Iodine deficiency disorders (IDD) were once more highly prevalent in China than in other countries across the world. In the 1960s, Japanese scholars reported the prevalence rate of goitre in Chengde City, Hebei Province, China to be 55–68 %(Reference Yu and Liu1). In the 1990s, endemic goitre was prevalent in thirty provinces, autonomous regions and municipalities directly under the Central Government of China, with the exception of Shanghai. In addition, endemic cretinism was prevalent in most cities and/or provinces, except for Shanghai and Jiangsu(Reference Yu2). At that time, the population within iodine-deficient areas in China was 425 million, which accounted for 66 and 40 % of the total population of iodine deficiency areas in Asia and in the world, respectively. Before implementation of large-scale prevention and treatment for IDD, there were about 35 million cases of goitre in China(Reference Yu2). Following the implementation of the universal salt iodisation (USI) strategy in 1995, the prevalence of IDD in mainland China has decreased from 20·4 to 5·8 % by 2002(Reference Yu and Liu1,Reference Ma, Skeaff and Pearce3) . IDD are the primary cause of endemic goitre and endemic cretinism. Despite the effort to prevent and control IDD, new cases of endemic cretinism were confirmed in 2006 in the Xinjiang region of China, specifically in Wushi, Baicheng County of Aksu Prefecture, and Lop County of Hotan Prefecture(Reference Chen4). As a result of geography and climate, the iodine content is low in soil and water in Xinjiang. Therefore, there is a serious environmental deficiency of iodine. Some sections of the population suffer from serious iodine deficiency, including farmers and herdsmen from remote areas of Southern Xinjiang(Reference Ma, Skeaff and Pearce3). This iodine deficiency has been further fuelled by poverty and the eating of soil salt, a soil from the Gobi Desert in southern Xinjiang, which contains sodium hydroxide, sodium chloride and a variety of harmful substances, but does not contain iodine.
The government of Xinjiang Uyghur Autonomous Region, with the support of central and local finances, has taken a series of measures in high-risk areas to prevent and control IDD. The primary measures taken were emergency iodine enhancement in key groups by supplementation with Lipiodol capsules – an organic iodine compound that combines vegetable oil with iodine and is used to prevent IDD such as endemic goitre and endemic cretinism – and free distribution of iodised salt to disadvantaged people in Xinjiang. There have been annual increases in the coverage rate of iodised salt and the consumption rate of qualified iodised salt in Xinjiang Uighur Autonomous Region after continuing financial subsidies(Reference Zheng, Xu and Gu5). Consequently, household consumption of qualified iodised salt in Xinjiang has exceeded 90·0 % in the past decade.
In 2012, the Centre for Endemic Disease Control, which is part of the Chinese Centre for Disease Control and Prevention, organised multidisciplinary experts to conduct a survey on the current state of prevention and control of IDD in Hotan and Kizilsu Kirghiz Autonomous Prefecture areas, both of which have a history of serious iodine deficiency. The results showed that in children aged 8–10 years and women of childbearing age (20–50 years), IDD had been effectively controlled, which demonstrated an obvious positive effect of prevention and control efforts(Reference Su, Shen and Yan6). In 2007, the WHO, the United Nations Children’s Fund (UNICEF) and the International Council for Control of IDD (ICCIDD) proposed that ‘when universal salt iodisation has been carried out effectively for more than 2 years (coverage of household use of qualified iodised salt >90 %), and the median urinary iodine of children is more than 100 μg/l, it can be considered that dietary iodine meets the needs of women of childbearing age, pregnant women, and lactating women. There is no need for additional iodine fortification’(Reference Andersson and de Benoist7). Iodine supplementation using Lipiodol capsules began in 2006 in iodine-deficient areas of Southern Xinjiang. However, after more than a decade of implementation, data are not available yet on this initiative. Therefore, the long-term benefits and safety of such supplementation in iodine-deficient areas of Xinjiang are uncertain. The purpose of this study was to determine the effects of long-term use of Lipiodol capsules by assessing iodine nutrition status, thyroid function and prevalence of goitre and thyroid nodules among three groups of women, including women of childbearing age (who were neither pregnant nor lactating at the time of survey), pregnant women and lactating women in four areas of Xinjiang. In addition, we evaluated the prevention and treatment efficacy of emergency iodine supplementation and fortification measures.
Materials and methods
Survey areas
Four areas of Xinjiang Uighur Autonomous Region in China, including Kashgar, Aksu, Turpan and Yili Prefectures, were selected for the present study. The selection of these areas was guided by reports on iodised salt coverage (>95 %) and administration of Lipiodol capsules from the 2014 technical report of surveillance and emergency iodine enhancement in high-risk areas of IDD in China(Reference Liu, Fan and Liu8). The median water iodine in all the three areas was <10 μg/l. Lipiodol capsules were administered twice per year in Kashgar (oral Lipiodol dose: 100 mg) and once per year in Aksu (oral Lipiodol dose: 200 mg). In Turpan, Lipiodol capsules were also administered once per year (oral Lipiodol dose: 200 mg), but this ended in 2015. Yili was used as a control in the present study since Lipiodol capsules were not administered in this area. In order to ensure the compliance with the subjects, all the women in Kashgar, Aksu and Turpan took Lipiodol capsules in maternal and child health stations during the implementation of iodine supplementation measures using Lipiodol capsules. The use of Lipiodol capsules by the women was monitored and evaluated by provincial and municipal health administrative departments and disease prevention and control agencies.
Sampling method
A multi-stage, stratified, random sampling method was used in this study. In the first stage, one county/district was randomly selected from each of the areas mentioned above. In the second stage, according to the expected sample size, three towns were randomly selected from each county/district. In the final stage, individuals meeting the inclusion criteria in the selected towns were randomly chosen as the subjects for the study.
Based on the sampling method, the following towns were selected: Xiaputule, Yingmaili and Hexiaawati towns in Peyziwat County of Kashgar Prefecture; Tuohula, Jiamu and Gule’awanti towns in Wensu County of Aksu Prefecture; Qiatekale, Aidinghu and Putao towns in Gaochang District of Turpan Prefecture and Tulupanyuzi, Wenyaer and Yingtamu towns in Yining County of Yili Prefecture. Fig. 1 shows the geographical distributions of the survey areas.
