Background
The coronavirus disease 2019 (COVID-19) pandemic has revealed new opportunities for stewardship of diagnostic tests in healthcare settings and has demonstrated the value of close collaboration between healthcare epidemiologists and clinical laboratorians to prevent infection transmission and optimize patient outcomes. Reference Fabre, Davis and Diekema1 Throughout the COVID-19 pandemic, clinicians have adapted to rapidly changing realities and recommendations to keep patients and healthcare personnel safe. Early on, testing was limited to a few high-complexity, commercial, or laboratory-developed molecular assays and was unavailable or in extremely short supply in many areas. Reference Sharfstein, Becker and Mello2 Testing was reserved for patients with fever and respiratory symptoms, often with turnaround times of a week or more, meaning that testing had little impact on treatment or infection prevention decisions. When it became apparent that asymptomatic and presymptomatic persons could transmit infection, indications for testing expanded to include detection of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) regardless of symptoms. Recommendations for incorporation of diagnostic testing to help prevent transmission of SARS-CoV-2 and subsequent infection in healthcare facilities soon followed; this guidance was extrapolated largely from experience with other respiratory virus outbreaks when diagnostic testing was limited. Reference Jones3
As more laboratory and point-of-care molecular and then antigen tests became available and new SARS-COV-2 variants emerged, healthcare facilities faced the challenge of determining how to deploy testing to diagnose infection in symptomatic patients who might qualify for specific pharmacologic therapies, as well as to identify patients who were asymptomatic or mildly ill but who could act as sources of nosocomial or occupational transmission. Considerations for infection prevention included (1) whom to test, (2) timing of initial and/or repeat testing, (3) test methodology (ie, nucleic acid versus antigen target, laboratory-based versus point-of-care), and (4) result interpretation. Although hundreds of molecular and antigen tests to detect SARS-CoV-2 eventually became commercially available, 4 use of testing often reflected availability of test kits, specimen collection supplies, and testing personnel at individual healthcare facilities, which varied greatly over time.
We highlight diagnostic stewardship lessons learned during the COVID-19 pandemic and discuss how diagnostic stewardship principles can inform future efforts to prevent transmission of emerging pathogens in healthcare settings. We focus on the approach to testing patients and healthcare personnel for infection prevention purposes in acute-care settings. Broader discussions of laboratory testing to diagnose COVID-19 are published elsewhere. Reference Hayden, Hanson, Englund and Lee5,6
Diagnostic testing strategies for preventing transmission of emerging pathogens in healthcare settings
We identified 4 factors that influence diagnostic stewardship decisions aimed at preventing transmission of emerging pathogens in acute healthcare settings (Table 1).
Table 1. Diagnostic Stewardship Considerations for Emerging Pathogens in Healthcare Settings
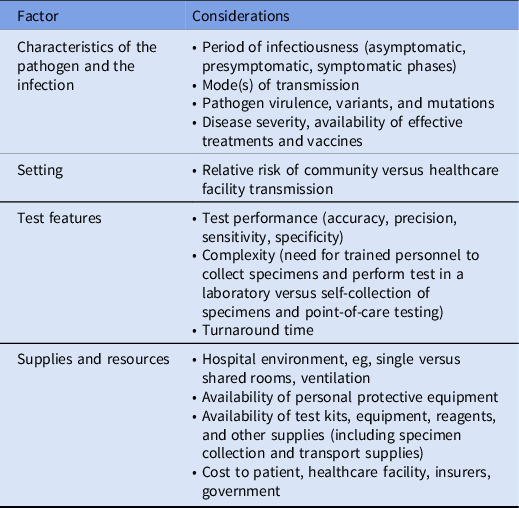
Characteristics of pathogen and infection
Period of infectiousness
The period of infectiousness is the time during which a person can transmit a pathogen, and it may include asymptomatic, presymptomatic, and symptomatic periods. The period of infectiousness can be determined only by careful epidemiologic studies, which include contact tracing and examination of transmission pairs. Reference Johansson, Quandelacy and Kada7,Reference Boucau, Marino and Regan8 Defining the period of infectiousness for SARS-CoV-2 has been challenging. It is estimated that transmission of SARS-CoV-2 during presymptomatic and asymptomatic infection accounts for 30%–60% of transmission events. Reference Johansson, Quandelacy and Kada7 Moreover, the viral variant and the vaccination status of the infected person may affect the duration of infectiousness. Reference Wu, Kang, Guo, Liu, Liu and Liang9 Both hospitalized patients and healthcare personnel may be unknowingly infected with SARS-CoV-2, and both may transmit the virus to other patients and healthcare workers if appropriate personal protective equipment (PPE) and other infection prevention measures are not employed. Reference Lucey, Macori and Mullane10
