hospitalization, or disability), (2) AEs related to the program, and (3) AEs leading to study withdrawal or program cessation. We also reported on falls as a process outcome to inform the implementation of a larger-scale version of this trial with falls as a secondary outcome.
Statistical Analyses
Participant characteristics, feasibility, falls, AEs, and process outcomes were summarized using mean (SD) for continuous variables and number (per cent) for categorical variables. Change in PA levels, physical performance, and quality of life over the 6 month program were reported as absolute change values and analyzed using paired t-test and Wilcoxon rank-sum test analyses (for non-normally distributed data). Per-protocol and intention-to-treat (ITT) analyses were performed for PA, physical performance, and quality of life data. Missing values analysis examined patterns of missing data. Multiple imputation procedures were used to impute the missing data values (fully conditional specification method, model for scale variables = linear regression, number of imputations = 5, maximum iterations = 25) and pooled results were reported. Barriers and facilitators to implementation were analyzed using thematic content analysis (Graneheim & Lundman, Reference Graneheim and Lundman2004) and the BCW framework (Michie et al., Reference Michie, West, Campbell, Brown and Gainforth2014). Participant feedback on the Mi-LiFE program was presented using descriptive feedback. Mi-LiFE program fidelity was reported using mean ratings of compliance. p values were reported to three decimal places, and statistical significance was defined as p < .05. No correction for multiple testing was made because of the exploratory nature of the analyses. Analyses were performed with SPSS Statistics v.24 (Armonk, NY, USA). A sample size calculation was previously reported (Gibbs et al., Reference Gibbs, McArthur, Milligan, Clemson, Lee and Boscart2015).
Results
Feasibility of Recruitment, Adherence, and Retention
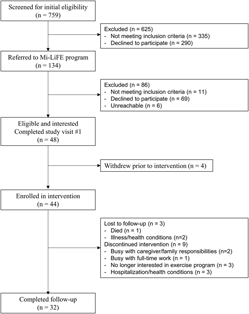
During the FHT geriatric screening, 44 per cent (335/759) of the individuals were not referred to the program because they self-reported regular PA (≥ 30 minutes of moderate-intensity PA on ≥ 5 days/week) and 38 per cent (290/759) did not consent to future contact (Figure 1). One hundred and thirty-four individuals were referred to the Mi-LiFE program, with most individuals being from the clinic (78.4%; n = 105) and a smaller proportion being from the FHT geriatric screening program participant pool (21.6%; n = 29). Physician referral was a more successful recruitment strategy (54.2%; n = 26) than nurse referral (29.2%; n = 14) and other strategies (16.7%; n = 8). Referrals were distributed similarly across the 13 local physician practices (mean = 3.2, total: 41) with a smaller proportion recruited from the five non-local practices (mean = 1.4, total: 7). Of those referred, 8 per cent were ineligible (contraindications to exercise – n = 4; participation in a similar exercise program – n = 2; moderate-to-severe lung disease/COPD – n = 2; dementia diagnosis – n = 1; physician chose not to refer – n = 2).

Figure 1: CONSORT study flow diagram
Of the 134 potentially eligible individuals referred to the program, 36 per cent agreed to participate (n = 48), while 52 per cent were not interested (access to exercise– n = 18; illness/health condition – n = 17; lack of transportation – n = 14; not interested in exercise – n = 12; too busy/lack of time – n = 4; away/travelling during program – n = 2; difficulty understanding program – n = 2), 8 per cent were ineligible (n = 11) and 4 per cent were unreachable by phone (n = 6) (Table 1). Forty-eight participants consented to enroll in the program (representing 39% of those eligible [48/123] and 6% of those screened [48/759]). Most uninterested individuals were referred by a nurse (79.8%; 59/75) and a smaller proportion were referred by a physician (20.2%; 16/75). Individuals who declined participation were 81 ± 5 years of age and 57 per cent (n = 43) were female. Six participants were a spouse or friend referred by another participant. Three caregivers attended the program to assist participants with cognitive/physical limitations but did not enroll. One female participant with multiple sclerosis and cognitive impairment who enrolled in the program with her spouse did not agree to data collection and subsequently withdrew from the program. Therefore, data from 47 individuals were analyzed for the secondary objectives.
