INTRODUCTION
The UK Armed Forces do not routinely screen for blood-borne viruses (BBVs) in recruits, but candidates self-declare their HIV status (with confirmation from their general practitioner), and those with a diagnosed HIV infection are barred from entry in accordance with Service Policy [1]. Continuing recruitment from Commonwealth countries into the UK Armed Forces has led to concern that the prevalence of BBVs may be increasing in military recruits. In 2006, there were 48 known HIV-positive Service personnel [2] of whom 30 were from Commonwealth countries. However, the prevalence in recruits is not known since infected individuals may be undiagnosed, or cared for by other non-Defence medical providers. The prevalence of BBVs in recruits to the UK Armed Forces is needed to inform policy discussions on whether screening and hepatitis B vaccination programmes are necessary for this population and, if they are, whether they should be implemented universally, or targeted at recruits with specific characteristics.
An unlinked anonymous survey [Reference Nicoll3] was conducted to measure the prevalence of selected BBV markers (for HIV, hepatitis B and C) to inform strategic discussions of future screening and hepatitis B vaccination policies.
METHOD
Between January 2007 and January 2008, recruits entering phase one (basic) training centres of the Royal Navy (RN), Army, and Royal Air Force (RAF) had blood samples taken for routine blood grouping. Residual blood samples were unlinked from identifying information and tested anonymously for markers of BBV infection. Posters were displayed in each training centre and recruits were able spontaneously to object to being included in the study.
Limited demographic data from personnel records were retained with the samples to assist with interpretation. These included: Service, commissioned vs. non-commissioned recruits, nationality, ethnicity, age group, and gender. All identifiers were irreversibly deleted and demographic data reconfigured to eliminate the risk of deductive disclosure. Separate data tables were retained for each variable, in such a way to prevent the cross-referencing of data.
Once the anonymization process was complete, laboratory testing commenced using markers for HIV, hepatitis B and hepatitis C. Specimens were pooled and screened for markers for HIV, hepatitis B and hepatitis C. Specimens were screened for antibodies to HIV types 1 and 2 (anti-HIV-1/-2) using the Abbott Murex HIV 1.2.gO assay (Abbott Diagnostics, UK). Repeatedly reactive specimens were then confirmed as anti-HIV positive using the Genelabs Diagnostics HIV Western Blot 2.2 (Abbott Diagnostics).
For hepatitis B, two markers were used: antibody to hepatitis B core antigen (anti-HBc) and hepatitis B surface antigen (HBsAg). Anti-HBc is a marker of past cleared hepatitis B infection while HBsAg is a marker of current hepatitis B infection. For anti-HBc, pooled specimens were tested using Monolisa Anti-HBc plus ELISA (Bio-Rad Laboratories, UK) to identify reactive specimens. Reactive specimens were also tested with the Abbott Murex Anti-HBc (total) EIA (Abbott Diagnostics) and reactivity in both EIA tests taken as definitive evidence of the presence of anti-HBc. Anti-HBc reactive specimens were screened with Abbott Murex HBsAg v.3 EIA (Abbott Diagnostics). Markers of prior hepatitis B vaccination were not examined.
For hepatitis C, specimens were screened for antibodies to hepatitis C virus using the HCV 3.0 SAVe ELISA (Ortho Diagnostics, UK). Reactive specimens were re-tested using the Monolisa Anti-HCV plus v.2 ELISA (Bio-Rad Laboratories). Specimens reactive by both methods were considered anti-HCV positive; those with discordant findings were considered to be indeterminate. χ2 and Fishers' exact tests were used to investigate the significance of findings where relevant.
The survey was approved by both a multi-regional research ethics committee (05/MRE02/85) and the Ministry of Defence research ethics committee.
RESULTS
Between 22 January 2007 and 31 January 2008, 14 663 samples were obtained from recruits, irreversibly anonymized and tested for HIV, hepatitis B and hepatitis C (Fig. 1). Overall, 0·65% (96/14 759) of recruits objected to being included in the survey. There were records (5·1%) to which a laboratory sample could not be linked. Limited demographic data could be linked to 59·6% (8291/13 915) of samples. The remaining 5624 samples could only be matched to training centre and Service. Forty-three samples were insufficient for hepatitis B testing.