To help mitigate this risk, multiple SARS-CoV-2 testing strategies have been implemented, with the goals of ensuring appropriate transmission-based precautions for patients infected with SARS-CoV-2, and of excluding infectious patients with nonurgent medical needs and infectious healthcare personnel from the workplace. 11 However, the utility of these approaches has been hampered by the lack of a laboratory marker of infectivity; the unwillingness of healthcare personnel to always comply with testing; and the cumbersome and operationally disruptive processes of contact tracing, testing, and furloughs of exposed healthcare personnel. Reference Iddins, Waugh and Buck12,Reference Hijano, Hoffman and Tang13 Furthermore, results of diagnostic tests, especially molecular tests, were found to be poor predictors of infectivity, particularly in asymptomatic individuals. Reference Talbot, Hayden and Yokoe14 The COVID-19 pandemic highlights the importance of determining the period of infectiousness for emerging infectious pathogens and of establishing laboratory markers of contagiousness.
Mode of transmission
SARS-CoV-2 is spread primarily through aerosols and droplets. 15 The COVID-19 pandemic highlighted the challenges of defining the medical procedures that generate aerosols and theoretically pose the highest risk to healthcare personnel for pathogen exposure. The Centers for Disease Control and Prevention (CDC), 16 the World Health Organization (WHO), 17 and several professional societies have published lists of putative aerosol-generating procedures. In turn, healthcare facilities often prioritized testing of patients before these procedures (eg, emergency intubation and extubation) to reduce transmission. Reference Mick and Murphy18 Additionally, due to concerns that asymptomatic or presymptomatic hospitalized persons may contribute to viral transmission via the airborne route, Reference Sah, Fitzpatrick and Zimmer19–Reference Duval, Palmer and Tudge21 many healthcare facilities routinely tested patients for SARS-CoV-2 infection at the time of hospital admission or prior to transfer to another healthcare facility, regardless of patient symptoms. This information was used to guide patient room assignment, especially in hospitals where single rooms were scarce. Some facilities took screening a step further and used in-house, laboratory-developed molecular tests to identify SARS-CoV-2 genotypes during periods when multiple variants of concern were circulating and used this information to cohort patients who were infected with the same variant. Reference Matic, Lowe and Ritchie22 Although diagnostic testing should improve prediction of the likelihood of COVID-19, evidence to support the value of testing asymptomatic persons in reducing transmission of SARS-CoV-2 in healthcare settings or preprocedure testing remains sparse and of low quality. Reference Talbot, Hayden and Yokoe14,Reference Brody, Shi and Shaffer23,Reference Moreno-Pérez, Merino, Chico-Sánchez, Gras-Valentí and Sánchez-Payá24
Pathogen variants and mutations
Mutations in the genetic sequence or antigenic composition of a pathogen can develop quickly and affect diagnostic test performance. 25,Reference Osterman, Badell and Basara26 The emergence of transmissible SARS-CoV-2 variants with shorter incubation periods relative to earlier strains Reference Lyngse, Kirkeby and Denwood27,Reference Allen, Vusirikala and Flannagan28 has also influenced the frequency and timing of testing (eg, postadmission vs postexposure) needed to identify presymptomatic or asymptomatic infections.
Disease severity
The risk of severe disease among patients may influence testing strategies in acute-care settings. Although most individuals diagnosed with COVID-19 have mild-to-moderate symptoms, older adults, those who have not been vaccinated, and those with underlying medical conditions or immune compromise are at higher risk for severe disease. Reference Booth, Reed and Ponzo29 Because hospitalized patients are generally more susceptible to developing severe disease compared with the general population, Reference Ponsford, Ward and Stoneham30,Reference Elkrief, Desilets and Papneja31 SARS-CoV-2 testing of hospitalized patients has been used to diagnose and treat infections as soon as possible and to reduce the risk of nosocomial transmission in this vulnerable population.
