Memory is an important constituent of higher-level cognition. Specifically, executive functioning is suggested to mechanistically influence, and be influenced by, numerous dimensions of memory, including episodic, working and semantic memory( Reference Carpenter, Just and Reichle 1 , Reference Mazoyer, Zago and Mellet 2 ). In terms of short- and long-term memory, episodic memory refers to the memory of an event or an ‘episode’. Working memory capacity and executive function share a common underlying executive attention that is strongly predictive of higher-level cognitive function, including episodic memory( Reference McCabe, Roediger and McDaniel 3 ). Executive function includes subcomponents of cognition, such as cognitive-related inhibition and reasoning. These parameters may help to facilitate basic cognitive functioning required to perform goal-directed behaviours (e.g. inhibiting and filtering distractive stimuli). Further, individuals with enhanced levels of executive function generally have the ability to maintain an appropriate mental state to fulfil a future goal( Reference Posner and DiGirolamo 4 ), which may include cognitive processes such as planning, filtering competing information, maintaining efforts despite distractions and inhibiting goal-inconsistent responses( Reference Glass, Buu and Adams 5 ). Lastly, semantic memory involves retrieval of factual information that is learned over a period of time (e.g. the definition of a word)( Reference Slotnick 6 ) and is not bound to any specific experience in which the memory was acquired( Reference Eichenbaum 7 ). Evaluation of factors influencing semantic memory is of particular importance as semantic functional MRI (fMRI) activation has been shown to serve as a better predictor of cognitive change when compared with episodic fMRI tasks( Reference Hantke, Nielson and Woodard 8 ).
Emerging work suggests that obesity is associated with worse memory function. For example, chronic obesity may detrimentally influence memory function through morphological brain changes, insulin resistance, neuroinflammation, TAG metabolism, circulating levels of glucocorticoids and cerebral metabolite concentrations( Reference Bruce-Keller, Keller and Morrison 9 ). In addition to obesity, research demonstrates that obesity-related diets, such as the ‘Western diet’ (high in saturated fats and simple sugars), has been shown to correlate with impairments in learning and memory( Reference Kanoski and Davidson 10 – Reference Titova, Ax and Brooks 14 ). Further, some work suggests that such memory impairments may be diet-induced, as opposed to be driven by changes in adiposity( Reference Coppin, Nolan-Poupart and Jones-Gotman 15 ). For example, results from recent animal studies demonstrate that spatial and working memory deficits are observable after only a few days of consuming a Western diet( Reference Kanoski and Davidson 16 ). Human studies also indicate diets high in vegetables, fruit, fish, soya products may benefit cognitive functioning in older individuals( Reference Jiang, Huang and Song 17 ).
Existing observational work examined dietary inflammatory index (DII®) scores and cognitive function over time. This includes evidence for pro-inflammatory diets to correlate with incident cognitive impairment and dementia among older women( Reference Hayden, Beavers and Steck 18 ), as well as middle-aged men and women evaluated 13 years following initial cognitive assessment( Reference Kesse-Guyot, Assmann and Andreeva 19 ). Related work( Reference Kesse-Guyot, Assmann and Andreeva 19 ) has examined the association of DII on semantic memory and working memory, although episodic memory performance was not included as an outcome of interest( Reference Kesse-Guyot, Assmann and Andreeva 19 ), which is a novel addition of our present study. We evaluated this topic further, by extending the investigation to include a nationally representative sample of US older adults, as this population is susceptible to age-related memory impairment( Reference Nyberg, Backman and Erngrund 20 ). Thus, the main purpose of this study was to examine the association between DII scores and a test battery of specific memory functions. In addition, to comprehensively evaluate the association between DII and memory, we examined this potential association while considering various memory parameters, including episodic memory, working memory and semantic memory.
Methods
Study design and participants
The National Health and Nutrition Examination Survey (NHANES) is an ongoing survey conducted by the Center for Disease Control and Prevention. NHANES employs a nationally represented sample of U.S. adults evaluated through a multistage, clustered probability design. Participants are initially interviewed in their homes and then, within 1–2 weeks, examined in a mobile examination centre (MEC). Details of the NHANES methodology is available on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm).