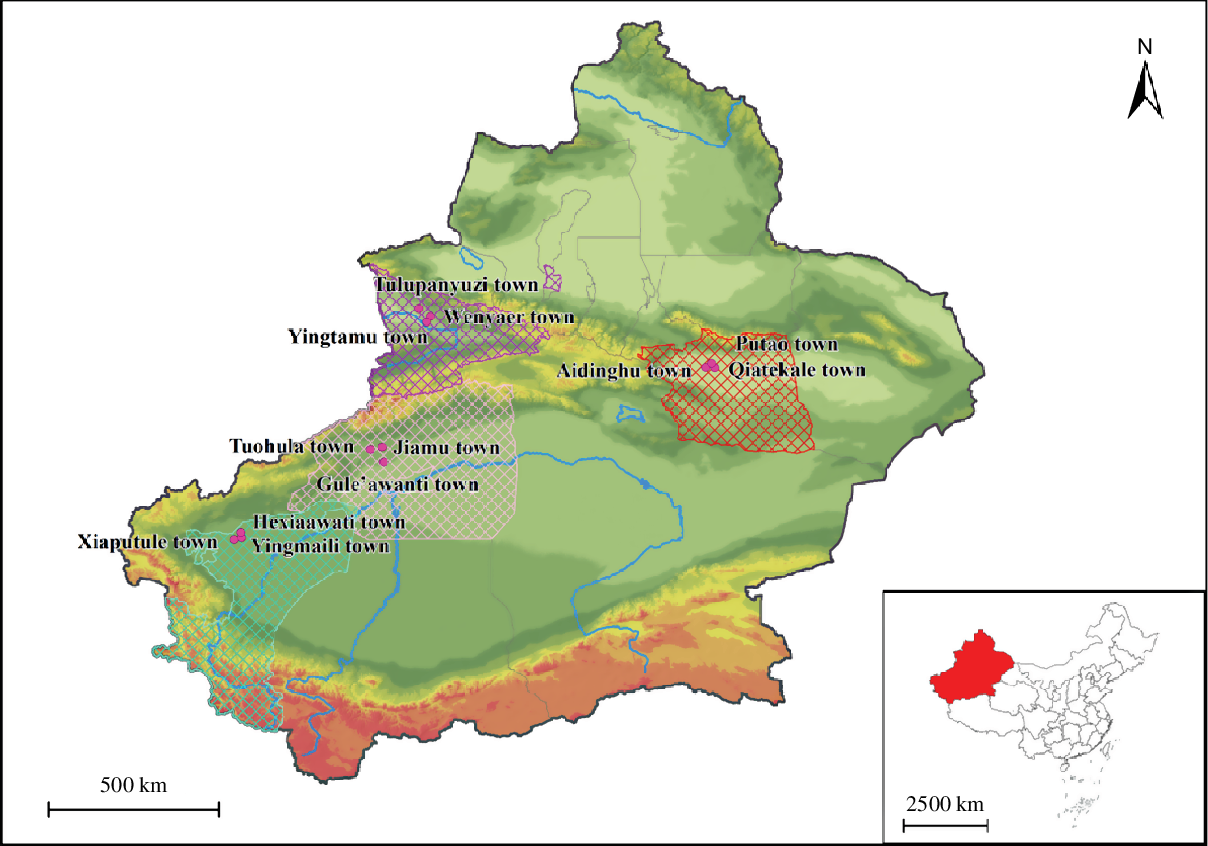
Fig. 1. The geographical distributions of the survey areas in Xinjiang Uygur Autonomous Region of China. ![]() , Kashgar Prefecture;
, Kashgar Prefecture; ![]() , Aksu Prefecture;
, Aksu Prefecture; ![]() , Turpan Prefecture;
, Turpan Prefecture; ![]() , Yili Prefecture;
, Yili Prefecture; ![]() , provincial boundary;
, provincial boundary; ![]() , city boundary;
, city boundary; ![]() , rivers. Elevation (m):
, rivers. Elevation (m): ![]() , 155–683;
, 155–683; ![]() , 684–1068;
, 684–1068; ![]() , 1069–1469;
, 1069–1469; ![]() , 1470–1999;
, 1470–1999; ![]() , 2000–2608;
, 2000–2608; ![]() , 2609–3248;
, 2609–3248; ![]() , 3249–3900;
, 3249–3900; ![]() , 3901–4586;
, 3901–4586; ![]() , 4587–5245;
, 4587–5245; ![]() , 5246–8611.
, 5246–8611.
Study design and participant selection
This study was cross-sectional by design and was conducted in line with the guidelines of the Declaration of Helsinki. Approval for the study was obtained from the Ethical Review Board of Harbin Medical University (ID no. hrbmuecdc20200501). Data collection was conducted from 1 June 2017 to 14 June 2017. Survey participants included women of childbearing age (20–50 years) who were neither pregnant nor lactating at the time of the survey, pregnant women and lactating women (breast-feeding within 2 years postpartum). We surveyed these groups for they are considered to be high risk for IDD. Moreover, iodine requirements during pregnancy and lactation are greatly increased relative to those for women who are not pregnant or lactating, owing to metabolic changes(Reference Yarrington and Pearce9,Reference Delange10) . To control for confounding factors, women in the following categories were excluded: those who were town residents for <5 years, those currently taking anti-thyroid drugs, those who had previously taken anti-thyroid drugs within 1 year preceding the survey, those who had eaten seafood within 3 d and those with a family history of thyroid diseases or congenital thyroid diseases.
The sample size for each area was determined by the variation in urinary iodine concentration (UIC); approximately 120 spot urine samples were needed to estimate the iodine level in a population with 95 % confidence within a precision range of ±10 %, and about thirty samples were needed for a precision range of ±20 %(Reference Andersen, Karmisholt and Pedersen11). Overall, a total of 1220 subjects were included, of which 311, 302, 300 and 307 were from Kashgar, Aksu, Turpan and Yili, respectively. Of the women of childbearing age, 104, 96, 96 and 99 were from Kashgar, Aksu, Turpan and Yili, respectively, of the pregnant women, 101, 102, 104 and 104 were from Kashgar, Aksu, Turpan and Yili, respectively; and of the lactating women, 106, 104, 100 and 104 were from Kashgar, Aksu, Turpan and Yili, respectively. According to the basic profile of the four areas in this survey, the four areas were similar with respect to socio-economic factors, including income and education. Moreover, all the subjects are of the Uyghur nationality of Xinjiang. Therefore, the eating habits in four areas were also similar. Written informed consents were obtained from all participants before data collection. The purpose of the study, including the risks and benefits of participating, was communicated to participants. In addition, in order to ensure the confidentiality and anonymity of the participants, all personal identifiers of respondents were removed. More importantly, all investigators signed a confidentiality agreement for the personal information of the respondents.