Setting
The utilization of diagnostic testing for infection prevention is driven largely by the incidence and risk of transmission in different settings. During the COVID-19 pandemic, the burden of community transmission influenced decisions to screen for COVID-19 among hospitalized patients. Reference Ponsford, Ward and Stoneham30 Early on, when the spread of COVID-19 was confined to certain areas of the country, selective testing of symptomatic hospitalized patients based on epidemiologic factors was common; however, results were often not available quickly enough to be used for decision making. Reference Elkrief, Desilets and Papneja31 Without prompt test results, decisions regarding infection prevention and isolation were often made using the pretest probability for COVID-19 (ie, based on presenting symptoms, exposures, clinical history, and community infection prevalence). Reference Hayden, Hanson, Englund and Lee5 The role of testing to prevent transmission has changed as we have learned how to protect healthcare personnel from respiratory exposure to the virus and determined that fomites and surfaces posed a low risk for transmission. We observed that work-related transmission among healthcare personnel was largely from socializing among colleagues. 17 Healthcare personnel-to-patient transmission of SARS-CoV-2 is mitigated when healthcare personnel appropriately adhere to basic infection-control mandates. Patient-to-patient spread of SARS-CoV-2 remains a concern in multibed hospital rooms, and diagnostic test screening in these settings may still be warranted (see the section on Supplies and other resources below).
Test features
Test performance
The goal of screening hospitalized patients using diagnostic tests for SARS-CoV-2 is to identify those who may be infectious so that measures can be taken (eg, isolation, enhanced PPE) to reduce the risk of nosocomial transmission. Both nucleic acid amplification tests and antigen detection tests have been used for this purpose, although each methodology has its limitations. Molecular assays are highly sensitive and detect very low quantities of virus. 6 Thus, the negative predictive value of molecular tests is quite high, both early and late in the disease course or when the prevalence of infection is high. In contrast, antigen tests have lower sensitivity but may have higher specificity, appearing to correlate better than molecular tests with results of viral culture. Reference Hayden, Hanson, Englund and Lee5 The accuracy of both molecular and antigen tests is lower in asymptomatic persons compared to symptomatic patients. Reference Hayden, Hanson, Englund and Lee5,6
These test performance features present challenges for determining whether to isolate asymptomatic hospitalized patients who test positive for COVID-19 or to quarantine exposed patients who test negative. Furthermore, test performance characteristics rely on the assumption that tests are being performed correctly. However, improper specimen collection or technical errors in laboratory or point-of-care test performance can lead to false-negative or false-positive results. Finally, different anatomic sites (nasopharyngeal swab vs saliva vs anterior nares swab) may harbor different concentrations of virus, which may affect test performance when different sites are sampled. 6,Reference Tsang, So, Ng, Cowling, Leung and Ip32 ,
Many discussions of test performance focus on analytic sensitivity and specificity or the ability of a test to detect or exclude a substance of interest. However, clinicians need information about clinical sensitivity and specificity, where tests results are correlated to populations of patients with or without disease. Reference Saah and Hoover33 For COVID-19, the determination of clinical performance of a test is particularly challenging because asymptomatic infection is common. With a high prevalence of COVID-19 infection in a community, the positive predictive value of a test for SARS-COV-2 is higher, and the likelihood of a false-positive result on hospital admission or preprocedural test is lower. However, during periods of low COVID-19 prevalence, the positive predictive value decreases, and falsely positive test results may lead to unnecessary isolation among hospitalized patients or unnecessary delays in surgery. Reference Hayden, Hanson, Englund and Lee5,6,Reference Srinivasan, Gohil and Abeles34,Reference Coffey, Diekema and Morgan35 Also of concern are false-negative results, which can occur especially with antigen tests in asymptomatic persons. A systematic review reported a pooled sensitivity of 81% (95% CI, 78%–84%) for antigen test detection of SARS-CoV-2 compared to standard nucleic acid amplification testing. Reference Hayden, Hanson, Englund and Lee5
Interpreting test results