NHANES procedures were approved by the National Center for Health Statistics institutional review board. Consent was obtained from all participants before data collection. Participant data from the 2011–2012 and 2013–2014 NHANES cycles were utilised, as these are the latest NHANES cycles with memory function data. The NHANES analytic sample included 1723 older adults 60–85 years (only those in this age range were eligible for memory assessment) of age who did not have one or more of the following chronic diseases: congestive heart failure, coronary artery disease, heart attack, stroke or physician-diagnosed diabetes.
Dietary inflammatory index
Dietary intake was assessed using the 24-h dietary recall interviews (24HR) that were validated by the Nutrition Methodology Working Group( 21 ). A single 24HR was used to calculate DII scores. The details of development of DII are described by Shivappa et al.( Reference Shivappa, Steck and Hurley 22 ). High sensitivity C-reactive protein (CRP) measurements were used to examine construct validity of the DII in a longitudinal cohort using 24HR and 7-d dietary recalls. Subsequently, the new DII also was validated in four studies among different populations with an extended number of inflammatory biomarkers (e.g. IL, IL-6, high-sensitivity C-reactive protein and TNF-α)( Reference Shivappa, Steck and Hurley 23 – Reference Ramallal, Toledo and Martinez-Gonzalez 27 ).
The DII consists of forty-five food parameters which include various macro- and micronutrients, flavonoids, spices and food items, each associated with an inflammatory effect score( Reference Shivappa, Steck and Hurley 22 ).These forty-five food parameters were based on findings contained in a total of 1943 articles that were reviewed and scored for their associations with these inflammatory biomarkers (CRP, IL-1β, IL-4, IL-6, IL-10 and TNF-α). A global database (food consumption from eleven populations globally) representing global daily intake for each of the forty-five parameters (i.e. foods, nutrients and other food components) was used as standard dietary intake to calculate the DII. A standard mean for each parameter from the representative world database was subtracted from the actual individual exposure and divided by its standard deviation to generate z scores. These z scores were converted to proportions (minimising effects of outliers/right- skewing). This value was then doubled and 1 was subtracted to achieve symmetrical distribution with values centred on 0. The resulting value was then multiplied by the corresponding inflammatory score derived from scoring the 1943 research articles for each food parameter and summed across all food parameters, to obtain the overall DII. To control for the effect of total energy intake, the DII was calculated per 1000 energy content of food consumed, which requires using the energy-standardised version of the world database. For the present study, twenty-six of the forty-five food parameters were available for DII calculation. Previous work indicated that there is no change in predictive ability of the DII in predicting inflammation when fewer food parameters are available (e.g. 26) compared with the full list of forty-five. In fact, rarely, if ever, do datasets have all available food parameters( Reference Shivappa, Steck and Hurley 22 ). These foods included carbohydrate, protein, fat, alcohol, fibre, cholesterol, SFA, MUFA, PUFA, niacin, thiamin, riboflavin, vitamin B12, vitamin B6, Fe, Mg, Zn, Se, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, β-carotene, n-6 and n-3.
Memory function
Several memory function assessments (episodic, semantic, and working memory/executive function) were employed, including the Consortium to Establish a Registry for Alzheimer’s disease( Reference Morris, Heyman and Mohs 28 ) (CERAD) Word Learning subset, the Animal Fluency test and the Digit Symbol Substitution Test (DSST).
The CERAD Word Learning subset has been used in several major epidemiological studies with diverse racial and cultural communities( Reference Fillenbaum, van Belle and Morris 29 – Reference Prince, Acosta and Chiu 32 ). This test specifically assesses episodic memory and consists of three learning trials, along with a delay trial (i.e. fourth trial). For the learning trials, participants read aloud ten unrelated words, one at a time, as they are presented on a computer screen. Following the tenth word, participants recalled as many words as possible. The order of the words changed across the three trials. The delayed trial occurred approximately 10 min after trial 1. The maximum score for each trial is 10.
The Animal Fluency test also has been employed in various epidemiologic cohorts( Reference Ramirez-Gomez, Zheng and Reed 33 – Reference Grundman, Petersen and Ferris 37 ), assessing verbal fluency( Reference Duff, Schoenberg and Scott 38 ) and semantic-based memory function. In this task, participants are asked to name as many animals as possible in 1 min. One point was given for each named animal.