Survey indicators and measurements
According to WHO recommendations, UIC and thyroid-stimulating hormone (TSH) levels were used to evaluate the iodine nutrition status of the population(12). However, we acknowledge some limitations of UIC and TSH as biomarkers; UIC is easily affected by diet, urine volume and collection time(Reference Wan, Qu and Wu13–Reference Yu, Zheng and Zheng15), and TSH is usually used to screen newborns for congenital hypothyroidism and is seldom used to assess iodine nutrition in the entire population(Reference Zhou, Lu and Pan16,Reference Pearce, Korada and Day17) . For that matter, we also included free triiodothyronine (FT3), free thyroxine (FT4), thyroglobulin antibody (TgAb) and thyroid peroxidase antibody (TPOAb) levels as novel indicators for testing. In addition, we collected at least 50 g of household table salt from all participants in clean, labelled Ziplock bags and tested for iodine content. In some instances, the total number of samples collected for each survey indicator did not correspond with the total number of participants; some participants were unwilling to produce samples or refused sample collection, especially urinary samples.
Water sample collection
Before the survey was conducted, water iodine levels in the areas were measured to determine whether the value was <10 μg/l cut-off that was used for inclusion in the present study. Each of the four areas has a centralised water supply system, and two parallel samples were collected from each survey area. The water iodine level for each area was recorded as the average value of the two samples.
Urine sample collection
From each participant, a fasting single-spot urine sample was collected in the morning (between 08.00 hours and 11.00 hours) in a clean, well-labelled plastic tube and stored at −20°C. The analysis was completed within 2 weeks of collection.
Thyroid volume measurements and goitre
Thyroid ultrasonography was performed by an experienced examiner using a 7·5 MHz transducer and mainly measured the thyroid volume, nodule diameter and echo. The depth (d), width (w) and length (l) of each lobe were measured, and the thyroid lobe volume was then calculated using the following formula: V (ml) = 0·479 × d × w × l (mm)/1000, and recorded as the sum of both lobes.
Blood sample collection
Two millilitres of venous blood (no anticoagulant) was collected from study subjects, allowed to stand at room temperature for 2 h and was then centrifuged at 3000 g . The serum was separated and stored at –80°C.
Determination of iodine in water, salt and urine samples
The iodine concentration of drinking water was determined using the method of As3+–Ce4+ Catalytic Spectrophotometry, as recommended by the Chinese National Reference Laboratory for Iodine Deficiency Disorders (NRLIDD) and the Chinese Centre for Disease Control and Prevention(Reference Wang, Liu and Li18). The iodine content in the salt samples was determined using the general test method of the salt industry(Reference Tong and Huo19). The UIC was measured according to the health standard method of China for determination of iodine in urine by As3+–Ce4+ catalytic spectrophotometry(Reference Zhang, Yan and Liu20).
Determination of thyroid hormones in blood samples
Levels of FT3, FT4, TSH, TPOAb and TgAb were determined using a chemiluminescent immunoassay (Elecsys and Cobas Diagnostics; Bayer Healthcare Company).
Reference standard
The standard for iodine content of edible salt in Xinjiang was 30 mg/kg, and the allowable range of qualified iodised salt was 21–39 mg/kg(21). According to WHO/UNICEF/ICCIDD recommendations, the adequate range of urinary iodine for adults, pregnant women and lactating women is 100–199, 150–249 and >100 μg/l, respectively. The reference values were 3·1–6·8 pmol/l (FT3), 12·0–22·0 pmol/l (FT4), 0·27–4·2 µIU/ml (TSH), 0–34 U/ml (TPOAb) and 0–115 U/ml (TgAb). Thyroid ultrasonography was performed according to Chinese health standards(Reference Liu, Chen and Jia22). Normal female thyroid volume is <18 ml.
Diagnostic criteria for thyroid disease
Hypothyroxinaemia was defined as FT4 < 12 pmol/l and TSH within the normal range; overt hypothyroidism was defined as TSH > 4·20 mIU/l and FT4 < 12 pmol/l; subclinical hypothyroidism was defined as TSH > 4·20 mIU/l and FT4 within the normal range; overt hyperthyroidism was defined as TSH < 0·27 mIU/l and FT4 > 22 or FT3 > 6·8 pmol/l; subclinical hyperthyroidism was defined as TSH < 0·27 mIU/l with FT3 and FT4 within the normal range; autoimmune thyroiditis was defined as TPOAb > 34 U/ml or TgAb > 115 U/ml, with overt or subclinical hypothyroidism; positive thyroid antibody titers were defined as TPOAb > 34 U/ml or TgAb > 115 U/ml; and goitre was defined by a thyroid volume >18 ml (female).
Statistical analysis
SPSS (version 17.0; SPSS Inc.) for Windows was used for data processing and descriptive statistical analysis. The continuous data were analysed by ANOVA. Normally distributed continuous data were presented as means and standard deviations; non-normally distributed data were expressed as medians and interquartile ranges. Variables with skewed distributions were assessed with a Kruskal–Wallis test. A χ 2 test was used to compare differences in rates between groups. A P value <0·05 was considered statistically significant; all tests were two-tailed.
Results
Demographic characteristics of the participants
The demographic characteristics of participants from the four areas are shown in Table 1. The median water iodine in Kashgar, Aksu, Turpan and Yili was 8·1, 1·5, 5·6 and 0·8 µg/l, respectively. The coverage rate of household consumption of qualified iodised salt exceeded 90 % in all areas except Aksu (74·2 %) and the coverage rate of iodised salt exceeded 95·0 % in all areas. There were no significant differences with respect to age or BMI between women from the four areas. Moreover, the composition, age and BMI of pregnant women at different trimesters in the four areas did not differ significantly (all P > 0·05).