Table 1: Feasibility of recruitment, adherence, and retention to the Mi-LiFE program (n = 48)
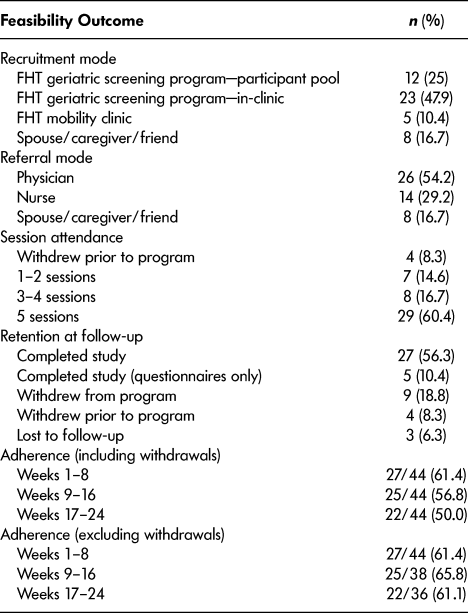
Note. FHT = family health team.
Most participants mentioned that referral from their physician/nurse was a factor in their decision to participate (see Table A1 in supplementary Appendix). Primary reasons for joining the program included to improve health and physical function, become more active, or prevent falls. Other incentives were to: join with caregiver, spouse, or friend; learn new information; try a new exercise program; participate in a low-intensity program; and join a free exercise program at their family physician’s practice (Table A1).
Participants attended (mean ± SD) 4±1 sessions, with 77 per cent (34/44) attending four or more sessions.
Adherence to the exercise program (including withdrawals) decreased across the program: 61 per cent (weeks 1–8), 57 per cent (weeks 9–16), and 50 per cent (weeks 17–24) (Table 1). However, the combined adherence to the session attendance (four or more sessions) and the home exercise participation (≥ 3 days/week over 6 months) was 36 per cent (16/44). Excluding withdrawals, adherence increased at the mid-point of the program and returned to its baseline level in the final weeks: 61 per cent (weeks 1–8), 66 per cent (weeks 9–16), and 61 per cent (weeks 17–24). Sixteen participants discontinued daily diary completion (discontinued exercises – n = 9, not agreeable or forgot to fill out forms – n = 5, started new exercise program – n = 2) and retrospectively self-reported adherence data. Two participants temporarily discontinued diary completion while on vacation and retrospectively self-reported their adherence. Four participants withdrew prior to the program. Of the remaining 44 participants, 9 discontinued the program and 3 were lost to follow-up. Retention at follow-up was 67 per cent, with 27 participants completing all follow-up assessments and 5 participants completing questionnaires only (Table 1).
Descriptive Characteristics
Twenty-nine women and 18 men were enrolled in the Mi-LiFE program with a mean (SD) age of 81 (5) years, body mass index (BMI) of 28 (5) kg/m2, and MVPA of 49 (87) minutes/week. Twenty-three per cent of participants reported one or more falls in the past year (Table 2). Most participants (74.5%) engaged in < 150 minutes/week of accelerometer-measured MVPA. However, participants self-reported 153 ± 159 minutes/week of aerobic PA (IPAQ-measured MVPA and light-intensity walking). More than half of the participants demonstrated a total SPPB score > 9 (62%) and ≥ 75 on the EQ5D VAS self-perceived health status (66%).
