Introduction
In the neotropics, many plant species rely on ants for protection, and in exchange, plants provide ants with extrafloral nectar (EFN), a liquid that is rich in carbohydrates (Del-Claro et al. Reference Del-Claro, Rico-Gray, Torezan-Silingardi, Alves-Silva, Fagundes, Lange, Dáttilo, Vilela, Aguirre and Rodriguez-Morales2016). Despite the amount of literature showing that ants exert a positive effect on plant growth and biomass, reduction of herbivory, and increases in fitness (Rosumek et al. Reference Rosumek, Silveira, de S Neves, de U Barbosa, Diniz, Oki, Pezzini, Fernandes and Cornelissen2009, Pereira et al. Reference Pereira, Boaventura, de Castro and Cornelissen2020), we cannot rule out the fact that these interactions are conditional over time and space where they take place (Thompson Reference Thompson2005, Jones & Koptur Reference Jones and Koptur2015). The literature is filled with data showing the influence of ants on herbivory in punctual periods, usually at the end of the leaf flush season (Coley et al. Reference Coley, Lokvam, Rudolph, Bromberg, Sackett, Wright, Brenes-Arguedas, Dvorett, Ring, Clark, Baptiste, Pennington and Kursar2005, Calixto et al. Reference Calixto, Novaes, Santos, Lange, Moreira and Del-Claro2021). The main problem with this approach is that by examining herbivory in only one specific period, the data might mask important variations in leaf area loss during the leaf flush season (Monique et al. Reference Monique, De Souza, Calixto and Silva2022). For instance, Nascimento and Del-Claro (Reference Nascimento and Del-Claro2010) showed that plants with ants had consistently lower herbivory levels throughout the four months of observation; in contrast, Kelly (Reference Kelly1986) revealed that plants with ants might show higher herbivory than plants without ants, depending on the census/sampling. In addition, Fuente & Marquis (Reference Fuente and Marquis1999) not only demonstrated that herbivory varies over time but also that it depends on the environment in which interactions occur (Del-Claro & Marquis Reference Del-Claro and Marquis2015). In this context, to enrich the knowledge of ant–plant interactions, it might be wise to examine how herbivory varies along the whole leaf flush season and if it is affected by both ants and different environments.
The interplay between the environment and mutualistic ant-partners is a main factor controlling the outcomes in ant–plant interactions (Nogueira et al. Reference Nogueira, Rey, Alcántara, Feitosa and Lohmann2015, Bächtold et al. Reference Bächtold, Silva and Del-Claro2016). Most of what we know about the spatiotemporal variation in ant–plant relationships comes from studies in natural areas; however, EFN plants also occur in fragments within urban settings, such as parks (Clarke et al. Reference Clarke, Fisher and Lebuhn2008, Silva et al. Reference Silva, Arnan, Andersen and Leal2019). In a broad sense, urban parks are considered isolated forest habitats surrounded by an urban matrix without terrestrial connections/corridors with natural areas and are susceptible to anthropogenic disturbances (Pacheco & Vasconcelos Reference Pacheco and Vasconcelos2007). In addition, these areas can be considered fragments and are consequently prone to edge effects, which create different microenvironmental conditions for species occurring on the edges and in the interior (Bolger et al. Reference Bolger, Suarez, Crooks, Morrison and Case2000).
For flying animals, parks are considered islands (McFrederick & LeBuhn Reference McFrederick and LeBuhn2006), but for terrestrial species, such as ants, parks can be regarded as refuges and encompass a subset of the species found in the forests (Santos et al. Reference Santos, Delabie and Queiroz2019). There is an assumption that the interactions between ants and plants in these places might be different from those in natural areas because the ant fauna of parks is restricted to a few species, most of which are non-specialist and opportunistic (Pacheco & Vasconcelos Reference Pacheco and Vasconcelos2007, Clarke et al. Reference Clarke, Fisher and Lebuhn2008). However, since EFN-drinking ants are generalists, interactions with plants are assumed to persist if EFNs are active (Silva et al. Reference Silva, Anjos, Bächtold, Lange, Maruyama, Del-Claro and Mody2020).
In this study, we investigated the spatiotemporal variation of ant–plant interactions in a fragment located in an urban setting. Our experiment consisted of a three-factor design with ‘time’ (the period that corresponds to the whole leaf flush season), ‘ants’ (present or absent), and ‘habitat type’ (the transects that incorporate the interior and the edges of the fragment). We addressed the following question: do plants with ants show lower herbivory levels, and does it depend on habitat type (interior or edges) and time of sampling (different dates of sampling within the leaf flush season)?