Table 1. Demographic characteristics in four areas*†(Normally distributed mean values and standard deviations; non-normally distributed medians and 25th and 75th percentiles (P25–P75); numbers and percentages)
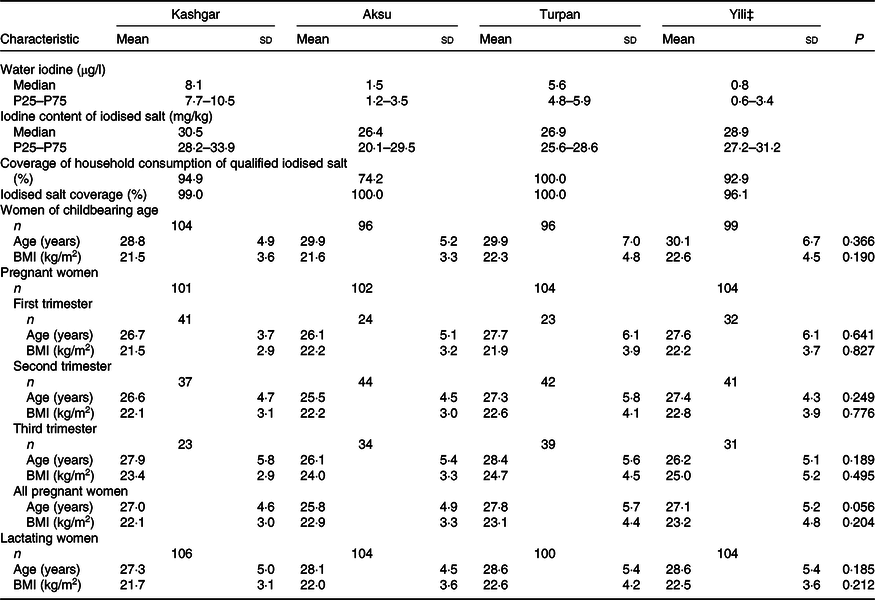
* One-way ANOVA was used for age and BMI. P < 0·05 was considered significant.
† There was no significant difference in the composition of pregnant women at different trimesters in the four areas (P = 0·064).
‡ Yili as the control in each area.
Iodine nutritional status
The UIC data for women from different groups across the four areas are shown in Table 2. According to the UIC measured in this survey, all women in all four areas were iodine sufficient. The UIC data showed a downwards trend in childbearing age, pregnant and lactating women in Kashgar, Aksu and Turpan.
Table 2. Urinary iodine concentration of different women in four areas (µg/l)† (Non-normally distributed medians and 25th and 75th percentiles (P25–P75))
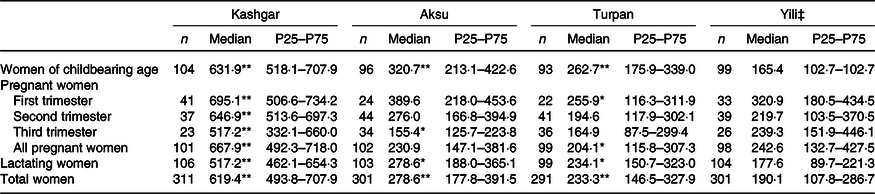
Median value was significantly different from that for Yili: * P < 0·05, ** P < 0·01.
† The Kruskal–Wallis test was adopted for urinary iodine concentration.
‡ Yili as the control in each area.
By the end of the study, Lipiodol capsules had been administered in Kashgar for <1 month. However, the UIC of childbearing age, pregnant and lactating women in that area was 631·9, 667·9 and 517·2 µg/l, respectively. For pregnant women in first, second and third trimesters, the UIC was 695·1, 646·9 and 517·2 µg/l, respectively. In Aksu, iodine supplementation with Lipiodol capsules had been ongoing for more than 6 months. We noted that, comparatively, the UIC of each of the three groups was significantly lower than that of Kashgar (women of childbearing age: 320·7 v. 631·9 µg/l, Z = −9·946, P < 0·001; all pregnant women: 230·9 v. 667·9 µg/l, Z = −10·694, P < 0·001; lactating women: 278·6 v. 517·2 µg/l, Z = −10·142, P < 0·001). At the same time, the level of UIC of pregnant women at different trimesters in the two areas also showed the same trend as all pregnant women taken together. The level of UIC in Aksu was lower than that in Kashgar (the first trimester: 389·7·9 v. 695·1 µg/l, Z = −6·006, P < 0·001; the second trimester: 276·0 v. 646·9 µg/l, Z = −6·387, P < 0·001; and the third trimester: 155·4 v. 517·2 µg/l, Z = −5·25, P < 0·001). UIC was higher in women of childbearing age and lactating women in Aksu compared with those in Turpan (320·7 v. 262·7 µg/l, Z = −2·992, P = 0·003; 278·6 v. 234·1 µg/l, Z = −3·083, P = 0·002) and Yili (320·7 v. 165·4 µg/l, Z = −6·155, P < 0·001; 278·6 v. 177·6 µg/l, Z = −6·804, P < 0·001). There were also significant differences in UIC between Turpan and Yili with regard to women of childbearing age (P < 0·001), pregnant women (all pregnant women, P = 0·021; pregnant women in first trimester, P = 0·033) and lactating women (P < 0·001). Nevertheless, although the UIC was higher for both women of childbearing age and lactating women in Turpan, it was lower for all pregnant women.
Thyroid hormones and autoimmune antibodies
Table 3 shows levels of thyroid hormones and autoimmune antibodies in women from Kashgar, Aksu, Turpan and Yili. There were significant differences in levels of FT3 (F = 6·193, P < 0·01), FT4 (F = 4·333, P = 0·005) and TSH (H = 16·924, P = 0·01) in women across the four areas. We found that thyroid hormone levels were significantly lower in Turpan women than in Yili women. However, we found no significant differences across the four areas with regard to TgAb positive rate, TPOAb positive rate or double antibody positive rate.