The DSST is a component of the Wechsler Adult Intelligence Test( Reference Wechsler 39 ), and has been used in large epidemiological and clinical studies( Reference Bienias, Beckett and Bennett 40 – Reference Proust-Lima, Amieva and Dartigues 42 ). The DSST relies on processing speed, sustained attention and working memory, and is frequently used as a sensitive measure of frontal lobe executive function( Reference Vilkki and Holst 43 , Reference Parkin and Java 44 ). The DSST was assessed using a paper-and-pencil format. At the top of the paper was a key containing nine numbers paired with symbols. Participants had two minutes to copy the corresponding symbols in 133 boxes that adjoin the numbers. A score was given for each correct match, with maximum score of 133.
Statistical analysis and covariates
Analyses (Stata®, version 12) accounted for the complex survey design employed in NHANES by utilising sample weights, primary sampling units and strata via the Taylor series (linearisation) method. Sample weights were re-weighted to account for the use of combined NHANES cycles. This was done by multiplying the 2-year cycles by 0·5. Information on the use of sample weights to generate population weighted estimates is available elsewhere( Reference Epstein, Temple and Roemmich 50 ).
Multivariable linear regression analyses were fit. Analytical assumptions of linear regression were checked and confirmed not to be violated. Models were computed separately for each memory outcome. In each model, DII was categorised into quartiles (lowest quartile as referent; see Table 1 for the mean DII values across the quartiles), with covariates including: age (years; continuous), sex (male/female), race-ethnicity (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black and other), measured BMI (kg/m2; continuous), self-reported smoking status (never, former, current), self-reported average hours of sleep each night (h/night; continuous), self-reported engagement in leisure-time moderate-to-vigorous physical activity (min/week) assessed from the Global Physical Activity Questionnaire( Reference Cleland, Hunter and Kee 45 , Reference Bull, Maslin and Armstrong 46 ), and Patient Health Questionnaire (PHQ-9) assessed depression symptomology (range=0–27; continuous). Statistical significance was established as a nominal α of 0·05.
Table 1 Weighed characteristics of the study variables (n 1723), National Health and Nutrition Examination Survey, 2011–2014 (Mean values with their standard errors)
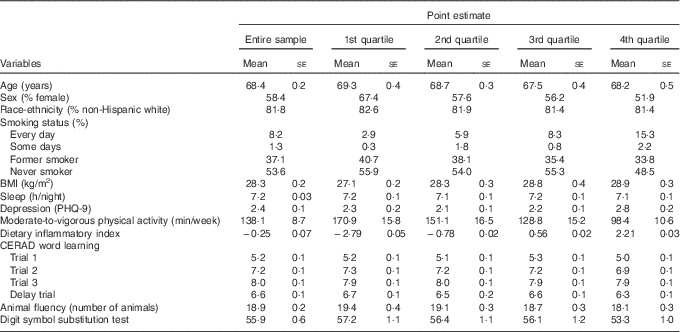
PHQ-9, Patient Health Questionnaire; CERAD, Consortium to Establish a Registry for Alzheimer’s disease.
Results
Table 1 displays the demographic characteristics of the study variables. Participants, on average, were 68·4 years, with the sample similarly distributed across sex (58 % female). Participants with a higher DII (i.e. 4th quartile) were more likely to be male, be a smoker, had a higher BMI score, and engaged in less physical activity.
In an adjusted model, and when DII was expressed as a continuous variable, DII was not statistically significantly associated with trial 1 (β=−0·02; 95 % CI −0·09, 0·03), trial 3 (β=−0·01; 95 % CI −0·07, 0·05) or the delay trial (β=−0·06; 95 % CI −0·15, 0·01) of the CERAD, but was significantly associated with Trial 2 of the CERAD (β=−0·08; 95 % CI −0·13, −0·01), the Animal Fluency test (β=−0·24; 95 % CI −0·42, −0·06) and the DSST (β=−0·64; 95 % CI −1·16, −0·13).