Our study plant was Inga laurina (Sw.) Willd (Fabaceae), an EFN-bearing legume species. Plants in this genus have long been studied with regard to their interactions with ants (Koptur Reference Koptur1984, Bixenmann et al. Reference Bixenmann, Coley and Kursar2013). Both the environment and ants seem to play an important role in the life history and herbivory of Inga species. For instance, habitat type and sunlight exposure are critical factors for the growth of I. vera; in addition, herbivory is greatly suppressed when ants are present (Kersch & Fonseca Reference Kersch and Fonseca2005). Thus, Inga species appear to be a good model for investigating how herbivory varies according to ants and the environment.
Materials and methods
Study area
The study was conducted between November 2019 and May 2020 at the Parque Ambiental Macambira (16°44′26.20″ S, 49°19′11.10” W, 827 m above sea level) in the city of Goiânia, Brazil. The park is used for recreation and educational purposes and lacks large-scale human disturbances, such as mowing, fires, and logging. The park contains a typical and conserved Cerradão with trees up to 10 m tall. The climate has two well-defined seasons: a dry winter from May to September and a rainy summer from October to April.
The park is an urban fragment with approximately 25 ha and is surrounded by avenues and houses all around its borders. The park itself is asymmetric; one part resembles an arrow, and the other a square, both of which are linked by a boomerang-shaped area of vegetation. The so-called west edge (16°44'26.20"S, 49°19'10.64"W) is limited by a matrix formed by deforested land containing a few shrubs and grasses; this matrix extends up to 150 m until the nearest street (Supplementary material 1). The so-called east edge (16°44'23.07"S, 49°19'6.63"W) ends in an avenue. The interior of the park is well preserved with large trees with canopies covering the understory and does not sustain perturbations other than storms that once in a while may provoke the fall of trees and branches. The most frequent plant species are Hymenaea courbaril L. (Fabaceae), Tapirira guianensis Aubl. (Anacardiaceae), Xylopia sericea A. St.-Hil. (Annonaceae), Schefflera morototoni (Aubl.) Maguire, Steyerm. & Frodin (Araliaceae), Mauritia flexuosa L. f. (Arecaceae), and Anadenanthera colubrina (Vell.) Brenan (Fabaceae).
Study plant
The genus Inga is restricted to the neotropics and contains approximately 300 species (Kursar et al. Reference Kursar, Dexter, Lokvam, Pennington, Richardson, Weber, Murakami, Drake, Mcgregor and Coley2009). The study plant, I. laurina, grows up to 20 m tall. The EFNs occur at the base of the opposite leaflets. The leaf flush is related to the onset of the rainy period (October), and the production of extrafloral nectar is continuous throughout the dry season, which lasts until April (end of the rainy season). From May to September, the plant does not produce any leaves, and the EFNs are non-functional. Several species of Inga are known to be visited by EFN-drinking ants, and studies have examined the potential of ants as antiherbivore defences in Inga, as well as the performance of plants in different environments (Koptur Reference Koptur1985, Kersch & Fonseca Reference Kersch and Fonseca2005, Bixenmann et al. Reference Bixenmann, Coley and Kursar2013). Two species of Inga are present in the park (I. alba (Sw.) Willd and I. nobilis Willd.), and together with I. laurina, these legumes are part of the park’s management plan.
Experimental design
We established a study design with 4 rows per 11 column-transects in the park; each row contained 22 plants and each column had eight plants. The largest linear transects were 200 m long (Figure 1) and were set from one edge to the other of the park (Supplementary material 1). A total of 88 seedlings of I. laurina were purchased from a commercial greenhouse located in Goiânia city, Brazil, transported to the park and transplanted into established transects. At this point, the seedlings were between 15 and 20 cm tall, and there was no significant difference in height, number of leaves, and number of EFNs between plants (p > 0.05 in all cases, details not shown here).

Figure 1. Representation of the transects established in the park to grow the individuals of Inga laurina. Each row sustained 22 seedlings, which were assigned as ant-present or ant-excluded treatments. Rows started from the very west edge and ended at the east edge (the limit between the park and the surrounding non-forested matrix). A clear delimitation of the extension of edges and the interior was made later, based on microclimatic data and statistical analyses (see Results section) (see also Supplementary material 1).
Each linear transect was 200 m long and was spaced 10 m from each other. A total of 22 seedlings of I. laurina were planted in pairs (one ant-present and another ant-excluded seedling) in each transect; each pair was 20 m distant from the other pair, and each plant from each pair was 1 m distant from the other (Figure 1).