Table 3. Thyroid hormone and antibody levels of different women in four areas†(Normally distributed mean values and standard deviations; non-normally distributed medians and 25th and 75th percentiles (P25–P75); numbers and percentages)
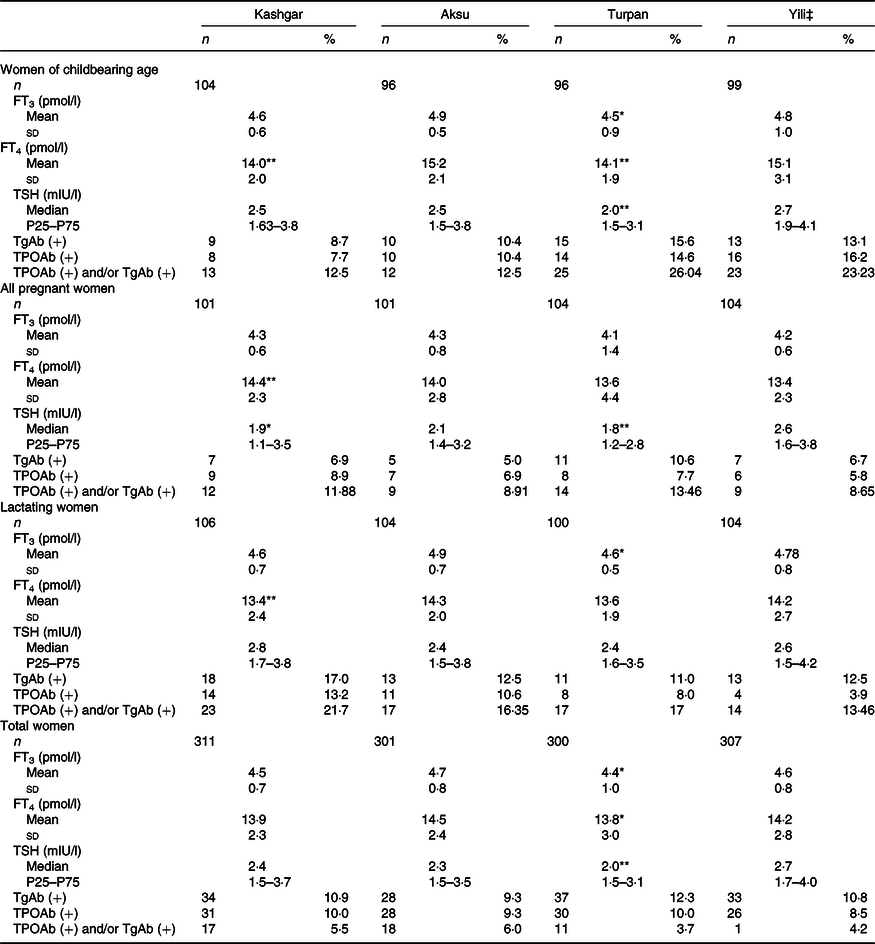
FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone; TGAb, thyroglobulin antibody; TGAb(+), test value >115 U/ml; TPOAb, thyroid peroxidase antibody; TPOAb(+), test value >34 U/ml.
Value was significantly different from that for Yili: * P < 0·05, ** P < 0·01.
† One-way ANOVA was used for FT3 and FT4; Kruskal–Wallis test was adopted for TSH; χ 2 test was used for TPOAb (+), TgAb (+), and TPOAb (+) and/or TgAb (+).
‡ Yili as the control in each area.
Further analysis by group revealed significantly lower FT4 levels in women of childbearing age and lactating women in Kashgar compared with those in Yili (14·0 v. 15·1 pmol/l and 13·4 v. 14·2 pmol/l, respectively). Furthermore, TSH levels of all pregnant women in Kashgar were significantly lower than those in Yili (1·9 v. 2·6 mIU/l). However, FT4 levels of all pregnant women in Kashgar were significantly higher than those in Yili (14·4 v. 13·4 pmol/l; P = 0·008). There were no significant differences in thyroid hormone levels between women in Aksu and Yili. There were significantly lower levels of FT3, FT4 and TSH in women in Turpan compared with women in Yili. As for thyroid autoimmune antibodies, there were no statistically significant differences between the groups with respect to TPOAb positive rate, TgAb positive rate and TPOAb and/or TgAb (Table 3).
Thyroid hormone and antibody levels of pregnant women at different trimesters in the four areas are shown in Table 4. As pregnancy progressed, the levels of FT3 and FT4 in each area tended to decrease, while the level of TSH reached the highest level in the third trimester. Except for the TgAb positive rate in Aksu (χ 2 = 9·674, P = 0·008) and the TPOAb and/or TgAb positive rate in Turpan (χ 2 = 6·760, P = 0·034), there was no significant difference in antibodies among other areas during different trimesters of pregnancy.
Table 4. Thyroid hormone and antibody levels of pregnant women at different trimesters in four areas†
(Normally distributed mean values and standard deviations; non-normally distributed medians and 25th and 75th percentiles (P25–P75); numbers and percentages)

FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone; TGAb, thyroglobulin antibody; TGAb(+), test value > 115 U/ml; TPOAb, thyroid peroxidase antibody; TPOAb(+), test value > 34 U/ml.
Value was significantly different from that for Yili: * P < 0·05, ** P < 0·01.
† One-way ANOVA was used for FT3 and FT4; Kruskal–Wallis test was adopted for TSH; χ 2 test was used for TPOAb (+), TgAb (+), and TPOAb (+) and/or TgAb (+).
‡ Yili as the control in each area.
The results of thyroid function in each trimester of pregnancy among the pregnant women in the four areas showed that there were statistically significant differences in the levels of FT4 and TSH between the four areas in the first and second trimester of pregnancy (FT4: F = 4·231, P = 0. 007 and F = 2·723, P = 0·046; TSH: χ 2 = 8·721, P = 0·033 and χ 2 = 8·156, P = 0·043). In contrast, the levels of FT3 only showed statistically significant differences among the four regions in the first trimester (F = 6·669, P < 0·001). For thyroid autoimmune antibodies, there were no statistically significant differences between the groups with respect to the TPOAb positive rate, the TgAb positive rate or the TPOAb and/or TgAb positive rate in pregnant women at different trimesters in the four areas (Table 4).