Table 2 displays the regression results examining the association between DII scores (categorised into quartiles) and memory function. Results were similar for the unadjusted and adjusted models. In addition, there was evidence of consistent inverse associations between DII scores and the different memory parameters. For episodic memory (CERAD Word Learning), those in the 4th (i.e. more pro-inflammatory) v. 1st (i.e. more anti-inflammatory) DII quartile recalled fewer words during the 10-min delay assessment (b adjusted=−0·39; 95 % CI −0·79, 0·00). Similarly, for semantic-based memory (Animal Fluency Test), those in the 4th v. 1st DII quartile listed fewer animal names (b adjusted=−1·18; 95 % CI −2·17, −0·20). Lastly, for the executive function and working-memory assessment (DSST), those in the 4th v. 1st DII quartile correctly matched fewer paired symbols (b adjusted=−2·80; 95 % CI −5·58, −0·02). Notably, there was no evidence of an interaction effect of sex and DII on any of the memory outcomes (results not shown).
Table 2 Regression results examining association between dietary inflammatory index (DII) and memory function (n 1723), National Health and Nutrition Examination Survey, 2011–2014Footnote * (Regression coefficients (b) and 95 % confidence intervals)
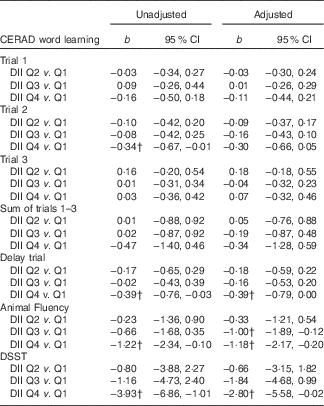
CERAD, Consortium to Establish a Registry for Alzheimer’s disease; Q, quartile; DSST, Digit Symbol Substitution Test; PHQ-9, Patient Health Questionnaire.
* In the adjusted model, covariates included age (years; continuous), sex (male/female), race-ethnicity (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black and other), measured BMI (kg/m2; continuous), self-reported smoking status (never, former, current), self-reported average hours of sleep each night (h/night; continuous), self-reported engagement in leisure-time moderate-to-vigorous physical activity (min/week) assessed from the Global Physical Activity Questionnaire and PHQ-9 assessed depression symptomology (range=0–27; continuous).
† Statistical significance (P<0·05).
Discussion
The purpose of this study was to examine the association between DII scores and a test battery of specific memory functions. The main finding of our study was that higher DII scores were associated with worse episodic memory, working memory, and semantic memory. Notably, however, DII was not consistently associated with the first three learning trials of the CERAD, but was significantly associated with the delayed trial of the CERAD. This suggests that dietary inflammation, as measured by the DII, may be less related to memory encoding, but may have a stronger influence on memory consolidation. Of course, future work is needed to evaluate this speculation.
Reduced cognitive performance has been evidenced in rats consuming an energy-dense, Western-style diet( Reference Kanoski and Davidson 10 ). Notably, cognitive functioning diminished following only 3 d of this high-fat dietary regimen, highlighting the plausible risk for deleterious cognitive outcomes to precede weight gain. This is a meaningful outcome, as not only are weight gain and chronic obesity associated with cognitive impairment, but acute deleterious changes in dietary practices may accelerate this risk of decline( Reference Kanoski and Davidson 10 ).
Although energetic requirements can be met by consuming a variety of healthful nutrients, Americans often consume foods high in refined carbohydrates and saturated fat( Reference Freeman, Haley-Zitlin and Rosenberger 47 , Reference Volkow, Wang and Baler 48 ). These foods are known to trigger dopaminergic reward pathways, strengthening learned associations between pleasurable food stimuli and immediate reinforcement( Reference Volkow, Wang and Baler 48 , Reference Higgs 49 ). Initially, ingestion of fatty and/or sugary meals increases the rate of dopaminergic firing, governed by the ventral tegmental area, along with dopamine release from the nucleus accumbens. Habitual intake of foods common to the Western diet, habituates the dopamine reward response to manifest even during the anticipation of experiencing food rewards( Reference Epstein, Temple and Roemmich 50 ). The hippocampus, an important subcortical memory structure, is also a key neuromodulator involved in the regulation of energy intake. Studies evaluating the influence of amnesic pathology in humans have shown that excessive eating behaviours may be accelerated following damage to the hippocampus( Reference Hebben, Corkin and Eichenbaum 51 – Reference Higgs 53 ). Other animal research suggests that hippocampal-dependent, episodic memory (including flexible memory) impairment may be attenuated by engaging in regular physical activity, despite concurrent consumption of a high-fat diet( Reference Klein, Jonas and Iggena 54 ). This finding lends further credence to the potential efficacy of physical activity to preserve memory functioning, counteracting diet-associated deficits in acute and delayed recall, attention, and processing speed( Reference Kanoski and Davidson 16 , Reference Francis and Stevenson 55 , Reference Holloway, Cochlin and Emmanuel 56 ). Our findings, however, demonstrate an association between DII and memory function, independent of self-reported physical activity behaviour.
