Introduction
Cardiovascular diseases, including hypertension and atherosclerosis, are among the most common causes of mortality and disability, bringing about a heavy economic burden for public health systems worldwide. The versatile etiology and complex pathological mechanism of cardiovascular diseases make them hard to be cured completely. It is well established that an imbalance of absorption and consumption of nutrients contributes to metabolism disorders, becoming the leading risk factor for cardiovascular diseases. Thus, a large proportion of cardiovascular diseases could potentially be prevented by controlling the risk factors.(Reference Li, Liu and Joseph1) Accumulated epidemiological surveys and animal experiments support the hypothesis of foetal origins of adult diseases, which states that exposure in early life to environmental adverse factors, especially malnutrition, programs the susceptibility to chronic diseases in adult life.(Reference Gluckman, Hanson and Cooper2,Reference Barker3) Several deadly famine events in history created unique cohort populations to reveal that famine-induced foetal and postnatal malnutrition increased the risks of hypertension and coronary heart disease later in life.(Reference Roseboom, de Rooij and Painter4–Reference Bercovich, Keinan-Boker and Shasha8) More animal models further linked cardiovascular diseases with poor nutrition in early life, highlighting the role of prenatal nutrition in long-term vascular health, which is often underestimated.
The Great Chinese Famine (GCF, 1959–1961) affected the entire country and populations and caused millions of deaths from hunger, making it the worst famine in modern history worldwide.(Reference Smil9) During the three years, the dramatic induction in crop production made pregnant women and newborns subject to nutritional deficiency. Given that the first 1,000 d in human life are developmental critical periods sensitive to environmental factors, this period includes embryo, foetal and infant or early child stages. However, previous human famine models, including the famous Dutch Hungry, were relatively short periods for investigations of the influence of the first 1,000 developmental days’ exposure on later life. In comparison, GCF lasted for about 3 years, it offers unique opportunities to study possible effects of sole foetal exposure or multi-period exposure to the famine. Over the last decade, several epidemiological investigations have found the potential associations between GCF exposure during early life and increased risks of hypertension,(Reference Wu, Feng and He10–Reference Yang, Hong and Li15) stroke,(Reference Tao, Yang and Wang16–Reference Zhou, Zhang and Fang18) heart disease(Reference Meng, Yu and Guo19,Reference Du, Zheng, Xu and Zhu20) and carotid atherosclerosis(Reference Huang, Liu and Yu21,Reference Liu, Huang and Lo22) in adulthood.
We have reported that prenatally exposure to GCF contributed to an increased risk of mid-age hypertension as well as glycolipid metabolic dysfunction.(Reference Wu, Feng and He10,Reference Zhang, Pu and Ding23) Nevertheless, there is still a lack of information on the various effects of different exposure periods during GCF on cardiovascular diseases. We hypothesised that different foetal-original cardiovascular diseases have different famine exposure windows. Therefore, the design of this study focused on comparisons and analysis among various developmental timing periods exposure to GCF, looking for new information that may lead to finding sensitive ‘window’ stages during the 1,000 d of early life in response to prenatal or postnatal insults with severe malnutrition.
Materials and methods
Participants
The present study used a retrospective cohort in Suzhou, Jiangsu Province, China. A total of 24,727 subjects aged between 55 and 63 years with medical examinations performed between 2016 and 2021 at the First Affiliated Hospital of Soochow University were recruited. In total, 1,118 subjects were excluded due to smoking (>1 package daily), drinking heavily (>50 g alcohol daily), familial history of cardiovascular diseases, as well as cancers. Totally, 12,557 participants born from 1 July 1958 to 31 December 1964 were included. In addition, subjects with repeated examinations in different years and missing information were excluded. Finally, 6, 662 participants were enrolled for the analysis (Fig. 1).

Fig. 1. Flow chart showing the step-by-step sample selection of the famine cohort.
Famine exposure
GCF started in the spring of 1959 and ended in later 1961 (9). The participants were divided into six groups, depending on their birth date: (1) unexposed group: born between 1 October 1962 and 31 December 1964 (n = 3,407); (2) the 1st trimester exposed group (n = 229); (3) tThe 1st and 2nd trimesters exposed group (n = 572); (4) whole gestation exposed group (n = 293); (5) whole gestation with early-childhood (up to 2-year-old) exposed group (n = 1,739); and (6) early-childhood (3 years of old) exposed group (n = 419).