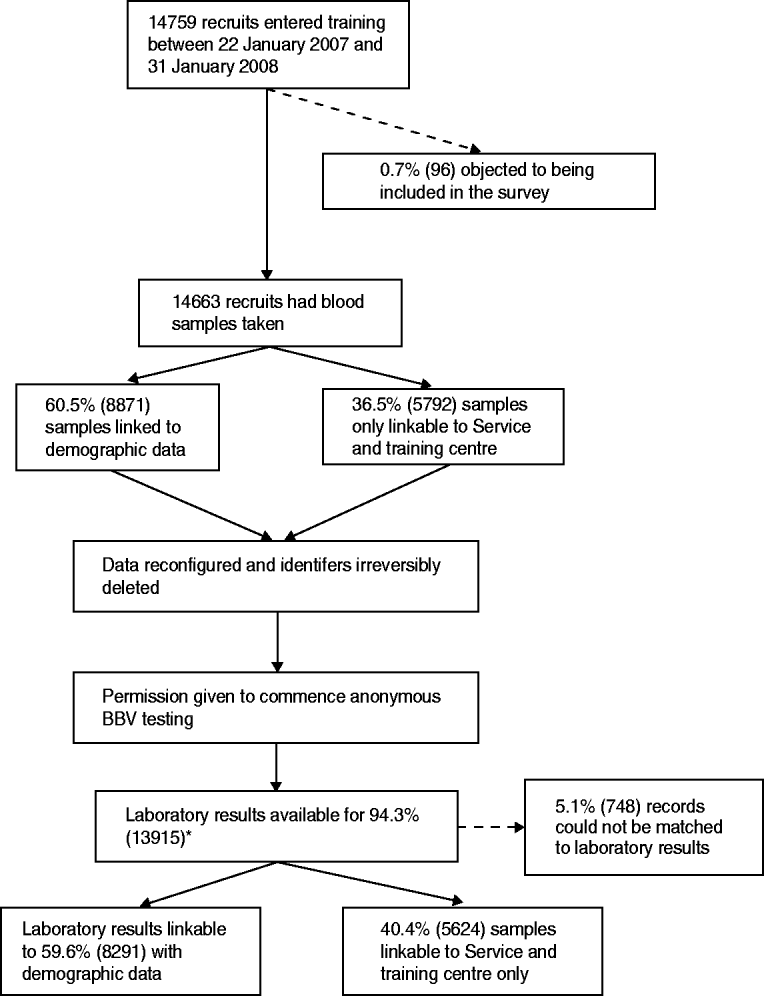
Fig. 1. Flow diagram of the unlinked anonymous survey of blood-borne viruses (BBVs) in military recruits. * Samples that were found to be indeterminate for markers of BBVs were excluded from the numerator and denominator.
Population characteristics
Of the 8291 recruits that had demographic data available, the sample population was predominantly young [89% (5703/6374), <25 years], male (92%, 5095/5545) and white (93%, 7491/8075). Ninety per cent (6435/7097) were UK nationals, and 4·6% (323/7079) were sub-Saharan African. Most sub-Saharan African recruits, (86%, 279/323) joined the Army. Non-UK nationals were more likely to be recruited at older ages (Fig. 2 a) compared to recruits of UK nationality (χ2 test for association, P<0·0001). The proportion of the total that was recruited as commissioned officers was 6·1% (849/13 065).