Our sample size was equal to 88 seedlings (11 plant pairs times 4 transects = 88 seedlings), which were divided into ant-present and ant-excluded individuals (n = 44 plants in each group). The restriction of ant access to plants was made with the use of nontoxic wax (Tanglefoot ©), which was applied to the foot of each ‘ant-excluded’ seedling. The ‘ant-present’ plants were left undisturbed, except by a thin layer of wax which was applied to a portion of the main stem to control for the effect of the wax; this did not restrain the access of ants to plant parts. Leaves and stems from each seedling were clipped to avoid the formation of vegetation bridges between plants.
In many ant-plant studies, scientists tag single individual plants and divide their bushes into experimental units where one bush receives the resin to avoid ants and a neighbour bush is assigned as a control unit (Alves-Silva et al. Reference Alves-Silva, Bächtold, Barônio, Torezan-Silingardi and Del-Claro2014, Aristizábal & Metzger Reference Aristizábal and Metzger2019). The advantage of this approach is that herbivores can rapidly assess the ant-free areas and forage accordingly. In addition, insects that whenever are harassed by ants in control bushes can rapidly fly to the nearest ant-excluded plant parts to keep feeding. In our study, the I. laurina plants were rather small to be divided into experimental bushes, so we preferred to grow two seedlings, one with and the other without ants, close to each other (1-m space), which covers the distance between experimental plant pairs in the study by Mody & Linsenmair (Reference Mody and Linsenmair2004).
We might also have chosen to not distribute the I. laurina in experimental pairs, but rather into ant-present and ant-excluded plants that were distributed evenly in the park and distant from each other. However, we were afraid that some plants might perish, which would affect the even sample size between experimental plants. In our current approach, if one plant from the pair perished, its counterpart was going to be excluded from the study as well; luckily this did not happen. To conclude, the decision to keep plant pairs 1-m spaced from each other was based on a logistic reason, since it permitted a quick evaluation of plants by one researcher in the field.
Data collection
Data collection commenced 21 days after plant acclimation in the area. The plants were scanned six times, from mid-December to late March, which corresponds to the wet season (see results for the dates). Field trips were carried out on December 16 (2019) and in the following dates of 2020: January 6, January 27, February 17, March 9, and March 30. In each field trip, the ‘ant-present’ seedlings were scanned for the presence of ants, and the ant-excluded plants were also checked to guarantee that no ants trespassed the wax barrier. Ants visiting the control plants were photographed to permit an initial classification to the genus level (this is possible for common genera, such as Camponotus, Cephalotes, and Crematogaster) (Supplementary material 1). At the end of the fieldwork, the ants were collected.
Our measure of herbivory was the ratio between the number of leaves and the number of leaves with herbivory (leaf area loss) in each seedling (González-Teuber et al. Reference González-Teuber, Silva Bueno, Heil and Boland2012). First, we aimed to measure herbivory as the leaf area loss (% and mm2 per leaf), using digital images. However, leaves usually had most of their areas and margins attacked by beetles and caterpillars, thus restraining us from measuring leaf area loss with accuracy (Supplementary material 1). Our measure of herbivory might not have been the most appropriate (i.e., damaged leaves divided by the total number of leaves; Alves-Silva & Del-Claro Reference Alves-Silva and Del-Claro2014; González-Teuber et al. Reference González-Teuber, Silva Bueno, Heil and Boland2012), but it was the only possible method to assess the herbivory levels in I. laurina. Nonetheless, Burger et al. (Reference Burger, Vondráčková, Skłodowski, Koid, Dent, Wallace and Fayle2021) did not notice differences between this method and the measure of leaf area loss. Thus, we believe our results are trustworthy.
Microclimatic data (temperature, humidity, and sunlight exposure) were collected to permit the classification of the edges and the interior of the fragment, because differences in temperature and humidity, for instance, are strikingly different on the edges and the interior, permitting its recognition and use in statistical analyses (Christianini & Oliveira Reference Christianini and Oliveira2012, Mendonça et al. Reference Mendonça, Russo, Melo and Durigan2015). Temperature and humidity data were recorded in the mornings (08:00 to 09:00 to avoid daily variation; Wang et al. Reference Wang, Lei, Li, Fan, Li, Sun and Chang2009) of sunny days using a digital thermo hygrometer (MTH-1300 Minipa). The device was placed between each pair of seedlings, and the data were recorded. The light incidence was measured with Google’s smartphone app Science Journal for Android OS (V. 3.5.329666436 from 2020; the app was discontinued by Google). By using the app, a single smartphone (model Samsung S9) was consistently used throughout the study, and it was held above and between the pairs of I. laurina. The camera then registered the luminosity in lux units.