Table 2: Baseline descriptive characteristics of participants in Mi-LiFE program
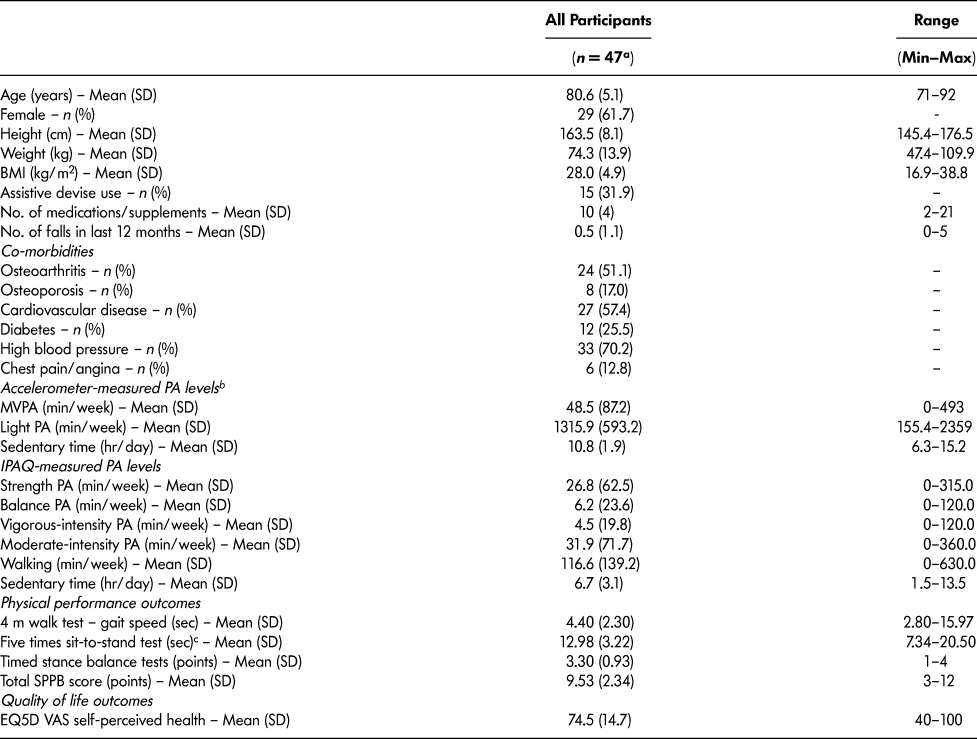
Note. BMI = body mass index; PA = physical activity; MVPA = moderate-to-vigorous physical activity; IPAQ = International Physical Activity Questionnaire; SPPB = Short Physical Performance Battery; EQ5D = EuroQol Five-Dimensional Questionnaire; VAS = visual analogue scale.
a 1 participant did not agree to data collection for secondary outcomes.
b 37 participants completed accelerometer data collection at baseline; data according to Freedson et al. (Reference Freedson, Melanson and Sirard1998) cut-points are presented.
c 6 participants used arms to stand or were unable to complete a single chair test and did not have data for five times sit-to-stand test.
Change in PA, Physical Performance, and Quality of Life
No statistically significant changes in accelerometer-measured PA outcomes were demonstrated at follow-up using per-protocol and ITT analyses (Table 3). For two participants, there was a change ≥ 200 minutes/week of MVPA over 6 months (representing possible outliers). Sensitivity analyses excluding these participants did not significantly influence the results for change in MVPA (mean change: 1.6 [16.5] minutes/week, p = .686; n = 19). Self-reported strength (mean change = 27.2 [70.3] minutes/week, p = 0.026, per-protocol; mean change = 41.6 minutes/week, p < .001, ITT) and balance activity increased from baseline to follow-up (mean change = 34.4 [55.0] minutes/week, p < .001, per-protocol; mean change = 51.2 minutes/week, p < .001, ITT). However, no statistically significant changes in self-reported MVPA, walking, and sedentary time were observed. Also, a small proportion of participants reported other strength and balance training (performed on their own or in exercise classes) at follow-up either alongside (19%, 6/32) or instead of the LiFE activities (19%, 6/32).
Table 3: Change in physical activity levels and physical performance outcomes in participants of Mi-LiFE program from baseline and follow-up (n = 32)—per-protocol analysis
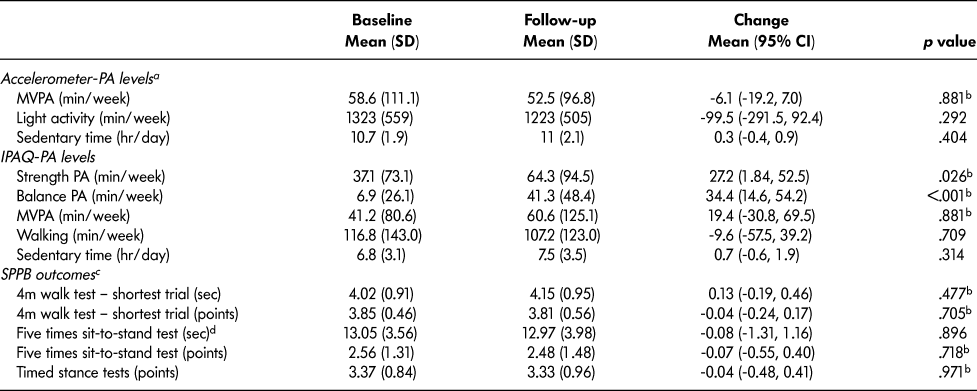
Note. PA = physical activity; MVPA = moderate-to-vigorous physical activity; IPAQ = International Physical Activity Questionnaire; SPPB = Short Physical Performance Battery.