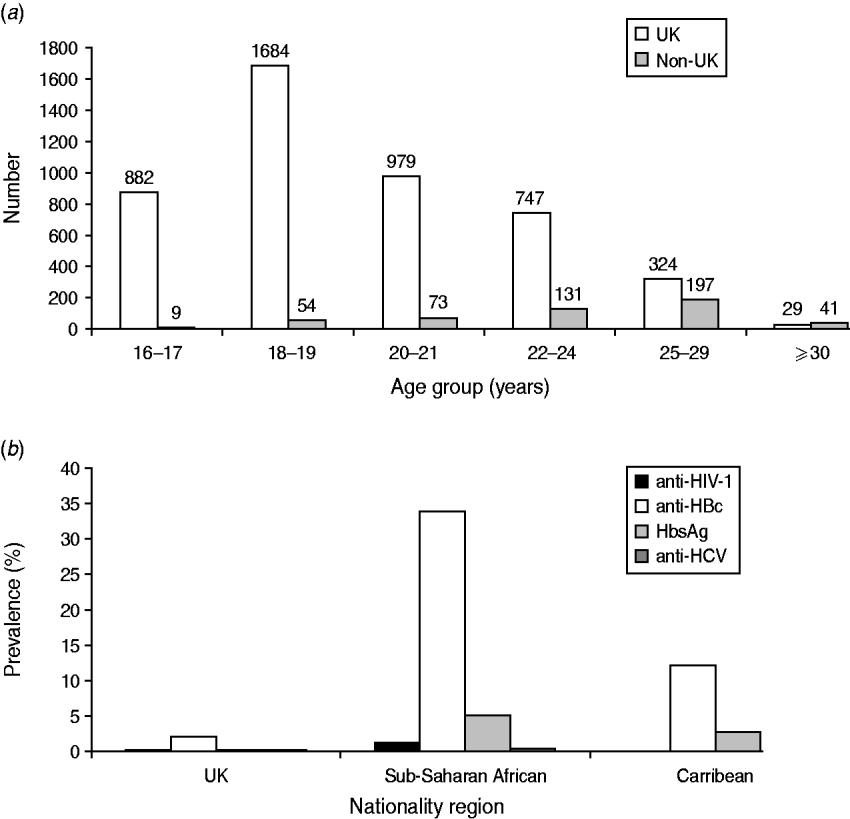
Fig. 2. Age distribution and blood-borne virus (BBV) prevalence by nationality. (a) Number of UK and non-UK military recruits entering training by age group. (b) Prevalence of BBV by nationality region.
Overall BBV prevalence
Overall, the prevalence of anti-HIV-1 was measured at 0·06% [8/13 914, 95% confidence interval (CI) 0·03–0·12]. The prevalence of anti-HBc and HBsAg was 3·63% (503/13 872, 95% CI 3·33–3·95) and 0·37% (51/13 872, 95% CI 0·28–0·49), respectively. The prevalence of anti-HCV was 0·06% (8/13 905, 95% CI 0·03–0·12). For both HIV and hepatitis C there was no significant difference in prevalence between the three Services (Table 1). For both anti-HBc and HBsAg, the prevalence was significantly higher in Army recruits compared to each of the other two Services (P<0·001).
Table 1. Prevalence by Service
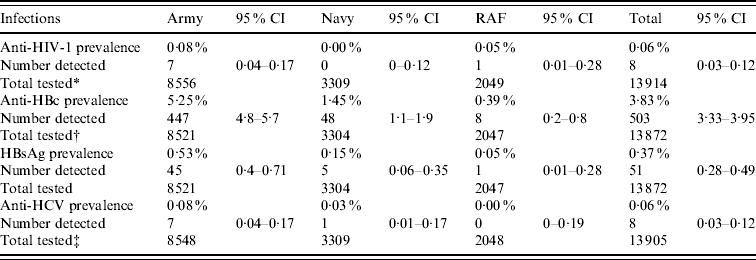
* One sample was indeterminate for HIV testing.
† Forty-three samples from the pilot were insufficient for HBV testing.
‡ Ten samples were indeterminate for HCV testing.
Prevalence by demographic and risk group
There were no statistically significant differences in BBV prevalence between laboratory samples that could be fully linked to demographic data, and samples that could be linked to Service and training centre only. The respective prevalences for the former and latter were: 0·07% vs. 0·04% for anti-HIV-1 (P=0·6); 3·47% vs. 3·85% for anti-HBc (P=0·28); 0·29% vs. 0·48% for HBsAg (P=0·09); and 0·04% vs. 0·09% for anti-HCV (P=0·36). The following section includes only the 8291 results from laboratory samples that could be linked to full demographic data.