Statistical analyses
The microclimatic data (light, temperature, and humidity) were visualized in different figures, and data were not linear, but rather varied depending on the location of the transects (Figure 2a, b, c). For instance, the last group of tagged plants (locate at a distance of 200 m along the transect – Figure 1), located on a border of the park (on the east), had the highest temperature and luminosity, and the lowest humidity. In contrast, some transects in the interior of the fragment had opposite values.
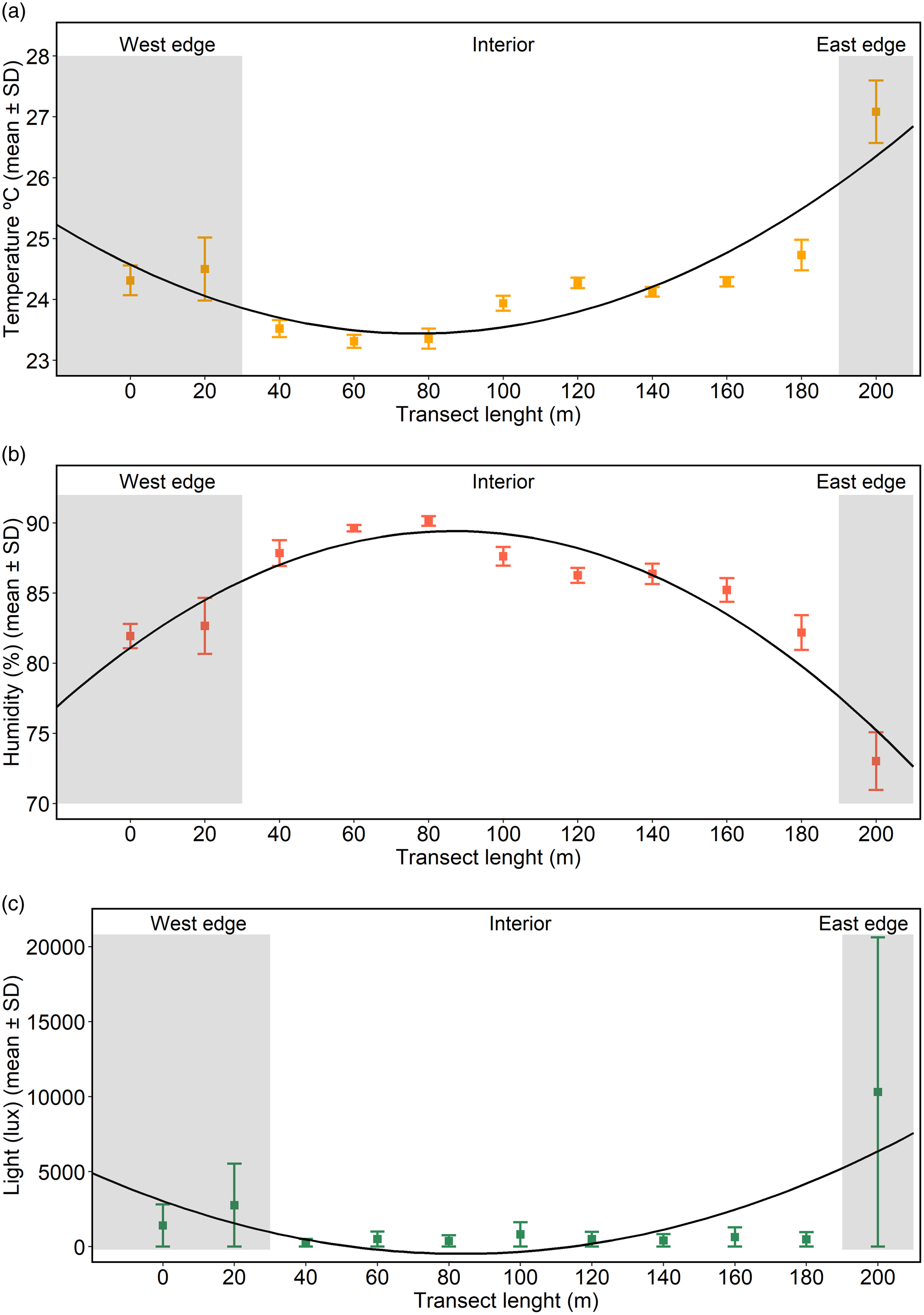
Figure 2. Variation of (a) temperature, (b) humidity, and (c) luminosity along the 200 m long transect within the park. In those places regarded as ‘edges,’ we noticed a visible contrast of environmental factors in comparison to the interior. The figure shows the mean and standard deviation.