a 21 participants completed accelerometer data collection at baseline and follow-up.
b Wilcoxon rank-sum tests were performed for non-normally distributed data.
c 27 participants completed SPPB data collection at baseline and follow-up.
d 3 participants failed the single chair stand test and did not complete five times sit-to-stand test.
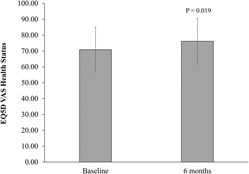
Total SPPB score did not significantly change in the per-protocol and ITT analyses (Figure 2). There were also no statistically significant changes in the SPPB individual components (4 m walk, five times sit-to-stand, timed stance tests) (Table 3). There was a significant increase in EQ5D VAS self-perceived health status (mean change = 5.33, p = .019, per protocol) (Figure 3). However, this finding was not statistically significant in the ITT analysis (mean change = 2.2, p = .282). Results for the EQ5D subscales were similar for most subscales at baseline and follow-up (Table 4) with minor increases in the number of participants who reported no problems in walking about and engaging in usual activities. Sixty-three percent of participants (n = 20) reported moderate pain/discomfort at baseline, which slightly increased to 78 per cent (n = 25) at follow-up.

Figure 2: SPPB scores in Mi-LiFE program at baseline and follow-up (n = 27)—per-protocol analysis.
Note. Thirty-two participants were retained at follow-up; five participants were unable/did not agree to complete in-person follow-up assessments (questionnaires only). Wilcoxon rank sum tests were performed for non-normally distributed data
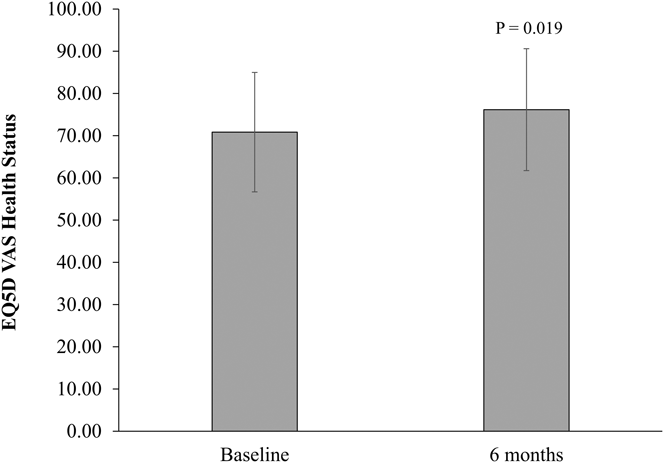
Figure 3: EuroQol Five-Dimensional Questionnaire (EQ5D) VAS of self-perceived health status in Mi-LiFE program at baseline and follow-up (n = 32)—per-protocol analysis
Table 4: EQ5D subscale results of Mi-LiFE program at baseline and follow-up (n = 32)

Falls and AEs during the Program
Ten participants (21.3%) fell during the 6 month program (19 falls with 6 resulting in non-serious injuries) with none related to the program. Seven participants reported one fall each and three participants reported one or more falls over 6 months. Four participants reported SAEs resulting in hospitalization (infection in big toe secondary to diabetes, worsening of COPD-related symptoms, severe chest/abdominal pain, diabetic episode) and one participant died (intracerebral hemorrhage) during the program. None of the SAEs were related to the program as adjudicated by the research staff, physician affiliated with the trial, and ethics boards. Of the SAEs, three led to permanent or temporary program discontinuation. Fourteen participants reported non-serious AEs, with two possibly related to the program (heel pain and hamstring strain) and 12 not related to the program (pneumonia/flu—n = 2, chronic leg pain—n = 2, fall-related injury—n = 2; low back pain, racing heart rate, strained rotator cuff, hip tendonitis, dementia diagnosis, heel spur, n = 1 each). Eight participants with non-serious AEs permanently or temporarily discontinued the program.