It has been suggested that consumption of inflammatory-related, high-fat, high-sugar diets may induce both transient and sustained neurological deficits, particularly dependent upon hippocampal and prefrontal cortex (PFC) function( Reference Volkow, Wang and Telang 57 ). Inhibitory control and global memory function may suffer as a result of neural disturbances within these regions. Coupled with a heightened dopamine response, impaired inhibition and memory functioning contribute to poor appetite regulation via incongruous hunger cues( Reference Francis and Stevenson 58 ), which may lead to overeating and continuation of negative dietary behaviours. This cyclical response has been observed directly in rats that demonstrate an inability to regulate hunger signals, becoming hyperphagic following hippocampal lesions( Reference Higgs 53 , Reference Davidson 59 – Reference Davidson, Kanoski and Walls 61 ).
Regarding the crucial importance for adequate functioning of the hippocampus and PFC to remain intact, the hemispheric encoding retrieval asymmetry model posits these structures are responsible for distinct roles within the complex domain of memory mechanisms. Retrieval of semantic information is governed by the left PFC, whereas the right PFC directs retrieval of episodic memory( Reference Nyberg, Cabeza and Tulving 62 ). The Trace Transformation theory proposes acute memory processes are encoded and organised in the hippocampus, reach neocortical storage, and may then be transformed into shared, hippocampal-neocortical representations( Reference Nader 63 , Reference Loprinzi, Edwards and Frith 64 ). Taken together, the differential impact of dietary behaviours on these (and other) highly-integrated structures warrants continued scientific exploration. Dietary inflammation must be regarded as a preventable function of poor diet. Therefore, increasing the quality and quantity of reputable research on this topic, will do much to expand empirical knowledge of the downstream impact of the obesity epidemic. High-fat, high-sugar diets may impart neural and systemic risks associated with weight gain, memory impairment, and cognitive dysfunction( Reference Volkow, Wang and Baler 48 , Reference Gunstad, Paul and Cohen 65 ). However, recent work admonishes scientists to consider variable inflammatory-induced metabolic and cognitive responses specific to white, beige, and brown adipose tissue( Reference Cohen, Levy and Zhang 66 ) when attempting to explain the specific correlates of body weight and cognition, as the relationship may be more nuanced than expected( Reference Hao, Dey and Yu 67 ). Nevertheless, our findings that higher DII scores (i.e. consistent with greater diet-related inflammation) were associated with worse episodic memory, working memory and semantic memory are noteworthy, and should prompt continued research on this exigent health concern. Such work should continue to explore the specific dietary-related neuro-inflammatory effects on key cellular pathways (e.g. long-term potentiation) that subserve memory function. For example, emerging work suggests reduced synaptic potentiation, long-term potentiation, and glutamate release are suggested to be associated with IL-1 mediated inflammatory responses( Reference Murray and Lynch 68 ). Pro-inflammatory cytokines, such as IL-1, are found in high concentrations in the hippocampus( Reference Cunningham, Wada and Carter 69 ), and may exert profound negative effects on memory and cognition. Exogenously applied IL-1 can inhibit calcium influx( Reference Plata-Salaman and ffrench-Mullen 70 ), protein kinase A (PKA)( Reference Plata-Salaman and ffrench-Mullen 70 ), and release of acetylcholine( Reference Rada, Mark and Vitek 71 ) and glutamate( Reference Huang, Huang and Lin 72 , Reference Murray, McGahon and McBennett 73 ) in the hippocampus, all of which play a key role in the cellular basis of episodic memory.