The COVID-19 pandemic has highlighted the pitfalls associated with inferring infectious status from results of a diagnostic test. Although demonstration of replication-competent virus in culture may be a minimum requirement for transmission, it correlates poorly with risk of transmission in real-world settings. Reference Boucau, Marino and Regan8 In clinical laboratories, viral culture has been replaced almost universally by molecular assays such as PCR, which have superior diagnostic performance characteristics and turnaround times. During the COVID-19 pandemic, clinicians and healthcare epidemiologists often used the cycle threshold (Ct) values that are generated during RT-qPCR to infer SARS-CoV-2 transmission risk. Ct values are inversely correlated with the quantity of nucleic acid target in a sample; a low Ct value correlates with a higher quantity of target, and a high Ct value correlates with a lower quantity of target. For SARS-CoV-2, viable, replication-competent virus is rarely cultured from samples with Ct values >30 on or after 14 days of illness, suggesting infectivity decreases with an increasing Ct value. Reference Binnickera36 However, Ct values can vary depending on the quality and volume of the sample, the type of commercial kit used, the timing of testing in the course of illness, and the virus variant. In a national proficiency testing survey conducted by the College of American Pathologists, the median Ct values reported by different FDA EUA test methods using identical control material varied by as many as 14 cycles (ie, 4,000-fold), and reproducibility of the control sample tested on the same testing platform differed by a median of 3 cycles (ie, 10-fold). Reference Rhoads, Peaper and She37 Notably, during the pandemic, many clinical laboratories have used multiple different molecular test methods simultaneously to meet high-volume testing demands and to account for supply shortages. Thus, Ct values may be different even when testing the same sample in a single laboratory. The Infectious Diseases Society of America (IDSA) and the Association for Molecular Pathology (AMP) recommend against the routine use of Ct values as markers of infectiousness or disease stage. 38 Antigen test results appear to correlate better with viral culture, particularly early in the course of disease when risk of transmission is greatest, although the correlation is still imperfect. Reference Hayden, Hanson, Englund and Lee5 Determination of a marker of infectivity remains an important research goal.
Supplies and other resources
For emerging pathogens, determining where and how transmission occurs is critical to developing infection prevention guidelines for hospitals. During the COVID-19 pandemic, healthcare settings were forced to convert spaces and environments to expand and optimize patient care and limit the risk of SARS-CoV-2 transmission. However, one of the most significant risk factors for SARS-CoV-2 transmission in hospital settings was shared patient rooms. Reference Trannel, Kobayashi and Dains39,Reference Klompas, Baker and Rhee40 Hospitals that were unable to exclusively use private rooms for patient care relied on widespread admission testing of patients and sometimes serial surveillance testing to reduce the risk of nosocomial SARS-CoV-2 transmission. Dedicated COVID-19 units were created to cohort patients and facilitate patient care.
The availability of adequate supplies, including PPE for healthcare personnel, is another determinant of the need for diagnostic testing for infection prevention. Early during the COVID-19 pandemic, many healthcare facilities did not have adequate PPE for healthcare personnel. The CDC published conventional/crisis guidance for healthcare facilities regarding how to conserve resources. 41 Healthcare facilities developed individual triage plans for PPE to mitigate COVID-19 risk. Healthcare personnel who did not have access to appropriate PPE were at higher risk for exposures, necessitating the need for testing and contact tracing among exposed employees and patients. At the same time, clinical laboratories struggled with shortages of specimen collection supplies, test kits, and testing equipment, limiting availability of testing. Reference Cornish, Bachmann and Diekema42 The release of rapid, point-of-care antigen testing later in the pandemic allowed for more timely testing of exposed patients and healthcare workers, but at a cost of lower analytical sensitivity. Considerations for future pandemics should include development of flexible, accurate point-of-care tests that could be tailored quickly to identify emerging pathogens streamlining regulatory review and approval of tests and assuring widespread and equitable availability of assays. Finally, who should pay for testing, including direct and indirect costs associated with the process, needs to be considered because even well-funded hospitals are unlikely to be able to sustain the burden for long periods.