Characteristics
Body weight, waist circumference and standing height were measured using calibrated instruments. The formula calculated body mass index (BMI, kg/m2): weight (kilograms) divided by height (meters squared). Blood samples were collected from participants who had fasted for a minimum of 8 hours and processed for fasting blood glucose (FBG), glycated hemoglobin (Hb1Ac), total cholesterol (TC) and triglyceride (TG) using an automatic biochemistry analyser.
Blood pressure and heart rate were measured by medical professionals using a sphygmomanometer in the sitting position between 7:00 and 9:00 a.m. Hypertension was defined as blood pressure≥140/90 mmHg. Normal blood pressure was defined as systolic blood pressure (SBP) <120 and diastolic blood pressure (DBP) <80 mmHg. Pre-hypertension was defined as 120 mmHg≤ SBP ≤139 mmHg or 80 mmHg≤ DBP ≤89 mmHg. Bradycardia was defined as heart rate <60 beats per minute, and tachycardia was defined as heart rate >100 beats per minute.
Assessment of atherosclerosis and stenosis
Color Doppler ultrasonography was operated by trained and experienced sonographers to measure blood flow velocity and intima-media thickness (IMT) of vertebral artery and carotid arteries, including common carotid artery, carotid artery bulb, external carotid artery and internal carotid artery. Intimal thickening was defined as 1.0 mm≤ IMT <1.5 mm, and the plaque was defined as IMT≥1.5 mm. Atherosclerosis was characteristic of intimal thickening or plaque.
Statistical analysis
All statistical analyses were performed using SPSS version 25 (SPSS Inc., Chicago, IL). All continuous variables were presented as mean ± standard deviation, and categorical variables were presented as a percentage. One-way analysis of variance was adopted to compare the intergroup difference with the Bonferroni correction. Independent sample T-test and Chi-square test were performed to determine differences between the unexposed group and exposed groups. For continuous variables, adjusted mean differences and 95% confidence interval (CI) were further obtained using the generalised linear model with age as a covariate. Wilcoxon rank sum test was applied to compare differences in hierarchical variables between the unexposed group and exposed groups.
To assess the association of GCF exposure in different developmental periods with dichotomous outcomes, odds ratio (OR) and 95% CI were estimated using binary logistic regression, adjusted with and without covariates. In Model 1, no variable was adjusted. Model 2 was adjusted for age. Model 3 additionally included sex, BMI, TC and blood pressure. All P values were two-sided, and statistical significance was defined as P-value < 0.05 unless otherwise indicated.
Ethical approval
The study was conducted following the principles of the Declaration of Helsinki, and all protocols were approved by the Institute Ethics Committee of the First Affiliated Hospital of Soochow University (ER0201601197). All participants gave written informed consent before participating in the study.
Results
Characteristics
The related data are shown in Table 1. Of 6,662 participants, 60.45% were male, and the average age was 57.16. The six groups had no significant differences in BMI, FBG, Hb1AC, TC and TG. The waist circumference in the 1st and 2nd trimesters group, whole gestation with early-childhood group was higher than that in the unexposed group. Heart rates (HR) in the gestation with early-childhood exposed group and early-childhood exposed group were significantly slower than that in the unexposed group.
Table 1. Baseline characteristics of different exposure groups

Data are presented as mean ± SD or percentage.
SBP, systolic blood pressure; DBP, diastolic blood pressure; Hb1AC, glycated hemoglobin; TC, total cholesterol; TG, triglyceride.
1 A one-way analysis of variance was applied in comparing the intergroup difference of measurement data. Statistical significance was defined as P-value < 0.003, and values without the same superscript letters are significantly different.