Thyroid diseases in the four areas
Thyroid diseases in the four areas are shown in Fig. 2. We noted significant differences in the detection rate of subclinical hypothyroidism between women in Kashgar, Aksu, Turpan and Yili (χ 2 = 14·532, P = 0·002). The detection rate of subclinical hypothyroidism was significantly lower in Kashgar and Turpan than in Yili (9·3 v. 16·6 %, χ 2 = 7·281, P = 0·007; 7·7 v. 16·6 %, χ 2 = 11·343; P = 0·001). We found no significant differences in the detection rates for hypothyroidism, hyperthyroidism or subclinical hyperthyroidism between the areas. However, significant differences were found between the areas for total detection rate of thyroid dysfunction cases; the total detection rate of thyroid dysfunction cases in Turpan was significantly lower than that in Yili (14·3 v. 25·4 %, χ 2 = 11·658, P = 0·001). The difference in the detection rate for hypothyroxinaemia across the four areas was statistically significant (χ 2 = 11·138, P = 0·011), with Aksu having the lowest detection rate of 9·6 %. The detection rates of autoimmune thyroiditis in women in Kashgar (5·8 %), Aksu (5·0 %) and Turpan (5·0 %) did not vary significantly, although the rates in these three areas were slightly higher than that in Yili (3·6 %; Fig. 2(d)).
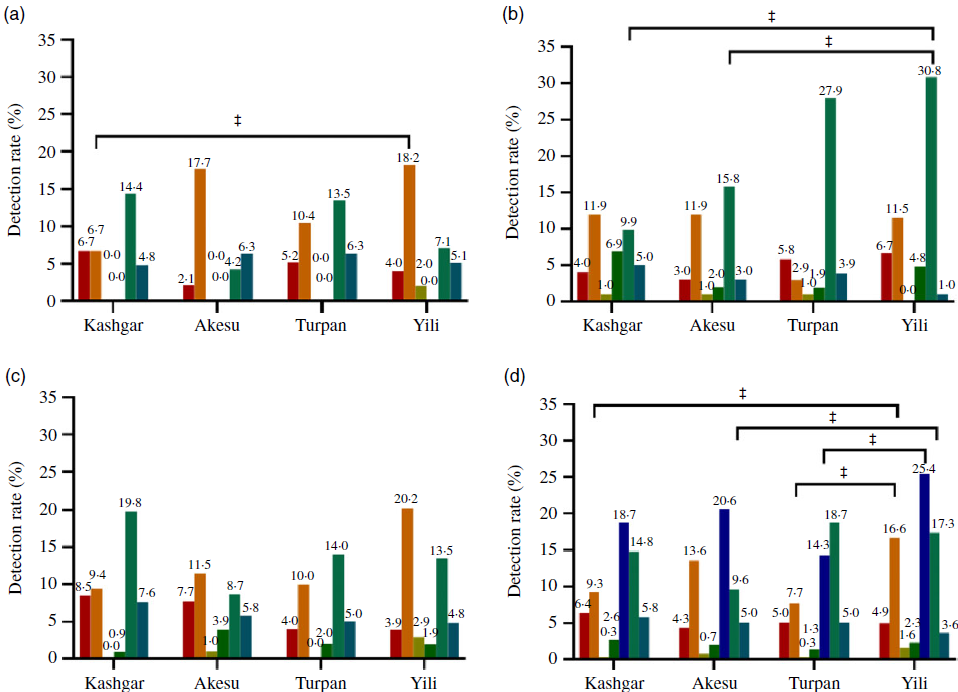
Fig. 2. Thyroid disease detection rates of different women in four areas. (a) Women of childbearing age; (b) all pregnant women; (c) lactating women; (d) total women; Yili as the control area and ‡P < 0·0167. ![]() , Overt hypothyroidism;
, Overt hypothyroidism; ![]() , subclinical hypothyroidism;
, subclinical hypothyroidism; ![]() , overt hyperthyroidism;
, overt hyperthyroidism; ![]() , subclinical hyperthyroidism;
, subclinical hyperthyroidism; ![]() , hyroid dysfunction cases;
, hyroid dysfunction cases; ![]() , hypothyroxinaemia;
, hypothyroxinaemia; ![]() , autoimmune thyroiditis.
, autoimmune thyroiditis.
The detection rates of thyroid diseases across the groups of women are shown in Fig. 2. There were significant differences in the detection rate of subclinical hypothyroidism in the different areas for women of childbearing age (χ 2 = 8·314, P = 0·04), and the detection rate was significantly lower in Kashgar than in Yili (6·7 v. 18·2 %, χ 2 = 6·159, P = 0·013; Fig. 2(a)). The detection rate of hypothyroxinaemia among the all pregnant women showed statistically significant differences across the four areas (χ 2 = 17·925, P < 0·001). The findings showed that the detection rate of hypothyroxinaemia in all pregnant women was lower in Kashgar and Aksu than in Yili (9·9 v. 30·8 %, χ 2 = 13·698, P < 0·001; 15·8 v. 30·8 %, χ 2 = 6·367; P = 0·012; Fig. 2(b)). By contrast, there were no significant differences between lactating women in the four areas with respect to thyroid diseases or the detection rate of autoimmune thyroiditis (Fig. 2(c)).
Table 5 shows thyroid ultrasound results for 1217 women in the four study areas. The total goitre rate in the four areas was 1·1 %, and the total detection rate of thyroid nodules was 5·9 %. No significant differences were observed in detection rate of goitre and thyroid nodules across the four areas (χ 2 = 2·461, P = 0·482; χ 2 = 1·411, P = 0·703).
Table 5. Detection rates of goitre and thyroid nodule in four areas* †
(Number of goitre and thyroid nodules and percentages)
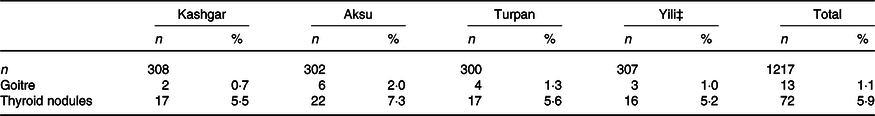
* Four areas were compared, the prevalence of goitre and the prevalence of thyroid nodules did not differ significantly.
† The χ 2 test was used for goitre and thyroid nodules.