A non-linear regression was made for each microclimatic variable, yielding polynomial models for temperature (intercept = 25.24, x = −0.74, x² = 0.08); humidity (intercept = 76.84, x = 4.72, x² = −0.44) and luminosity (intercept = 4930.0, x = −2086, x² = 201.5). We fit an adjusted curve along the fragment’s transects which demonstrated a parabolic trend of temperature, humidity, and luminosity. Regardless of the microclimatic variable, all of them showed that the inner part of the fragment (the interior) had different conditions in comparison with the borders. In fact, the adjusted parabolic curve allowed us to recognize three distinct areas within the fragment, which were labelled as ‘west edge’, ‘interior’, and ‘east edge’ (Figure 2a, b, c). The visual estimation and classification of edges and the interior were appropriate in our study as figures, and the polynomial adjusted regression curves were used to quantify how physical variables varied along the transects.
A more traditional approach would be to pairwise-compare each part of the transect to classify them onto edges and interior; however, this is not feasible due to the characteristics of the fragment. For instance, the temperature in the 20 m and 180 m transects was similar (Figure 2a) and one might assume that both parts of the transect should be put in the same categorical factor; nonetheless, they are 160 m away from each other and are separated by parts with lower temperatures. The 180 m transect is also very different (in terms of temperature, humidity and luminosity) from the subsequent transect (200 m) (Figure 2a). The adjusted parabolic curve revealed that the edges had increased values of temperature and light in comparison to the interior; in contrast, the humidity was conceivably lower on the edges. According to our classification, the west edge accounted for two transects (0 to 20 m, n = 16 plants), the interior for eight transects (40 to 180 m, n = 64 plants) and the east edge for one transect (200 m, n = 8 plants) (Figure 2a, b, c).
In order to statistically compare the microclimatic data among these three habitat types indicated by Figure 2, we performed Kruskal–Wallis test (non-parametric data) followed by Dunn’s test (a posteriori) for the values of temperature, humidity and sunlight exposure in each habitat type. Once we realized that the physical data of these three sites were statistically different (see Results section), the west edge, the east edge, and the interior of the fragment were incorporated into the subsequent analyses as categorical data.
General linear mixed model tests (GLMM) were used to examine the influence of plant groups (with or without ants), the location of plants in the transects, and dates of sampling (employed as a fixed and also as the random factor) on herbivory (% of attacked leaves per plant). Tukey’s post hoc tests were made whenever necessary to investigate in detail results that were statistically significant.
To verify if the herbivory data were spatially correlated, which might influence the results, we made Moran’s and Mantel tests, as well as a linear mixed-effects regression with spatial data of plants. None of the analyses were significant (results not shown); thus, we rule out the spatial location of plants as a variable of influence in our study.
Statistical procedures were performed using R statistical software, version 4.2.2.
Results
Plants up to 20 m from the west edge and the plants at the opposite site, at the very end of the transect (200 m), were considered to be on the edges (see Figure 1 for details on plant distribution). Figure 2 shows that the temperature, humidity, and light incidence at these sites were different from the so-called interior. The adjusted curve in the figure shows decreases in temperature and luminosity from the edges to the interior and an increase in humidity. The comparison among the edges and the interior yielded significant results (P < 0.0001 in all cases) (Figure 3). The east edge had the highest temperature, lowest humidity, and highest luminosity (Figure 3). The interior had the highest humidity, but the temperature was milder, and luminosity was the lowest in comparison to the edges (Figure 3). The physical variables were all related (Spearman’s correlation values: temperature and humidity = −0.94; temperature and luminosity = 0.91; humidity and luminosity = −0.86).

Figure 3. Comparison of (a) temperature, (b) humidity, and (c) light incidence in the sites classified as edges and interior of the urban fragment. Lowercase letters upon bars indicate statistical differences based on the Kruskal–Wallis test and on Dunn’s test (a posteriori).
Leaf herbivory was significantly influenced by the presence of ants (plants with ants had on average 10% less herbivory than plants without ants), the time of sampling, the location of plants along the transects (herbivory was higher on the edges and lower in the interior of the fragment), and by the interaction effects among the variables examined (Table 1). The interaction effect between ants and transect length indicates that not only herbivory depended on ant presence/absence but also on the location of plants along the transect (Figure 4). In fact, a significant effect of ant presence was noted for plants at the beginning of the transect (0 metres from the west edge); in contrast, at the end of the transect results were just the opposite (Figure 4). In the interior of the fragment, results varied as ants either had or had not a positive effect (i.e., reduction) on herbivory levels (Figure 4).