Implementation Results
Physical and psychological capability represented a barrier and facilitator from the participant’s perspective, such that chronic illness or injury and difficulty understanding the goals of the program affected their participation (Table 5). One participant reported that, “I am trying to follow through on the exercises, but my back gets quite tired and my energy levels are not good.” Another participant felt “The purpose of the exercises was sometimes unclear. Is it the number of times or how long we do the exercises that matters?” Barriers related to physical opportunity included limited one-on-one attention and lack of home-based visits. One participant explained, “I would have preferred the PT come to my home to teach me exercises. My husband gets taught exercise in our home and that works for us.” PT instruction and caregiver support were identified as facilitators. Additionally, the group-based program facilitated social and learning opportunities (e.g., “…being a part of a group and seeing other people’s points of view and issues they are dealing with”). However, certain participants would have preferred individual exercise prescription over the group approach (e.g., “Need to be careful with a group…don’t want to lose people or emphasize competition with others”). Behaviour change techniques (e.g., activity planning, positive reinforcement) encouraged reflective motivation, including: “a greater appreciation of balance and coordination” and a “change in my mental attitude toward exercise”. Yet, some participants had difficulty planning activities or preferred structured exercise prescription. One participant stated, “I still haven’t quite figured out the best way to incorporate them into my daily activities but would like to be able to eventually.” By modifying their home environments and daily routines, participants reported some habit change that led to automatic motivation. For example, one participant explained, “I have a paper route and would incorporate the tandem walk and the stairs into my route.” A detailed description of the participants’ feedback on the program is provided in Table A1.
Table 5: Thematic analysis of barriers and facilitators to implementation of the Mi-LiFE program using the BCW framework
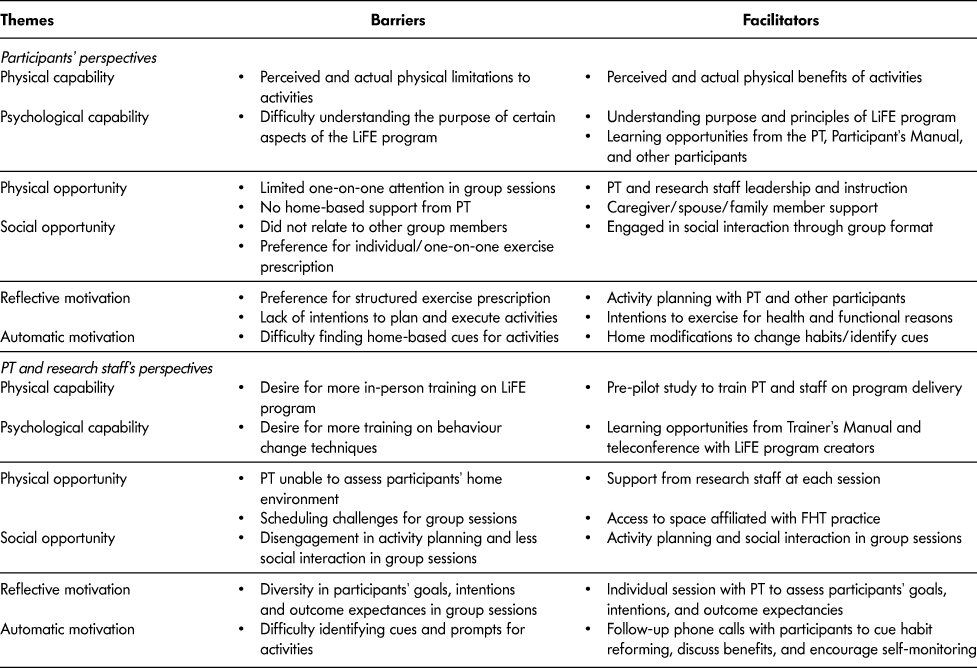
Note. BCW = behaviour change wheel; PT = physical therapist; FHT = family health team.