Association of perinatal exposure to GCF with blood pressure during the early stage of aging
Different effects of the various prenatal stages of exposure to GCF on offspring blood pressure
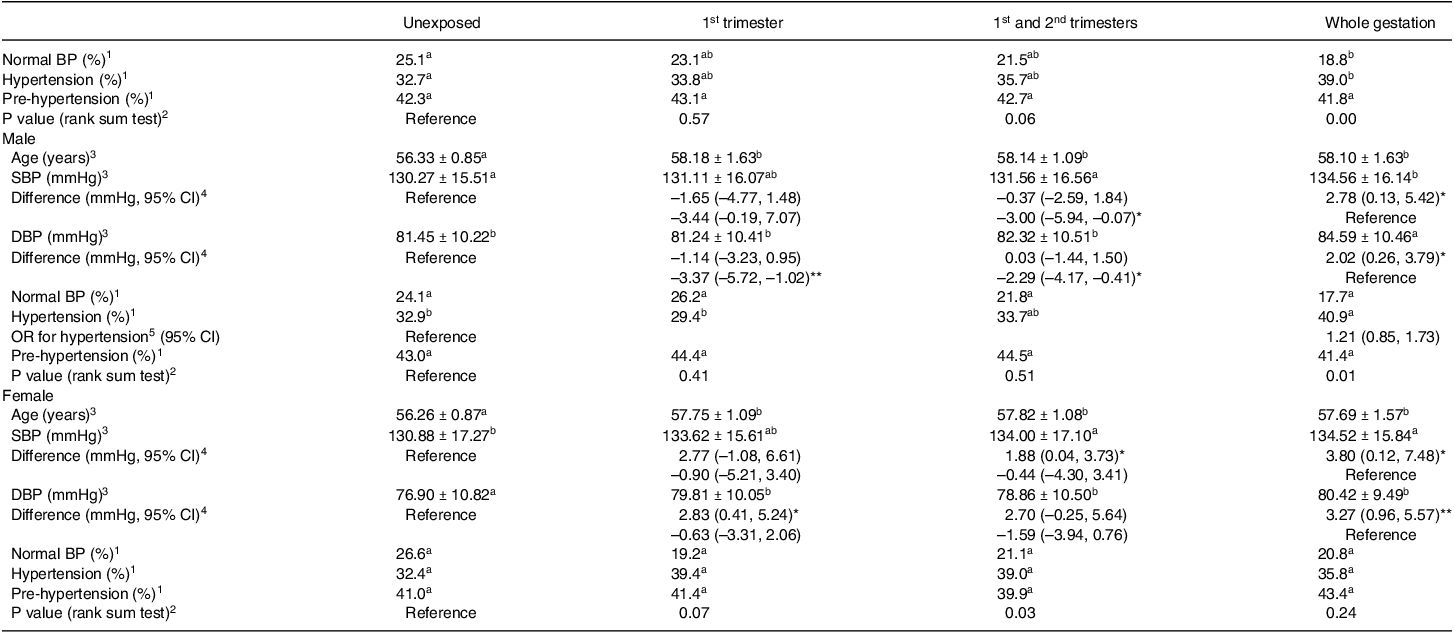
As shown in Table 1, both SBP and DBP were significantly higher in the whole gestation exposed group than that in the unexposed group. Blood pressure was categorised further as normal BP, pre-hypertension and hypertension. The prevalence of normal blood pressure in the whole gestation exposed group was significantly lower than that in the unexposed group (unexposed group: 25.1%, whole gestation exposed group: 18.8%, P = 0.02). Incidence of hypertension was elevated (unexposed group: 32.7%, whole gestation exposed group: 39.0%, P = 0.03). Consistent with the above findings, the distribution of the three levels was statistically different between the entire gestation exposed group and the unexposed group (Table 2). Compared with unexposed group or whole gestation exposure group, there were no significant differences in proportions of normal BP, prehypertension and hypertension in 1st trimester exposure group and 1st and 2nd trimesters exposure group.
Table 2. Blood pressure of subjects exposed to GCF during gestation
Data are presented as mean ± SD or percentage.
1 Chi-square test was applied.
2 P value in Wilcoxon rank sum test to evaluate distribution of hierarchical variables (normal BP, pre-hypertension and hypertension) between the unexposed group and exposed groups.
3 One-way analysis of variance was applied.
4 Mean differences and 95% CI between exposed groups and unexposed group, adjusted for age. CI, confidence interval.
5 OR of hypertension and 95% CI were estimated using binary logistic regression, adjusted with age. OR, odds ratio.
*P < 0.05, **P < 0.01, values without the same superscript letters are significantly different (P < 0.008).