Table 1. Variation of leaf herbivory in Inga laurina according to ants (presence and absence), date of sampling (six occasions), and transect length (200 m) in a tropical urban park. The interaction effect between ants*transect length*samplings was not conducted as it decreased the fit of the GLMM model.


Figure 4. Variation in the herbivory levels of Inga laurina according to transect length and plant groups (presence or absence of ants). The bars indicate the mean and SD values. Superscript asterisks indicate statistically significant differences according to Tukey’s post hoc tests.
Further comparisons considering each side of the park (west and east edges, and the interior of the fragment) showed that on the west edge, the plants without ants had an almost 2-fold higher herbivory rate, on average, than plants with ants (35% and 18%, respectively). The herbivory was similar between plant groups in the interior of the fragment, and plants with ants on the east edge showed higher herbivory (on average 25% more). The interaction effect of ants and dates of sampling occurred because herbivory varied throughout the leaf flush season in plants with and without ants (Supplementary material 2).
The plants did not sustain one single ant species consistently, as during fieldwork, we noticed shifts of ants in each seedling. Therefore, it was not possible to examine the effect of each ant on herbivory, and we considered the community of ants as a whole. We came across ants belonging to genera Camponotous, Crematogaster, Cephalotes, Solenopsis, and Ectatomma, and all of them visited the EFNs of I. laurina.
Discussion
Here, we found evidence of different microenvironments within the study area. Data on temperature, humidity, and luminosity revealed three distinct sites, two edges, and the interior. These sites, together with ants, were responsible for contrasting herbivory levels in I. laurina. On the west edge, plants with ants had the lowest herbivory levels, a result expected according to ant–plant interaction models of mutualism, because EFN plants deprived of ant-partners may experience increased herbivory levels (Kost & Heil Reference Kost and Heil2005, Katayama & Suzuki Reference Katayama and Suzuki2011). Studies have shown that the production of extrafloral nectar is higher in EFN plants susceptible to direct sunlight exposure (Millán-Cañongo et al. Reference Millán-Cañongo, Orona-Tamayo and Heil2014) and embedded in an environment with other plants (Alves-Silva & Del-Claro Reference Alves-Silva and Del-Claro2013); thus, it is expected that ants will be more frequent in such unshaded plants and defend the plant from herbivores (Alves-Silva & Del-Claro Reference Alves-Silva and Del-Claro2013, Bächtold et al. Reference Bächtold, Silva and Del-Claro2016). This situation was observed on the west edge.
On the east edge, results were the opposite, as plants with ants had higher herbivory than their counterparts. The east edge receives a huge influence from the surrounding non-forested matrix; in fact, the very east edge faces a main city avenue. Conversely, the west edge has no such abrupt change in the environment, as the matrix is formed by a deforested area with grasses and seedlings, which belongs to the park’s limit of occupation. Therefore, the east edge can be assumed to be severely disturbed in comparison to the west edge. The east edge had the highest temperature, luminosity, and lowest humidity in comparison to the other sites. Plants in highly stressed environments, such as the east edge, may be more susceptible to herbivore attack (García-Jain et al. Reference García-Jain, Maldonado-López, Oyama, Fagundes, de Faria, Espírito-Santo and Cuevas-Reyes2021). This occurs because plants cannot maintain their homeostasis when they are subjected to harsh conditions; in this case, their tolerance surpasses the limits of their phenotypic plasticity, incurring several changes, both physiological and morphological (Nikiforou & Manetas Reference Nikiforou and Manetas2017, Aguilar-Peralta et al. Reference Aguilar-Peralta, González-Rodríguez, Maldonado-López, Fagundes, Faria, Ávila-Cabadilla, Álvarez-Añorve and Cuevas-Reyes2020).
The study plant, I. laurina, is not a pioneer species, but rather a secondary and late-successional species (Uriarte et al. Reference Uriarte, Canham, Thompson and Zimmerman2004, Polli et al. Reference Polli, Romagnolo, Souza and Pastorini2020). Thus, by being exposed to such a harsh habitat as the east edge (in comparison to the other sites), both the plants and the ants might have faced detrimental effects (Kersch & Fonseca Reference Kersch and Fonseca2005, Yamawo et al. Reference Yamawo, Tagawa, Hada and Suzuki2014). Insect herbivores benefit from highly stressed plants, both by the plant’s reduced levels of defences and because of the disruption of top-down control, as the composition of the natural enemies of herbivores varies on spatial scales (Wirth et al. Reference Wirth, Meyer, Leal and Tabarelli2008, Santos et al. Reference Santos, Alves-Silva, Cornelissen and Fernandes2013, Pereyra et al. Reference Pereyra, Pol and Galetto2015). In the interior, a shaded habitat, ants reduced the herbivory in some plants, but not in others; nonetheless, the pooled results revealed no difference in herbivory according to plant groups. Usually, shade-tolerant and late-successional plants (such as I. laurina; Uriarte et al. Reference Uriarte, Canham, Thompson and Zimmerman2004; Polli et al. Reference Polli, Romagnolo, Souza and Pastorini2020) may have increased secondary compound defences in the shade, which is their ideal environment; this prevents herbivore attack (Coley Reference Coley1988, Dudt & Shure Reference Dudt and Shure1994).