From the PT and research staff’s perspectives, training was important to facilitate physical and psychological capability, including the experience of trialing the program, the LiFE Trainer’s Manual, and teleconference meetings with the LiFE program creators (Table 5). The LiFE Trainer’s Manual was used by the PT “to inform how to manage and lead the group, the organizational factors—where to sit and where to stand.” The pre-pilot test was beneficial to both the PT and research staff to “see if this would logistically work, give [us] the opportunity to familiarize ourselves with the program.” The PT and research staff would have preferred more intensive training on the behaviour change strategies central to the LiFE program, ideally in person. Barriers to physical opportunity were the scheduling complexity, single deliverer and inability to assess the participants’ home environment (e.g., “…they would have to imagine what their house looked like and where they could do it because we were not in their house”). Access to space and resources affiliated with the FHT practice facilitated implementation and program oversight. Activity planning and social interaction in the group sessions were considered facilitators by the PT and research staff. For example, “There was one group that attended every session together…they did the protocol exactly how you would intend for it to be. Everyone was engaged, supporting one another.” However, the PT found it challenging to promote behaviour change when participants were disengaged in activity planning or social interaction (e.g., “Some people were good at just fitting in with new people. There was the odd person that struggled with it more than others…they felt new and behind even if they weren’t.”) Diversity in participants’ goals, intentions, and plans were identified as a barrier for the PT in the group sessions (e.g., “Especially seeing that this person is very high functioning and someone else is very low functioning, so how am I going to address that in a group setting?”) Yet the individual session provided an initial assessment to guide program adoption and “get a sense of what they do every day”. Follow-up phone calls allowed the PT to cue exercise progression and positive reinforcement, especially when participants “stopped doing [the activities] as often and would say ‘Now that you called me I’m going to start doing them again’ or ‘That’s a good idea, I’m going to try that.’” All mean ratings of program fidelity by the PT were ≥ 1.8 (/2) with 62 criteria (/68) met in the individual sessions and 33 criteria (/34) met in the groups sessions.
Discussion
The present pilot study suggests that the Mi-LiFE program delivered in a primary care-based FHT practice is feasible because of acceptable recruitment and attendance rates alongside modifications to address retention and adherence challenges (Table 6). Like other primary care-based exercise interventions (Elley, Kerse, Arroll, & Robinson, Reference Elley, Kerse, Arroll and Robinson2003; Fortier et al., Reference Fortier, Hogg, O’Sullivan, Blanchard, Sigal and Reid2011; Pinto, Goldstein, Ashba, Sciamanna, & Jette, Reference Pinto, Goldstein, Ashba, Sciamanna and Jette2005), no changes in MVPA and sedentary time were observed, yet increases in self-reported strength and balance-related activity were found in per-protocol and ITT analyses. Mi-LiFE was associated with a significant increase in health-related quality of life in those participants who completed the program, with no statistically significant changes in physical performance, including gait speed, lower-extremity strength, and balance. High program fidelity and participant satisfaction were observed, with participant and deliverer capability, social and environmental support, and behaviour change techniques to increase motivation and habit change emerging as key themes for implementation. Program modifications, such as an initial home visit, more intensive follow-up counselling using the LiFE model of behaviour change, and targeted screening to enroll older adults at higher risk of falls and functional disability, may improve future implementation of Mi-LiFE.
Table 6: Tips for implementing group-based exercise programming in primary care

To our knowledge, our pilot pragmatic trial represents the first to evaluate the feasibility of implementing a group-based version of the LiFE program in an interdisciplinary primary care practice. Our findings suggest that the recruitment and attendance rates to the Mi-LiFE program are feasible, yet like most exercise interventions in real-world settings, retention and adherence to home exercise remain challenges. In-clinic recruitment involving the physician was the most feasible referral strategy compared with recruitment through a retrospective participant pool with 48 participants enrolled (representing 39% of those eligible and 6% of those screened). The FHT geriatric screening program involving self-reported PA classification resulted in a representative sample of older adults with 75 per cent engaging in fewer than 150 minutes/week of MVPA. Our retention rate was comparable to other exercise trials longer than 6 months wherein fewer than 75 per cent were retained (Iliffe et al., Reference Iliffe, Kendrick, Morris, Masud, Gage and Skelton2014; Pahor et al., Reference Pahor, Guralnik, Ambrosius, Blair, Bonds and Church2014). Fleig et al. (Reference Fleig, McAllister, Chen, Iverson, Milne and McKay2016) reported a 23 per cent drop-out rate from their group LiFE program in a smaller sample of 13 older women (mean age 66 years) over 4 months. The withdrawal reasons reported by our participants were consistent with prior exercise trials in older adults (e.g., change in health status, loss of interest, too busy, having moved) (Clemson et al., Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012; Fleig et al., Reference Fleig, McAllister, Chen, Iverson, Milne and McKay2016; Iliffe et al., Reference Iliffe, Kendrick, Morris, Masud, Gage and Skelton2014; Pahor et al., Reference Pahor, Guralnik, Ambrosius, Blair, Bonds and Church2014). Adherence rates vary widely across studies (18–100%) (Shier, Trieu, & Ganz, Reference Shier, Trieu and Ganz2016) and are typically higher in supervised, laboratory settings than in the real world using self-report methods (Morey et al., Reference Morey, Dubbert, Doyle, MacAller, Crowley and Kuchibhatla2003). It was not surprising that 77 per cent of our participants attended four or more supervised sessions; whereas the daily diary-documented adherence was much lower (50%) and reflective of the challenges of engaging older adults in unsupervised PA. More intensive behaviour change counseling during follow-up with a focus on long-term self-monitoring, habit reforming, and coping with barriers is advised to improve adherence for future implementation (Clemson & Munro, Reference Clemson, Munro and Pachana2017).