To determine the possible influence of sex differences, participants were divided into two subgroups, and the results per gender are shown in Table 2. Like the results of mixed sexes in the whole gestation-exposed group, the SBP and DBP were significantly higher than the unexposed group independent of sex differences. Given the significant age difference between exposed groups and unexposed group occurring in males and females (all P values < 0.001), the generalised linear model analysis was performed on the difference in blood pressure using age as a covariate. Compared with the unexposed group, the adjusted mean differences of male SBP and DBP in the whole gestation exposed group were 2.78 mmHg (95% CI: 0.13, 5.42) and 2.02 mmHg (95% CI: 0.26, 3.79) after correcting age with similar risk of hypertension (OR: 1.21, 95% CI: 0.85, 1.73). For females, the higher SBP and DBP in the whole gestation exposed group were also independent of age (adjusted mean difference: 3.80 mmHg (95% CI: 0.12, 7.48) for female SBP, 3.27 mmHg (95% CI: 0.96, 5.57) for female DBP). In female subgroups, more significant differences were found between other exposed groups and unexposed group. For example, DBP of the females whose mothers were exposed to GCF during 1st trimester was significantly higher than unexposed group independent of age (2.83 mmHg (95% CI: 0.41, 5.24)) after correcting for age. The adjusted mean SBP rather than DBP in 1st and 2nd trimesters exposed group was 1.88 mmHg higher (95% CI: 0.04, 3.73) than that in unexposed group.
It seems that female blood pressure in the early stage of aging is more sensitive to early pregnancy exposure to GCF than males, which was supported by a comparison among prenatal exposure groups. Compared with whole gestation exposure group, there was no difference in female SBP and DBP in 1st trimester exposure group and 1st and 2nd trimesters exposure group, indicating early and mid-pregnancy exposures have the same effects in blood pressure-raising effects on female offspring as the exposure throughout pregnancy. By contrast, male blood pressure of whole gestation exposure group was significantly higher than that of early and middle pregnancy exposure group (3.01 mmHg higher (95% CI: 0.07, 5.94) of SBP, 2.29 mmHg higher (95% CI: 0.41, 4.17) of DBP with age adjusted) and that of early pregnancy exposure group (3.37 mmHg higher (95% CI: 1.02, 5.74) of DBP with age adjusted), indicating that the effect of entire pregnancy exposure on blood pressure in the male offspring was stronger than early or/and mid-pregnancy exposures.
Different effects of postnatal exposure to GCF with or without prenatal exposure on offspring blood pressure
As shown in Table 3, the proportion of normal BP in the whole gestation with early-childhood exposure group was significantly lower than that in the unexposed group (unexposed group: 25.1%, whole gestation and early-childhood exposed group: 18.7%, P < 0.001), while hypertension incidence was higher (unexposed group: 32.7%, whole gestation exposed group: 36.6%, P = 0.008). The normal BP, pre-hypertension and hypertension distribution significantly differed between those two groups (P < 0.001). In both male and female subgroups, blood pressures of the whole gestation with early-childhood-exposed group were significantly higher than those of unexposed group. The difference in blood pressure was age -corrected to consider the age difference between unexposed group and the whole gestation with early-childhood- exposed group. Compared with unexposed group, the adjusted mean differences of male SBP and DBP in the whole gestation with early-childhood-exposed group were 2.27 mmHg (95% CI: 0.82, 3.72) and 1.14 mmHg (95% CI: 0.19, 2.08) from the unexposed group. The adjusted female DBP in the whole gestation with early-childhood-exposed group was 1.51 mmHg higher (95% CI: 0.29, 2.72).
Table 3. Blood pressure of subjects exposed to GCF after birth with or without gestation exposure

1 Chi-square test was applied.
2 P value in Wilcoxon rank sum test to evaluate distribution of hierarchical variables (normal BP, pre-hypertension and hypertension) between the unexposed group and exposed groups.
3 One-way analysis of variance was applied.
4 Mean differences and 95% CI between exposed groups and unexposed group, adjusted for age. CI, confidence interval.
5 OR of hypertension and 95% CI was estimated using binary logistic regression, adjusted with age. OR, odds ratio.
*P < 0.05, **P < 0.01, values without the same superscript letters are significantly different (P < 0.017).
In contrast, there was no significant difference in blood pressure between early-childhood exposed group and unexposed group. In line with that, the adjusted mean male DBP in the whole gestation with early-childhood-exposed group was significantly higher than that in the postnatally exposed group (2.02 mmHg (95% CI: 0.49, 3.55)) (Table 3). But the difference in female DBP between the two groups was not observed, indicating there might exist a slight effect on BP -raising for the postnatal exposure in the women.