HIV prevalence was significantly lower in UK recruits compared to sub-Saharan African recruits [0·02% (1/6434), 95% CI 0–0·1 vs. 1·24% (4/323), 95% CI 0·48–3·14, respectively] (P<0·0001). Anti-HIV-1 was not detected in recruits of any other nationality region (where nationality was known) (Fig. 2 b).
Significant differences were also found between UK and sub-Saharan African recruits, respectively, for anti-HBc [2·03% (130/6414) vs. 34% (109/322) (P<0·0001)] and for HBsAg [0·05% (3/6414) vs. 5% (16/322) (P<0·0001)]. The prevalences of anti-HBc and HBsAg in Caribbean recruits were measured at 12·1% (9/74, 95% CI 6·53–21·52) and 2·7% (2/74, 95% CI 0·74–9·3), respectively.
The prevalences of anti-HCV were 0·03% (2/6432, 95% CI 0·01–0·11) and 0·31%, (1/323, 95% CI 0·05–1·7331) in UK and sub-Saharan African recruits, respectively (P=0·14). Anti-HCV was not detected in recruits of any other nationality region, (where nationality was known).
There was a statistically significant difference in anti-HBc prevalence in commissioned and non-commissioned recruits [0·8% (7/849) vs. 3·8% (496/13 023); P<0·0001]. There were no statistical differences between commissioned and non-commissioned officers for anti-HIV-1 [0·0% (0/849) vs. 0·1% (8/13 065); P=0·6], HBsAg [0·1% (1/849) vs. 0·4% (50/13 023); P=0·173] or anti-HCV [0·0% (0/849) vs. 0·1% (8/13 056); P=0·6]. Statistically significant differences were also observed for HIV and anti-HBc between younger and older age groups (where age group was known) [HIV: 0·6% (4/683), ⩾25 years, and 0% (0/5051), <25 years (P=0·002); anti-HBc: 14·6% (99/679), ⩾25 years and 3·0% (151/5032), <25 years (P<0·001)].
DISCUSSION
This unlinked anonymous survey of BBVs in military recruits in 2007 tested samples from nearly 14 000 subjects. Overall, serological evidence of HIV and hepatitis C was found in 0·06% and 0·06% of recruits, respectively. Evidence of past cleared and current hepatitis B infection was found in 3·63% and 0·37% of recruits, respectively. HIV infections were found in recruits to the Army and RAF, hepatitis C in the Army and RN, and hepatitis B in all three Services. Both past and current hepatitis B infection was present at a significantly higher rate in Army recruits than in the other two Services.
While the overall HIV prevalence was relatively low, there was a statistically significant difference in HIV prevalence between UK (0·02%) and sub-Saharan African (1·24%) recruits. This difference probably reflects the higher HIV prevalence in sub-Saharan African countries compared to the UK. National prevalence estimates for the UK population aged 15–59 years indicated that 0·09% of the white UK population and 3·7% of the black African UK population were living with diagnosed HIV infection in 2007 [4]. While national prevalence estimates are higher than the prevalence observed in military recruits, the latter group are a much younger population, and the observed prevalence probably predominantly reflects undiagnosed HIV infection (those with diagnosed HIV infection being excluded from recruitment). The higher HIV prevalence observed in older recruits probably reflects the higher average age of recruitment associated with non-UK recruits (Fig. 2 a), a substantial proportion of whom were of sub-Saharan African nationality.
Interpretation of the anti-HBc prevalence of 3·63%, and an HBsAg prevalence of 0·37% within a general UK context is more difficult since there are few UK populations with appropriate hepatitis B prevalence data for comparison. Results are consistent with an unlinked anonymized survey of adults conducted in 1996 in England and Wales [Reference Gay5] which found an anti-HBc prevalence of 3·9%. More recently, in women attending antenatal clinics in England, the prevalence of HBsAg was 0·44% in 2007 (HPA CfI, personal communication), but data were not collected on anti-HBc prevalence in this population. Of injecting drug users (attending services in England, Wales and Northern Ireland [6]), anti-HBc prevalence was measured at 15% in 2007. In the same year, an HBsAg prevalence of 0·03% was found in new blood donors [7].