Real-world diagnostic stewardship scenarios in acute healthcare settings
We posed real-world clinical dilemmas in the setting of emerging pathogens and list considerations for determining the testing approach based on each of 4 factors: (1) features of the pathogen; (2) healthcare setting; (3) type of test; (4) facility and supply variables.
1. Should a healthcare facility perform admission testing of all patients?
-
(1) Features of the pathogen: Considerations include the period of infectiousness, including an asymptomatic or presymptomatic infectious period, and the possibility of widespread transmission through droplet or airborne routes, as well as severity of illness.
-
(2) Setting: Considerations include the risk of severe disease among hospitalized patients, the extent of the outbreak (ie, affecting the entire population or only a specific subgroup?), the ability to identify high-risk patients based on clinical or exposure history alone, and whether certain patients should be prioritized for testing.
-
(3) Type of test: Considerations include the availability of a low-complexity, analytically sensitive and specific test with a rapid turnaround time, the type of specimen, the need for collection by trained personnel versus patient self-collection, the ability of the test to detect presymptomatic or asymptomatic patients, and the availability of testing at the point of care.
-
(4) Facility and supply variables: Considerations include the availability of adequate PPE and training for healthcare personnel, the ability to isolate patients, including the presence of behavioral health units, and the availability of tests and laboratory personnel.
Diagnostic stewardship discussion
Admission testing (in addition to primary infection control interventions) of all patients may be considered if the disease is widespread, highly transmissible including in presymptomatic or asymptomatic patients, disease severity is high, there are an adequate number of accurate tests and laboratory support. Alternatively, selective testing may be considered if availability of tests is limited, the pathogen is less transmissible, disease severity is mild, or high-risk patients can be identified based on clinical or exposure history.
2. Should preprocedural or presurgical testing of patients be performed (to prevent healthcare-associated transmission)?
-
(1) Features of the pathogen: Considerations include the period of infectiousness (including whether there is an asymptomatic or presymptomatic period), mode of transmission, and whether certain procedures increase transmission risk (eg, intubation and extubation for airborne pathogens).
-
(2) Setting: Considerations include the extent of the outbreak (ie, is this affecting the entire population or only a small proportion?), the ability to identify high-risk patients based on clinical or exposure history alone, and whether certain patients should be prioritized for testing (eg, admission to semiprivate room is anticipated post-procedure).
-
(3) Type of test: Considerations include turnaround time, sensitivity and specificity, and whether there is information regarding the optimal time that patients might be tested prior to the procedure.
-
(4) Facility and supply variables: Considerations include adequate PPE and training for healthcare personnel, the ability to isolate patients, the availability of tests and laboratory support, patient consequences of delaying procedures or operations.
Diagnostic stewardship discussion
Preprocedure screening may provide minimal additional benefit when other effective infection control strategies are in place but may be considered if the disease is widespread, highly transmissible, PPE supplies are limited or unavailable, and the procedure increases the risk of transmission. Facilities should balance potential benefits with consequences for patients if procedures are canceled or delayed due to positive tests.
3. Should healthcare personnel be tested routinely?
-
(1) Features of the pathogen: Considerations include the period of infectiousness (including whether there is asymptomatic or presymptomatic period), whether certain groups of individuals are at higher risk for severe disease, mode of transmission.
-
(2) Setting: Considerations include the extent of the outbreak and spread among healthcare personnel, from healthcare personnel to patients, or from patients to healthcare personnel.
-
(3) Type of test: Considerations include the availability of a sensitive and specific test with a rapid turnaround time, the ability of the test to detect presymptomatic or asymptomatic patients, specimen type, availability of home testing.
-
(4) Facility and supply variables: Considerations include the availability of PPE and training for healthcare personnel, availability of testing supplies and laboratory support (if testing is laboratory based).
Diagnostic stewardship discussion
Routine testing of healthcare personnel could be considered if there is a high likelihood that healthcare personnel transmit the infection to coworkers or to patients, the disease is widespread, transmitted during presymptomatic or asymptomatic periods, and an adequate number of tests with short turnaround times are available. If healthcare personnel-to-patient transmission is a concern, selective testing of healthcare personnel could be considered for those working with patients at high-risk for acquiring infection and/or severe disease. If staffing is at crisis levels, then healthcare worker testing may not be actionable.