To compare the effects of pregnancy exposure, postnatal exposure and a combination of the two exposures on blood pressure, we set the whole pregnancy exposure group as the control group. As shown in Table 4, blood pressure in the group exposed during pregnancy accompanied by postnatal exposure was not significantly different from that in the group exposed during pregnancy alone. In contrast, blood pressure in the postnatal exposure group was significantly lower than that in the pregnancy exposure group independent of sex difference (2.07 mmHg (95% CI: 0.37, 3.78) for male DBP, 3.28 mmHg (95% CI: 0.30, 6.25) for female DBP).
Table 4. Comparison of blood pressure between subjects undergoing prenatal and postnatal exposure

1 Chi-square test was applied.
2 P value in Wilcoxon rank sum test to evaluate distribution of hierarchical variables (normal BP, pre-hypertension and hypertension) between the unexposed group and exposed groups.
3 One-way analysis of variance was applied.
4 Mean differences and 95% CI between exposed groups and unexposed group, adjusted for age. CI, confidence interval.
* P < 0.05, values without the same superscript letters are significantly different (P < 0.017).
Effects of exposure to GCF on heart rates
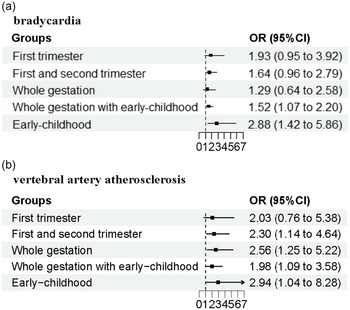
HR in the early-childhood-exposed group or gestation with early-childhood-exposed group were significantly slower than that in the unexposed group (Table 1). Furthermore, the risks of bradycardia were also associated with those two exposure groups (Fig. 2). Further analysis showed, for the male, HR in early-childhood exposed group and whole gestation with childhood exposed group were decreased significantly compared with that in the unexposed group, dropping by 3.39 beats per minute and 2.61 beats per minute (adjusted for age), respectively (Table 5). Consistently, the incidences and risks of bradycardia but not tachycardia were increased in postnatal exposure groups with or without foetal exposure. After adjustment for age, BMI, blood pressure and TC OR (95% CI) of bradycardia was 1.61 (1.07, 2.44) for whole gestation with childhood exposure and 2.34 (1.04, 5.27) for childhood exposure. In general, the heart rate in late middle-aged women was higher than that of men of the same age. Although lower HR in whole gestation exposed group, as well as the childhood exposed group with or without foetal exposure, the risk of bradycardia was only associated with childhood exposure (OR:6.04, 95% CI:1.37, 26.72) but not gestation exposure (OR: 2.17, 95% CI: 0.62, 7.64) and gestation with childhood exposure (OR: 1.31, 95% CI:0.64, 2.69).

Fig. 2. The association of GCF exposure with cardiovascular disease. (a) Bradycardia; (b) vertebral artery atherosclerosis. Squares represent point estimates for odd ratios (OR), and horizontal lines represent 95% confidence interval (CI). The multivariable model was adjusted for age, gender, systolic blood pressure, diastolic blood pressure and total cholesterol.
Table 5. Effect of GCF exposure during early life on heart rate

Data are presented as mean ± SD or percentage.
a Mean differences and 95% CI between exposed groups and unexposed group, adjusted for age. CI, confidence interval.
b Adjusted for age, gender, DBP, SBP and TC. OR, odds ratio.
*P < 0.05, ***P < 0.001.
Impact of perinatal exposure to GCF on atherosclerosis
Since hypertension or elevated BP is a significant risk factor for atherosclerosis, we also examined the association of perinatal exposure to GCF with atherosclerosis. Compared with the unexposed group, the prevalences of carotid artery atherosclerosis in gestation-exposed group with and without early-childhood exposure were significantly increased (Table 6). The result from logistic regression analysis without adjustment (Model 1) showed that the crude ORs for the two groups were 1.32 (95% CI: 1.03, 1.43) and 1.16 (95% CI: 1.01, 1.32), compared to the unexposed group. But no statistically significant difference was seen between the GCF exposed groups and the unexposed group in Model 2 (adjusted for age) and Model 3 (adjusted for age, sex, BMI, blood pressure and TC).
Table 6. Relative risks of carotid artery atherosclerosis in exposure groups to GCF

OR, odds ratio; CI, confidence interval.
Model 1 without adjustment.
Model 2 adjusted for age.
Model 3 adjusted for age, gender, DBP, SBP and TC.
* P < 0.05.