Like other primary care exercise interventions, the Mi-LiFE program was not effective at increasing MVPA, despite increases in self-reported strength and balance activity (Elley et al., Reference Elley, Kerse, Arroll and Robinson2003; Fortier et al., Reference Fortier, Hogg, O’Sullivan, Blanchard, Sigal and Reid2011; Pinto et al., Reference Pinto, Goldstein, Ashba, Sciamanna and Jette2005). Notably, the effects on self-reported strength and balance activity align with the findings from the LiFE efficacy trial (Clemson et al., Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012), and reflect compliance with the program’s training principles and behaviour change techniques. The focus of the LiFE program is to integrate strength and balance exercises into daily lifestyle activities through activity planning and habit reforming, which may explain the lower incentive to increase structured, aerobic-based MVPA. Also, accelerometers have a limited capacity to objectively detect increases in strength and balance exercise, which may be classified as light-intensity physical activity or even sedentary time. Several other trials have found a discrepancy in effectiveness to change physical activity depending on the assessment measures (Elley et al., Reference Elley, Kerse, Arroll and Robinson2003; Fortier et al., Reference Fortier, Hogg, O’Sullivan, Blanchard, Sigal and Reid2011; Pinto et al., Reference Pinto, Goldstein, Ashba, Sciamanna and Jette2005). Therefore, overestimation of PA using self-report measures, experimental bias related to accelerometer use, and contrasts in PA dimensions are all considerations when interpreting our findings.
Improvements in physical performance and quality of life have been well documented following strength and balance exercise interventions in older adults (Clemson et al., Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012; Martin et al., Reference Martin, Wolf, Moore, Rolenz, DiNinno and Reneker2013; Pahor et al., Reference Pahor, Guralnik, Ambrosius, Blair, Bonds and Church2014). Improvement in health-related quality of life following the Mi-LiFE program was expected given its socially interactive nature and lifestyle-focused approach. However, the lack of effectiveness to improve physical performance conflicted with the home-based LiFE efficacy trial wherein static balance, tandem walk time, and lower limb strength significantly improved after 6 months (effect sizes = .40–.63) (Clemson et al., Reference Clemson, Fiatarone Singh, Bundy, Cumming, Manollaras and O’Loughlin2012). The home-based LiFE efficacy trial recruited a higher risk group who had a history of two or more falls or an injurious fall, and they had a lower perceived health status at baseline (EQD5 VAS < 75). Pahor et al. (Reference Pahor, Guralnik, Ambrosius, Blair, Bonds and Church2014) also observed a significant increase in total SPPB score (1.1 points) following 6 months of moderate-intensity PA in sedentary older adults at risk for major mobility disability (Life Study Investigators et al., 2006). Yet, similar to our study, Fleig et al. (Reference Fleig, McAllister, Chen, Iverson, Milne and McKay2016) reported no significant changes in SPPB outcomes after 4 months of a group LiFE program. It is possible that the Mi-LiFE program was associated with maintenance of already reasonable mobility and functional status wherein 62 per cent of participants had an SPPB score > 9, reflective of ceiling effects with the measure (Guralnik et al., Reference Guralnik, Simonsick, Ferrucci, Glynn, Berkman and Blazer1994). Also, the intensity of the strength and balance activities may have been insufficient to elicit significant results in some participants, especially those at the highest end of the functional spectrum. Qualitative feedback from participants reflected potential benefits related to confidence while walking, ability to perform daily activities, and perceived balance and strength improvements. Therefore, an exercise program involving higher load strength training of all major muscle groups alongside teaching the LiFE balance and strength principles may be an alternate approach for more functionally capable and less at-risk older adults.