4. Should a facility perform contact tracing and/or routine surveillance testing of patients and healthcare personnel?
-
(1) Features of the pathogen: Considerations include the period of infectiousness, including whether there is an asymptomatic or presymptomatic period, incubation period between exposure and infection, whether certain groups of individuals are at higher risk for severe disease, as well as mode of transmission.
-
(2) Setting: Considerations include whether spread of disease within a facility justifies testing.
-
(3) Type of test: Considerations include the availability of a sensitive and specific test with a rapid turnaround time, the ability of the test to detect presymptomatic or asymptomatic patients if needed, as well as specimen type.
-
(4) Facility and supply variables: Considerations include an adequate number of healthcare personnel to perform contact tracing, the ability to quarantine patients and/or healthcare personnel, and the availability of private rooms or areas to quarantine patients, as well as testing availability and laboratory support.
Diagnostic stewardship discussion
Contact tracing often requires significant resources and may be of low value if there are many potential sources of community exposure and transmission. Contact tracing or surveillance testing might be considered if the disease has limited spread, is transmitted during presymptomatic or asymptomatic periods, has a long incubation period, there are adequate tests available with short turnaround times or there is evidence of spread of disease within a facility.
Research recommendations for diagnostic stewardship in emerging infectious diseases
Extreme day-to-day demands on healthcare epidemiologists and infection preventionists during the COVID-19 pandemic limited the opportunities to conduct research, and many questions remain. Key needs exist both to optimize COVID-19 prevention in healthcare settings and to inform future pandemic preparedness. First, systematic analysis of the impact of widespread testing among hospitalized patients is needed. Most published studies cannot answer whether there are benefits to testing, much less identify the best diagnostic stewardship factors, including optimal populations, indications, and frequency of testing. Second, certain outlier situations do not fit into published criteria for COVID-19 testing. For example, among severely immunocompromised patients, SARS-CoV-2 virus may be detected using both molecular and antigen tests for extended periods following an initial diagnosis. Because live virus has been recovered from immunocompromised patients for >10 days following an initial diagnosis, the CDC recommends a ‘test-based strategy’ to determine the duration of isolation for patients with SARS-COV-2 infection who are moderately to severely immunocompromised. 43 It is likely that testing strategies for future pandemic pathogens will also have to be modified for immunocompromised patients or other groups (eg, infants, elderly adults) whose period of test positivity or infectiousness may differ from that of the otherwise healthy population. The period of infectiousness for patients with COVID-19 has not been demonstrated; the period of infectiousness should be better characterized and may change in the future as the virus continues to evolve. Determining whether any tests that are currently available correlate with infectiousness would improve diagnostic stewardship for prevention of transmission, although new tests may be needed. Finally, comparing the results of systematic contact tracing using different assays and testing approaches while considering the incubation period of the infection may help to improve diagnostic stewardship decisions by identifying the optimal balance between test turnaround time and performance characteristics. For COVID-19 and future pandemic pathogens, research should determine the relative impact and cost of different diagnostic testing strategies.
In conclusion, the COVID-19 pandemic has presented challenges for testing and diagnostic stewardship in healthcare settings. The rationale and expected impact of different approaches to testing was often not clear when policies were adopted. We have identified factors that influence diagnostic stewardship decisions aimed at preventing transmission in healthcare settings (eg, pathogen characteristics, test features, settings, supply, and resources), and we have proposed a framework for considering testing decisions for a new pathogen. Infection prevention teams can use the lessons learned from the COVID-19 pandemic to help guide future diagnostic stewardship decisions to prevent transmission of emerging pathogens in healthcare settings.
Acknowledgements
We have endorsement from IDSA Diagnostics Committee, Society of Hospital Medicine, Society of Infectious Diseases Pharmacists, and the Pediatric Infectious Diseases Society.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
Dr. Diekema reports consulting fees regarding development of molecular diagnostics from OpGen, Inc and a bioMerieux research contract for clinical trials of new antimicrobial susceptibility testing devices. Dr. Hayden reports payment for service on a clinical adjudication panel for an investigational SARS-CoV-2 vaccine manufactured by Sanofi. All other authors report no conflicts of interest relevant to this article.