Table 7 shows the risk of vertebral artery atherosclerosis in exposure groups to GCF. Vertebral artery plaque was found in 1.7% of participants in the unexposed group, and the percentage in three exposed groups (1st and 2nd trimesters exposed group, gestation with early-childhood exposed group, and early-childhood exposed group) was higher than that in the unexposed group. In model 1 of logistic regression analysis, OR (95% CI) of vertebral artery atherosclerosis was 2.54 (1.51, 4.28) for 1st and 2nd trimesters exposed group, 2.06 (1.31, 3.25) for gestation with early-childhood exposed group, 3.83 (2.23, 6.57) for early-childhood exposed group. After adjusting for age (Model 2), GCF exposure during the entire gestation, as well as the three exposure groups mentioned above, was associated with the risk of vertebral artery atherosclerosis. When more covariates (age, sex, BMI, blood pressure and TC) were included in the models: adjusted OR (95% CI) was 2.30 (1.14, 4.64) for 1st and 2nd trimesters exposed group, 2.56 (1.25, 5.22) for the gestation exposed group, 1.98 (1.09, 3.58) for gestation with the early-childhood exposed group, and 2.94 (1.04, 8.28) for the early-childhood exposed group (Fig. 2). The differential consequence of maternal undernutrition on the risk of atherosclerosis was present between carotid arteries and vertebral arteries, possibly due to the difference in basic incidence rate (47.1% for carotid artery in the unexposed group vs 1.7% for vertebral artery).
Table 7. Relative risks of vertebral artery atherosclerosis in exposure groups to GCF

OR, odds ratio; CI, confidence interval.
Model 1 without adjustment.
Model 2 adjusted for age.
Model 3 adjusted for age, gender, DBP, SBP and TC.
*P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The exposure to GCF was categorised with several early developmental periods in the present study to determine whether effects of perinatal malnutrition on later life would be various or the same, which might be able to offer new information on sensitive ‘window’ periods for undernutrition increased risks of the cardiovascular diseases in developmental origins. With blood pressure, heart rates and artery atherosclerosis as outcomes, this study first revealed the association between GCF exposure during early life periods and risks of cardiovascular diseases in the offspring between 55 and 60 years of old based on exposure time, blood vessel types and sexes. Specifically, prenatal exposure is more harmful to elevated blood pressure than postnatal exposure. Female blood pressure is more sensitive to maternal undernutrition during early intrauterine development (before late pregnancy) than males. However, the detrimental effect of postnatal exposure on the risk of bradycardia was more substantial than that of prenatal exposure to GCF. Compared to carotid atherosclerosis, vertebral artery atherosclerosis is more vulnerable to GCF exposure, regardless of prenatal or postnatal exposure.
It has long been known for more than three decades that adverse nutritional conditions during pregnancy may permanently change the functions of specific organs in the offspring, leading to chronic adult diseases. This concept is currently referred to as the Developmental Origins of Health and Disease (DOHaD) and has been supported by a growing number of studies using famine models.(Reference Roseboom, de Rooij and Painter4,Reference Meng, Yu and Guo19,Reference Kyle and Pichard24–Reference Abate, Abdulahi and Abdulhay26) Among those models, Dutch Famine was the most famous one as it was among the first famine models used to research DOHaD in humans.(Reference Roseboom, van der Meulen and Ravelli27,Reference Roseboom, van der Meulen and Osmond28) As for hypertension, one of the most concerned vascular diseases, the results in different famine models were inconsistent, which was unsurprising since the conditions and factors among various human famine models differed. Roseboom reported a series of studies on the offspring exposed prenatally and postnatally to Dutch Famine and found that famine can lead to increased risk of various diseases, but not hypertension.(Reference Roseboom, van der Meulen and Ravelli29–Reference Painter, de Rooij and Bossuyt31) They also considered that a maternal unbalanced protein/carbohydrate ratio rather than an absolute reduction of intake was linked to hypertension risks of the offspring.(Reference Roseboom, van der Meulen and Ravelli27) Conversely, a number of other subsequent studies observed that foetal exposure to the famine contributed to increased blood pressure in the offspring.(Reference Stein, Zybert and van der Pal-de Bruin5,Reference Hult, Tornhammar and Ueda6,Reference Zhang, Pu and Ding23,Reference Abate, Abdulahi and Abdulhay26) Although those studies and results are interesting, all human famine models used in medical research have their strengths as well as limitations. Thus, when this study used GCF for investigation, we must consider what has been known from Dutch and other famine models, and what is different about GCF. Notably, the Dutch famine lasted about 6 months only, which even could not cover the whole pregnancy in humans. The GCF famine was much longer than the Dutch famine model, at three years. This provides opportunities to study the reciprocal effects of different foetal stages and child periods for exposure to famine in the offspring.