‡ Yili as the control in each area.
Discussion
Growing evidence suggests that thyroid hormone disorders can be caused by either insufficient or excessive iodine intake(Reference Sang, Wei and Zhao23–Reference Sang, Chen and Shen25). Therefore, we investigated the iodine nutrition status of women in Xinjiang after more than a decade of iodine supplementation with Lipiodol capsules. The results showed that in each of the four areas, median water iodine was <10 μg/l and the iodised salt coverage was >95·0 %. These data imply that, despite these areas being iodine-deficient, there was effective coverage of iodised salt in the population. However, a high population coverage of iodised salt does not always translate into high consumption of qualified iodised salt. Indeed, we noted low household consumption of qualified iodised salt in Aksu. This observation may have resulted from the unstable quality of local iodised salt due to poor storage, as well as inadequate health education and promotion of iodised salt consumption(Reference Ma26).
The study investigated the iodine nutrition status for women of childbearing age, pregnant women and lactating women in Turpan and Yili and found that these women were in a non-iodine deficiency state. The median UIC of women of childbearing age, pregnant women and lactating women in Kashgar was in excess of the recommended levels of median UIC (>500·0 μg/l). Similarly, women of childbearing age in Aksu were also in a state of iodine excess (320·7 μg/l). It should be noted that the composition, age and BMI of pregnant women at different trimesters in our study were also well comparable in the four areas. Furthermore, the UIC levels of pregnant women at different trimesters showed the same trend as the UIC levels of all pregnant women among the four areas. This finding may be explained by the short duration between the time of supplementation and data collection, which likely caused a transient increase in UIC among the sampled population(Reference Ma27). Specifically, Lipiodol capsules were administered to women in Kashgar in May 2017, and data were collected in June 2017; in Aksu, iodine supplements were administered in December 2016, 6 months prior to data collection. Interestingly, in Aksu, we found iodine nutrition to be adequate among pregnant women (230·9 μg/l) and sufficient among lactating women (278·6 μg/l), which may reflect an increased utilisation of iodine during pregnancy and lactation(Reference Yarrington and Pearce9,Reference Delange10) . A study in Gansu Province of China showed that the iodine nutrition status of pregnant and lactating women was significantly improved after a single oral administration of 200 mg Lipiodol capsules(Reference Zhen, Wang and Li28). However, in the same study, the level of urinary iodine peaked at the first day after taking Lipiodol capsules. The quantity of urinary iodine excreted within 21 d was greater than that at 1 month after taking Lipiodol capsules. Then, from 3 to 9 months after taking Lipiodol capsules, the UIC gradually tended to be >150 μg/l, the ideal cut-off for pregnant women proposed by the WHO(12). Also, a study from Qinghai Province of China, where emergency iodine supplementation with Lipiodol capsules was implemented, demonstrated that the pregnant and lactating women took Lipiodol capsules continuously for 3 years, which was effective in prevention and treatment of IDD(Reference Chen, Luo and Wen29). The above data suggest that the national policy of iodine supplementation and fortification has effectively improved the iodine deficiency state of the population in this survey area, although there was evidence of excess iodine intake. This finding was similar to a case–control study by Isa et al. from Malaysia, which demonstrated that oral iodised oil can effectively reduce thyroid size and improve iodine nutrition levels among schoolchildren and pregnant mothers in endemic goitre areas. However, the related long-term effects need to be monitored more closely(Reference Isa, Alias and Kadir30).
The results of thyroid function of women in the survey areas showed that levels of FT3, FT4 and TSH were significantly different in Turpan compared with Yili. However, there were no significant differences in FT3, FT4 or TSH levels between women in Kashgar, Aksu and Yili. The results of thyroid function in pregnant women at different trimesters showed that the levels of FT3 and FT4 were significantly lower in the third trimester than in the first trimester, while the level of TSH increased in the third trimester. Previous studies(Reference Yan, Dang and Dang31,Reference Yang, Zheng and Li32) have suggested that after pregnancy, the synthesis of thyroxine binding globulin in the liver increases due to the rise in oestrogen. This leads to the increase of Triiodothyronine (T3)/Thyroxine (T4) binding to thyroxine binding globulin and ultimately a decrease in FT3 and FT4 serum levels. Our findings are consistent with these previous studies. Furthermore, no significant differences were noted in the positive rates of TgAb and/or TPOAb among the four areas including pregnant women in different trimesters. Nevertheless, the detection rate of autoimmune thyroiditis among all women was slightly higher in Kashgar, Aksu and Turpan, where Lipiodol capsules were administered, compared with Yili, where iodine supplementation with Lipiodol capsules was not implemented. When iodine supplementation or fortification is administered in an iodine-deficient state, there is a risk for overdose if supplementation is continued after iodine supply improves. This claim has been supported by empirical studies which reported that long-term fortification of iodine after iodine deficiency would have an impact on thyroid autoimmune status and could result in the production of abnormal thyroid antibodies in susceptible people(Reference Lind, Kumnig and Heinisch33,Reference Pedersen, Knudsen and Jørgensen34) . Similarly, a study of schoolchildren from areas with severe iodine deficiency in Azerbaijan showed that thyroid autoantibody (TPOAb) was significantly higher when taking 190 mg Lipiodol capsules twice a year (at intervals of 6 months) than once a year(Reference Markou, Georgopoulos and Makri35). Therefore, iodine supplementation and fortification programmes must be monitored and evaluated regularly to avoid excess iodine intake and the associated adverse effects.