While the unlinked anonymous survey in 1996 is probably the best comparator, none of these groups represent fair comparisons with military recruits. For instance, antenatal women represent an older age group than military recruits, injecting drug users represent a group with particularly high-risk behaviours for acquiring hepatitis B, while blood donors are at particularly low risk for hepatitis B due to the donor screening process that precedes donation. As with HIV, the elevated hepatitis B prevalence in Army recruits of sub-Saharan African nationality probably reflects the high prevalence of hepatitis B in countries of that region. Similarly, the higher prevalence in older age groups probably reflects the older age of recruitment of non-UK recruits.
The prevalence of hepatitis C for military recruits was 0·06%. There were no significant differences in prevalence between nationalities or ethnic groups. This estimate is lower than that found in an unlinked anonymous sample of adults conducted in 1996 (0·7%) [Reference Balogun8]. The prevalence estimate for hepatitis C in the population of England and Wales aged 15–59 years was 0·72% in 2003 [9]. The difference in prevalence probably reflects the younger age of military recruits, compared to the England and Wales population and the reduced likelihood that injecting drug users enter the Armed Forces (recruits self-declare that they do not inject drugs on entry).
There are some limitations to the survey results. The demographic profiles of the 96 recruits who objected to being included in the study were not known. However, 76% (73) of the objectors originated from one training centre, which did not differ from other training centres in the distribution of ethnicity and nationality of recruits; this, and the small proportion of objectors, suggests the exclusion of the objectors is unlikely to have biased the sample. Only 60% of laboratory samples could be linked to full demographic data due to incomplete electronic personnel records and difficulty in matching between recruits. However, there was no significant difference in BBV prevalence between laboratory results that were fully linkable to demographic data and results that could be linked to Service and training centre only. Consequently the 8291 results fully linkable to demographic data can reasonably be extrapolated to the entire sample. While the general completeness of demographic variables able to be matched to demographic data was good, completeness was poorer in RN recruits for age group and gender variables. This was due to difficulties in obtaining electronic data for these variables.
Steps to eliminate deductive disclosure disallowed the cross-referencing of results between different demographic and risk groups and, consequently, multiple-regression analyses. However, in anticipation of this, demographic data were analysed prior to data reconfiguration to assess the degree of association between demographic variables. This allowed the identification of associations between nationality and age group.
It is not current policy to screen recruits for BBVs on entry into the UK Armed Forces, or to conduct interval testing of Armed Forces personnel (e.g. on return from operations abroad). All recruits entering the United States (US) Military Services have been screened for HIV since 1985 [Reference Bautista10]; the data measure prevalence at 0·7% in this population which is much higher than in UK recruits. The age distribution of recruits between the two countries is similar (53% aged <20 years in the US Armed Forces compared to 50% for the UK) but the USA has a higher proportion of non-white recruits (21% vs. 7%, respectively) [Reference Bautista10].
Our findings suggest that those recruited into the UK Armed Forces from sub-Saharan Africa could be selectively screened on entry into the UK Armed Forces. The presence of hepatitis B in all Services, regardless of nationality and commissioned status, and the additional risks associated with active service, demonstrates the need for further discussion about routine hepatitis B vaccination within the Armed Forces. Future policy on screening and vaccination requires assessment to ensure such measures are cost-effective, and do not discourage or prevent potential recruits from high-prevalence countries from being recruited to the UK Armed Forces.
ACKNOWLEDGEMENTS
We thank the staff at phase one training centres for implementing the survey at their sites and providing demographic data. Thanks are also extended to laboratory staff at National Blood Service Bristol for processing the samples before anonymous testing. We also express our gratitude to Bharati Patel and Sharon Barnett for their valuable contribution to this project through the laboratory testing for blood-borne viruses.
DECLARATION OF INTEREST
None.