In the current study, female blood pressure was elevated significantly by 1st trimester exposure as well as 1st and 2nd trimesters exposure, supporting that embryonic and early foetal development is a critical window of programming vascular disease. Although the information on birth weight was not available for this study, other studies have found that exposure to famine in early pregnancy coupled with the resumption of nutritional intake in late pregnancy can result in a birth weight comparable to that of the unexposed group.(Reference Roseboom, van der Meulen and Ravelli27) Even though the birth weight was comparable, early pregnancy exposure was shown to increase blood pressure of adult offspring.(Reference Wang, Wang and Lei7) Therefore, the reprogramming mechanisms of early pregnancy exposure might not be related to growth retardation only, but an epigenetic modification of critical genes. It is worth noting that hypomethylation of several genes (IGF2, FAM150B, LEP) was specific for early gestation exposure to famine,(Reference Heijmans, Tobi and Stein32,Reference Tobi, Slieker and Stein33) suggesting early gestation rather than middle or late gestation is identified as a critical period for adult DNA methylation changes.(Reference Tobi, Slieker and Stein33) Investigation in this study highlights sensitive windows of early pregnancy, which appeal to more candidate methylated genes related to the acquisition of cardiovascular diseases-susceptible traits in humans to be uncovered.
We and other researchers have found higher cardiovascular risks (elevated blood pressure in the present study) in women exposed to famine during early or mid-gestation(Reference Painter, de Rooij and Bossuyt31,Reference van Abeelen, Veenendaal and Painter34,Reference Li and Lumey35) than men exposed at the same stage. It is widely accepted that the incidence of cardiovascular diseases such as hypertension is lower in women than in men before the age of 50–60, but the incidence rises in post-menopausal women and eventually surpasses that of age-matched men due to the lack of estrogen protection.(Reference Lawes, Vander Hoorn and Law36,Reference Taddei, Virdis and Ghiadoni37) Epigenetic dysregulation, especially DNA methylation, can also be proposed to explain the gender preference of the effects of maternal exposure on offspring. For example, methylation differences of LEP were restricted to women, paralleling the sex-related consequences of prenatal famine exposure.(Reference Tobi, Slieker and Stein33)
The three-year-long famine in China has led to a division of the exposed population into not only pre-birth and post-birth exposures but also a special category of people who continue to suffer from nutritional shortages after birth following pre-birth exposure. As described above, blood pressure of postnatal exposed group was comparable to that of unexposed group and lower than that of foetal exposed group. Unlike postnatal exposure, postnatal exposure accompanied by whole gestation exposure elevated significantly blood pressure of male and female offspring, and the male blood pressure was higher than that in postnatal exposure group. On the other hand, there was no difference in increasing blood pressure range between whole gestation exposure group and postnatal exposure with whole gestation exposure group. Those new findings indicate the effect of foetal exposure followed by postnatal exposure is similar to only foetal exposure, suggesting a relatively weak impact of postanal exposure on blood pressure in late middle-aged offspring.
In contrast with blood pressure, heart rate decreased significantly among offspring exposed to GCF after birth but not those exposed during gestation. What is more, postnatal exposure was associated with a risk of bradycardia. A recent study observed that risks of bradycardia, atrial fibrillation and atrioventricular block increased in offspring suffering from GCF (including prenatal exposure combined with postnatal exposure).(Reference Zheng, Pu and Rui38) Foetal heart rate falls physiologically as the pregnancy progresses and throughout early childhood due to resorptive degeneration of SAN as well as the vague nerve which matures after 20 weeks of gestation.(Reference Heuser39) In rats, a maternal low-protein diet was found to aggravate sympathetic bradycardia and elevate arterial pressure in the male offspring.(Reference Barros, De Brito Alves and Nogueira40) Although an increase in blood pressure can result in a decrease in heart rate via baroreceptors in the peripheral nervous system,(Reference Heuser39) risk of bradycardia occurred in early-childhood exposure group whose blood pressure was normal in the present study and whole gestation exposure to GCF elevated blood pressure of offspring but not risk of bradycardia. The asynchronisation between increased blood pressure and bradycardia suggests some non-neurogenic or neurogenic mechanisms involved in bradycardia due to postnatal overnutrition. Whether it is the remodeling of SAN or the imbalance of the autonomic nervous system that accelerated heart rate decline in aging has not yet been resolved.