The two edges of the park showed contrasting results in terms of herbivory according to ant presence/absence. We may conclude that ant-plant interactions were not affected by a mild stress (west edge), and in fact, gaps may strengthen the ant visitation to plants and the reduction of herbivory rates (Alves-Silva & Del-Claro Reference Alves-Silva and Del-Claro2013). Nonetheless, a harsh environment such as the east edge might have disrupted the interactions between ants and the non-pioneer species I. laurina. In this site, ants might have changed their foraging behaviour and abandoned the plants during some periods, or the plants might have been visited by species of ants that offered no protection from herbivores (Palmer & Brody Reference Palmer and Brody2007, Nogueira et al. Reference Nogueira, Guimarães, Machado and Lohmann2012, Leal et al. Reference Leal, Andersen and Leal2015, Andersen Reference Andersen2019). Some plants that are located in harsh environments (e.g., edges, proximity to roads) and are subjected to full sunlight exposure face severe water stress which in turn is a detrimental factor for extrafloral nectar production (Newman & Wagner Reference Newman and Wagner2013, Kunert et al. Reference Kunert, Aparecido, Higuchi, Santos and Trumbore2015, Cruz Rocha et al. Reference Cruz Rocha, Cristaldo, Santos Lima, Dos Santos, Do Sacramento, Santana, Dos Santos Oliveira, Bacci and Albano Araújo2019). Thus, ants might have had no benefit in visiting the plants, making them susceptible to herbivory attack. The ant-plant specificity could unravel some of these assumptions, but I. laurina was visited by several ant species, thus limiting our knowledge.
In our study, we quantified herbivory in I. laurina throughout the leaf flush season, and we registered the periodical variations in herbivory and whether they varied according to site (edges and interior) and ant presence/absence. This approach is uncommon in ant–plant studies, with results either showing that the effects of ants are consistent or not depending on the census (Kelly Reference Kelly1986, Nascimento & Del-Claro Reference Nascimento and Del-Claro2010). In I. laurina, we covered the whole leaf flush season and examined in different periods how plants were performing with and without ants. In the end, we found a significant interaction effect between ants and sampling date, indicating that herbivory was higher or lower depending on the presence of ants and the date of sampling. This result is in accordance with Monique et al. (Reference Monique, De Souza, Calixto and Silva2022) who show that plants with ants experienced large variations in flower production during the reproductive season. Thus, it is clear that the true effect of ants on plants could only be examined if one takes into consideration how plants with ants experience higher o lesser performance in a given period of its phenology, especially when ants are supposed to patrol the plants (i.e., when extrafloral nectaries are active).
Urban parks have been regarded as unimportant places for ant biodiversity, both because of their relatively low areas, and the existence of only a subset of species that occur in natural areas (Menke et al. Reference Menke, Guénard, Sexton, Weiser, Dunn and Silverman2011). Nonetheless, we showed that the same mechanisms that influence ant-plant interactions in natural areas, such as plant phenology, microclimatic gradients, ant presence, and spatial scales (Nogueira et al. Reference Nogueira, Baccaro, Leal, Rey, Lohmann and Bronstein2020, Calixto et al. Reference Calixto, Novaes, Santos, Lange, Moreira and Del-Claro2021, Monique et al. Reference Monique, De Souza, Calixto and Silva2022), are also present in the study park. In this context, not only parks are important to increase our understanding of ant-plant interactions, but it is also surprising that these relationships in such (so-called) disturbed habitats do persist, revealing potential micro hotspots of ant-plant interactions. Our understanding of the biodiversity in tropical urban parks in the tropics is scarce (< 10% of studies in parks) (Nielsen et al. Reference Nielsen, van den Bosch, Maruthaveeran and van den Bosch2014). In Brazil, most investigations in urban areas evaluate the abundance and community composition of epigaeic ants; in addition, most studies were conducted in the Atlantic Forest biome (Munhae et al. Reference Munhae, Bueno, Morini and Silva2009, Reference Munhae, Souza-Campana, Kamura and Castro Morini2015, Melo et al. Reference Melo, Koch, Andrade, Travassos, Peres and Delabie2022). To the best of our knowledge, ours is the first study in Brazil to evaluate how herbivory in an urban park (with the matrix surrounded by a city) is affected by ant-partners, space and time.