Memory deficits, impaired neuronal growth and proliferation, and inhibited brain-derived neurotrophic factor activity also are driven by diet-induced endothelial cell dysfunction, and subsequent IL-1 release across the blood-brain barrier. Further, neuroinflammation linked with microglial phenotypic changes engendered by chronic systemic inflammation may limit the efficiency of long-term hippocampal potentiation( Reference Freeman, Haley-Zitlin and Rosenberger 47 , Reference Liu, Wu and Hayashi 74 ).
Diet-induced obesity also has been shown to impair dopaminergic signalling( Reference Johnson and Kenny 75 ). Diet-associated adiposity may impair memory as dopamine plays an important role in memory function( Reference Jay 76 ). For example, dopamine receptor-mediated signals (e.g. via D1 and D2 receptors) are important in facilitating long-term potentiation( Reference Frey, Matthies and Reymann 77 , Reference Frey, Schroeder and Matthies 78 ), a critical cellular basis of memory function (particularly episodic memory)( Reference Lynch 79 ). Dopamine regulation of long-term potentiation may occur via the D1/cAMP/PKA pathway, where the D1 receptor coupled to adenylate cyclase (AC) increases AC activity( Reference Jay 76 ). This leads to the formation of cAMP that activates PKA, which in turn can phosphorylate transcription factors (e.g. CREB) as well as phosphorylate both AMPA and NMDA receptors( Reference Jay 76 ), key receptors in memory-related long-term potentiation.
A limitation of this investigation was our use of a single measure of dietary intake, assessed across a time-period of 24 h. However, this measure has been validated (i.e. correlated with other markers of inflammation) in previous research in large populations( Reference Shivappa, Steck and Hurley 23 – Reference Ramallal, Toledo and Martinez-Gonzalez 27 ), and, thus, was an appropriate method to estimate DII for the purposes of the present study. Another limitation is that the NHANES memory assessments, particularly the DSST, does not exclusively measure memory function, as it also evaluates other sub-cognitive parameters such as cognitive processing speed and executive function. However, the consistent findings of an association between DII and the three memory-related assessments provides credence to these observations. In addition, NHANES did not collect data on cognitive impairment or neurocognitive disorders; thus, we were unable to take this into account when interpreting our findings. Further, like all epidemiological studies, it is not possible to fully discount the potential effects of residual confounding bias. We are also not able to infer causality of our observed associations given the cross-sectional design of our study. Thus, we cannot discount the potential reverse causality, or possible bi-directionally of DII and memory function. Lastly, based on the available NHANES data, we were only able to include twenty-six of the original forty-five parameters when calculating DII. Thus, our calculated DII may be an underestimate of the participant’s true DII, and as a result, our observed association between DII and memory may be underestimated. To our knowledge, this is the first study assessing older US men and women ages 60+ from a nationally representative sample. In addition, as no studies have explored the specific relationship between DII on a multitude of memory parameters, this paper is a robust first step to more comprehensive research. Future work should track dietary behaviour over multiple days to provide a more inclusive representation of changes in dietary behaviour across time.
In conclusion, we observed a consistent inverse association between DII and various memory types. Future experimental work confirming our findings are warranted. Our findings underscore the importance of eating an anti-inflammatory diet, not only for cardiovascular reasons, but for cognitive purposes as well. Future work investigating potential molecular mediators of the DII-memory relationship is also warranted.
Acknowledgements
Drs M. D. W., N. S. and J. R. H. were supported by grant no. R44DK103377 from the National Institutes of Health’s (NIH’s) National Institute of Diabetes and Digestive and Kidney Diseases. The NIH had no role in the design, analysis or writing of this article.
E. F. prepared part of the initial draft of the manuscript. N. S. assisted in the calculation of the DII. P. D. L. computed the analyses. E. F., N. S., J. R. M., J. R. H., M. D. W. and P. D. L. helped conceptualize the study and provided feedback on various drafts of the manuscript.
Dr J. R. H. owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the DII from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. Drs M. D. W. and N. S. are employees of CHI.