Besides blood pressure and heart rate, atherosclerosis in birth cohorts was investigated. Although the risks of carotid artery atherosclerosis were minor in all exposed groups, risks of vertebral artery atherosclerosis were significant in three types of gestation exposures (1st and 2nd trimester’s exposure, whole gestation exposure, whole gestation with early-childhood exposure) and postnatal exposure. Notably, as a kind of damage occurring in blood vessels, atherosclerosis and hypertension reinforce each other. What is new and exciting in the present study is that the analysis revealed the sensitive window timing periods to undernutrition insults on different cardiovascular diseases are various.
Previous studies also reported that famine could lead to changes in blood pressure and heart rate in the offspring adults and increase risks of arrhythmia(Reference Zheng, Pu and Rui38) and atherosclerosis.(Reference Huang, Liu and Yu21) This study first revealed that prenatal periods were more sensitive to undernutrition than early childhood stage in terms of hypertension or blood pressure regulation system associated with vascular abnormalities. On the other side, postnatal exposure to GCF affected heart beat more than that of the prenatal exposures. In other words, the information translated from those findings indicates that embryo and foetal periods are sensitive windows of the vascular systems by GCF; and infant stages are more sensitive window for GCF affecting the heart in this study. This new information achieved from the present study has important clinical implications. For example, following future studies further confirming our findings, prevention strategies in cardiovascular protection can gain new directions. To patients with prenatal undernutrition history, corresponding prevention may be able to mainly aim at vascular protection, while for people with postnatal exposure history, preventive measures may select those to protect the heart for normal cardiac rhythm.
However, the present study still has several limitations. For example, the ambiguous timing of GCF or food supply shortage allowed for some errors in the exposure subgroups to remain, which may have narrowed the variability of the groups. In addition, this study did not include questionnaires on the population’s education, dietary habits and exercise habits, which might influence the occurrence of cardiovascular diseases.
In summary, the present study showed the various effects of GCF exposure in early life on different cardiovascular diseases with gender preference, highlighting the importance of the critical time window of exposure to GCF. Thus, it appears that the long-term threat of early-life malnutrition to cardiovascular health in offspring may be involved in different molecular mechanisms in various organs of different populations at distinct developmental periods of exposure. Early intervention may offset the programming process to prevent the development of cardiovascular diseases, but individuals with a history of undernutrition early in life should be given special attention according to exposure timing and gender.
Abbreviations
GCF: Great Chinese Famine; BMI: body mass index; FBG: fasting blood glucose; Hb1Ac: glycated hemoglobin; TC: total cholesterol; TG: triglyceride; BP: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure; IMT: intima-media thickness; CI: confidence interval; OR: odds ratio; HR: Heart rates; DOHaD: Developmental Origins of Health and Disease.
Acknowledgements
We thank Dr. J. Pu’s colleagues for the medical examinations and records.
Authorship
Conceptualisation, Xiuwen Zhou and Zhice Xu; Data curation and Xiuwen Zhou; formal analysis, Xiuwen Zhou; funding acquisition, Xiuwen Zhou, Jianhong Pu and Zhice Xu; investigation, Yumeng Zhang and Qiutong Zheng; methodology, Xiuwen Zhou and Yumeng Zhang; project administration, Xiuwen Zhou; resources, Daiyi Zhang and Jianhong Pu; Software, Yi Ding; supervision, Jianhong Pu and Zhice Xu; validation, Xiuwen Zhou, Yumeng Zhang and Qiutong Zheng; visualisation, Xiuwen Zhou; writing – original draft, Xiuwen Zhou; writing – review and editing, Zhice Xu all authors have read and agreed to the published version of the manuscript. We thank the colleagues of Dr. J. Pu for the medical examinations and records.
Financial support
This work was supported by the National Key R&D Program of China (Z.X., grant number 2019YFA0802600); Wuxi Taihurencai Project Fund (Z.X.); Wuxi Key Laboratory of Perinatal Bio-Medicine (Z.X.); Suzhou Natural Science Foundation (X.Z., grand number SKJY2021068); Health Care Issues for Cadres in Jiangsu Province (J.P., grant number BJ2009); Health science and technology innovation for Suzhou Science and Technology project (J.P., grant number SKY2022044).
Declaration of interests
The authors declare none.