Our pilot study provides valuable insight into barriers and facilitators to the real-world implementation of the evidence-based LiFE intervention into practice using the BCW framework (Michie et al., Reference Michie, West, Campbell, Brown and Gainforth2014). Certain themes that emerged were consistent with previous research on translating exercise interventions into practice (e.g., physical and psychological capability of participants and deliverers, environmental support, resources) (Flocke, Crabtree, & Stange, Reference Flocke, Crabtree and Stange2007; Yarnall, Pollak, Ostbye, Krause, & Michener, Reference Yarnall, Pollak, Ostbye, Krause and Michener2003). However, our findings also highlight new perspectives on the importance of social support, behavior change techniques, and training for deliverers of group exercise in a FHT context. The training resources for the LiFE program (e.g., Trainer’s and Participant’s Manuals) (Clemson & Munro, Reference Clemson and Munro2014a, Reference Clemson and Munro2014b) were identified as facilitators by deliverers and participants, and would allow widespread delivery of the program. Future implementation should consider more physical support through in-person/online training of the deliverers and at least one instructional session in the participants’ homes, if feasible. As expected, social support was a key factor in implementing the group program (Burke et al., Reference Burke, Carron, Eys, Ntoumanis and Estabrooks2006), such that the participants’ level of social interaction during the sessions may have influenced their response (Corbett et al., Reference Corbett, Rejeski, Tudor-Locke, Glynn, Kritchevsky and McDermott2017). The smaller group size in the Mi-LiFE program likely affected the group dynamics, and adaptation for larger group classes (more than five people per session) may present with different results. Inclusion of interactive activities to enhance teamwork and positive social participation would be beneficial in future implementation.
Strengths of our pilot trial include its theory-driven behaviour change strategies and evidence-based training elements, comprehensive evaluation frameworks (RE-AIM, BCW) and pragmatic design with generalizable eligibility. Using both accelerometer and self-report measures of PA provide valuable pilot data on the effects of the Mi-LiFE program on PA levels in inactive older adults. Also, our exercise delivery approach (including the training) could be implemented per protocol by other rehabilitation professionals, such as kinesiologists, occupational therapists, and exercise physiologists, working in interprofessional primary care settings using the LiFE Trainer’s and Participant’s manuals (Clemson & Munro, Reference Clemson and Munro2014b). The lack of a control group and shorter follow-up period represented limitations, which precluded our ability to report the long-term effectiveness and maintenance of our program within the FHT context. Because our pilot feasibility trial was implemented in one primary care practice with one expert deliverer, we were unable to examine adoption of the Mi-LiFE program at the organization level (Glasgow et al., Reference Glasgow, Vogt and Boles1999).
In conclusion, Mi-LiFE represents a feasible exercise program to implement in a primary care-based FHT practice for older adults with an emphasis on in-clinic screening involving a physician, individualized and group exercise training elements, and opportunities for caregiver participation. Recommended modifications to improve retention and adherence include an individual session in the participant’s home to assess environmental support, targeting people at higher risk of falls/disability, and incorporating more intensive follow-up counselling using the LiFE model of behaviour change. As expected, increased self-report strength and balance activity and health-related quality of life were observed, but no clinically significant improvements in MVPA and functional outcomes were demonstrated. For clinical populations with better function and no specific fall risk, a hybrid group approach could be considered where more than 150 minutes/week aerobic MVPA and higher load strength training would be added to the LiFE balance and functional principles. Deliverer training, social dynamics, and longer-term behaviour change strategies are key components of the implementation process for future lifestyle-based group exercise programs in primary care for older adults. Prior to scale-up and widespread implementation, a multi-centre pragmatic trial is required to evaluate acceptance and readiness to adopt the modified Mi-LiFE program in FHT practices, variation across deliverers and sites, and real-world effectiveness versus a comparator.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0714980818000739