One potential limitation of the study was the lack of ant–plant specificity, as more than one ant species was observed visiting the same individual plant during the study; this prevented us from examining the role of each species in plant protection. Nonetheless, this was a characteristic of the study, and since the interaction between EFN plants and ants is facultative, plants can be visited by several ant species (Anjos et al. Reference Anjos, Caserio, Rezende, Ribeiro, Del-Claro and Fagundes2017). Moreover, the stability of ant-plant mutualism in the tropics depends on only a few arboreal species of ants (Lange et al. Reference Lange, Dáttilo and Del-Claro2013, Fagundes et al. Reference Fagundes, Dáttilo, Ribeiro, Rico-Gray, Jordano and Del-Claro2017, Câmara et al. Reference Câmara, Leal, Blüthgen, Oliveira, Queiroz and Arnan2018), which coincidentally are species that occur in parks (Aranda et al. Reference Aranda, Tibcherani, Nacagava, de Carvalho and de Souza2022). In this context, the authors might consider the effect of the ant community on plants rather than analysing each ant species separately (Franco & Cogni Reference Franco and Cogni2013, Raupp et al. Reference Raupp, Gonçalves, Calixto and Anjos2020). Another potential limitation of the study was the sampling of only one urban area, and this is justified by logistics and the period of fieldwork, that was time-consuming, as we conducted an experimental approach based on ant presence/absence covering a whole plant phenophase, which is the same approach used in natural areas to investigate plant-ant interactions (Fagundes et al. Reference Fagundes, Dáttilo, Ribeiro, Rico-Gray, Jordano and Del-Claro2017, Monique et al. Reference Monique, De Souza, Calixto and Silva2022). The sampling of ants in one or few areas is not uncommon for urban habitats (Ribeiro et al. Reference Ribeiro, Sibinel, Ciocheti and Campos2012, Cantone Reference Cantone2018, Souza-Campana et al. Reference Souza-Campana, Carvalho, Canali, Silva, Morini and Fujihara2020), and besides, most of what we know about ant-plant interactions in natural areas comes from studies conducted in single areas as well. Nonetheless, the interpretation of our results should consider the conditions of the study park, and we expect that further comparable studies could enhance our knowledge of plant-ant interactions in urban areas.
The outcomes of the interplay between the spatiotemporal effect of ants on herbivory and the concomitant effect of the environment (fragmentation, edge effects) and time are difficult to generalize, given the variability of possible results (e.g., the interactions among all variables can lead to various different scenarios) (Yamawo et al. Reference Yamawo, Tagawa, Hada and Suzuki2014, Pereyra et al. Reference Pereyra, Pol and Galetto2015, Câmara et al. Reference Câmara, Almeida, Tabarelli, Andersen and Leal2017). Here, we provide further information on this system by showing that leaf herbivory in an urban park depends both on space and on time. There is not much literature on edge effects in the Cerrado biome, let alone investigations of edge effects and ant–plant interactions in urban settings (Santos et al. Reference Santos, Delabie and Queiroz2019). Our study highlights how the outcomes of ant–plant interactions are conditional. In the study park, a 200 m spatial variation was enough to display variations in the effectiveness of ants as plant guards of I. laurina.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0266467423000044
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Should the manuscript be accepted for publication, the data supporting the results will be archived in an appropriate public repository and the DOI will be included accordingly.
Acknowledgements
We appreciate the comments by Andre Luis da Silva Castro, Daniel de Paiva Silva, Bruno de Sousa Lopes, Diego V. Anjos, the editors of Journal of Tropical Ecology and six anonymous reviewers on early versions of the study. We also thank Mr. João Lopes Rodrigues, manager of Green Areas, Mr. Antônio Junio Gonçalves da Cruz, manager at the Gerência e Monitoramento Ambiental de Goiania, the Agência Municipal do Meio Ambiente and the Departamento da Gerência e Monitoramento Ambiental de Goiania for logistics and permitting the conduction of the fieldwork at the Macambira park. We thank the Instituto Federal Goiano for financial support and Capes (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for their support in the CRENAC (Programa de Pós-Graduação Profissional em Conservação dos Recursos Naturais do Cerrado) post-graduation programme. We have no conflict of interest.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors
Competing Interest Declaration
The authors declare none