According to our results, the total detection rate of thyroid dysfunction cases was 18·7, 20·6, 14·3 and 25·4 % in Kashi, Aksu, Turpan and Yili, respectively. The detection rate of hypothyroidism among women in Kashgar was 6·4 %. Although this was slightly higher than the detection rate observed in the other three areas, it was closely aligned with the prevalence rate of hypothyroidism among lactating women (6·8 %) reported in 2014 by the Centre for Endemic Disease Control, Chinese Centre for Disease Control and Prevention(Reference Liu, Fan and Liu8). Among the four areas, the detection rate of subclinical hypothyroidism varied significantly, with the highest rate of 16·6 % recorded among women in Yili. Notably, Yining County of Yili is located in the north of Xinjiang, where living standards and economic conditions are comparatively better than in other regions. Iodine levels in the water of the villages and towns of Yining County range from 0·1 to 7·2 μg/l and the external environment are in a state of iodine deficiency. In 2006, the coverage rate of iodised salt in Yining County was 58·33 %, the median UIC of children was 91·9 μg/l and the goitre rate was 15·9 %; none of these measures met the national standard for eliminating IDD(Reference Zhang, Chen and Ge36). In recent years, intervention by the Chinese government resulted in remarkable progress in the prevention and control of IDD in Yining and the coverage rate of iodised salt has increased significantly. National reports showed that iodised salt coverage and consumption of qualified iodised salt in Yining exceeded 95·0 % in 2015 and 2016, which is the standard for the elimination of IDD(Reference Zhang, Chen and Ge36). In 2017, surveillance results of IDD in Yining County, Xinjiang showed the median UIC of children was 120·43 μg/l and the goitre rate in children was 1·0 %. Thus, the iodine nutrition level of children in Yining County has improved to an iodine-sufficient state, and the goitre rate in children has reached the elimination standard of IDD. Although the present study found the median UIC of women in Yili to be in an adequate state, the detection rate of subclinical hypothyroidism in Yili was the highest of any survey region, indicating a need for further investigation.
It has been reported that increased prevalence of goitre, thyroid nodules and hyperthyroidism is the main consequences of long-term iodine deficiency in adults(Reference Khattak, Ittermann and Nauck37). The goitre rates were <5 % in all four areas surveyed, and detection rates of hyperthyroidism and thyroid nodules were relatively low. It is evident that with the annual increase of iodised salt coverage rate, iodine nutrition is becoming more adequate for the population. The iodine deficiency in the four areas was greatly improved. A controlled trial in an iodine-deficient area of Zaire demonstrated that after 2 years of supplementation with Lipiodol, the age–sex standardised total goitre prevalence in the population decreased from 64 to 54 %(Reference Phillips and Osmond38). According to a study by Phillips and his colleagues, the effects of a single oral Lipiodol dose can last up to 8 months(Reference Phillips, Lusty and Osmond39). It is worth mentioning that the detection rates of thyroid nodules among women were 5·5, 7·3, 5·6 and 5·2 % in Kashgar, Aksu, Turpan and Yili, respectively; these rates are lower than those reported in other areas of China. In 2014, it was reported that the detection rate of thyroid nodules was 23·36 % in women and 16·04 % in lactating women in the Beihai area of Guangxi Province(Reference Liu, Wang and Liu40). Investigation of iodine levels in Juye County of Shandong Province showed that the detection rate of thyroid nodules in adults was 16·5 %(Reference Chen, Meng and Liu41). A survey conducted by Du et al. in areas of iodine deficiency, iodine sufficiency and iodine excess found detection rates of thyroid nodules in adults to be 10·8, 22·2 and 8·66 %, respectively(Reference Du, Gao and Meng42). A large number of epidemiological studies have shown that the use of high-resolution B-ultrasound can detect nodules in 19–67 % of randomly selected people, mostly in women and the elderly(Reference Yan43). The above surveys reported detection rates of thyroid nodules that were higher than those found in this survey, which may be explained by differences between Xinjiang and other areas in mainland China with respect to external environment iodine nutrition, eating habits, lifestyle, work stress or other factors.
Xinjiang spans across a wide area and has an uneven distribution of rivers. Historically, IDD in southern Xinjiang was more serious than in northern Xinjiang. Of the four areas surveyed, Kashgar and Aksu are located in the south of Xinjiang, Turpan is located in the central and eastern parts of Xinjiang and Yili is located in the north of Xinjiang. In Turpan, both the iodised salt coverage and consumption rate of qualified iodised salt were 100 %. At the time of this study, it had been a year and a half since the last oral administration of Lipiodol capsules and the effect of these supplements was no longer evident(Reference Zheng and Bai44). The survey showed that, in Turpan, iodine nutrition status was sufficient in women of childbearing age (UIC: 262·7 μg/l), adequate for pregnant women (UIC: 204·1 μg/l) and sufficient for lactating women (234·1 μg/l). Moreover, the total detection rate of thyroid dysfunction cases was lower in Turpan than in the areas surveyed. These data suggest that consumption of iodised salt provides necessary iodine nutrition for the population in these areas and that there is no need for additional iodine supplementation or fortification.
According to the results of this survey, IDD has been effectively controlled in Kashgar and Aksu. Therefore, it is appropriate to provide free supply of iodised salt for disadvantaged people along with emergency iodine supplementation with Lipiodol capsules for people in special need. These preventions and control measures should be adopted in the areas where the coverage rate of iodised salt, and the consumption rate of qualified iodised salt do not meet recommended standards, and the price subsidy of iodised salt should continue for the key population. Since either iodine deficiency or iodine excess can have adverse effects on the human body, it is important to note that iodised salt alone is shown to be adequate for women who are not pregnant or lactating and there is no need for supplementation with Lipiodol capsules in this population. In order to avoid negative effects of iodine excess, we suggest that the use of Lipiodol capsules in women with special needs should be gradually discontinued based on the existing iodine nutrition levels of local women aged 20–50 years.
Strengths and limitations
This study has several strengths. First, it is the first to evaluate the effect of Lipiodol capsules on women aged 20–50 years in iodine-deficient areas over the course of 10 years. This type of study is critical to guide follow-up implementation and emergency use of Lipiodol capsules for key populations in iodine-deficient areas. Second, the subjects of this study are of the Uyghur nationality of Xinjiang. At present, there are few studies on the effect of long-term use of Lipiodol capsules in Chinese ethnic minorities. Our results provide a theoretical reference for the prevention and treatment of IDD in minorities. Despite the notable strengths of the present study, there are also some limitations. First, dietary habits were not surveyed to determine possible food-based sources of iodine. Second, a small number of urine and blood samples could not be collected, resulting in inconsistencies in the numbers of urine and blood test results across groups. Therefore, future research should focus on the collection of basic information and samples. Third, the diagnostic criteria of thyroid diseases in pregnant and lactating women were based on the general population in this study. The reference range for thyroid hormone in pregnant and lactating women has not been established in China, especially in Xinjiang. The residents of this study area are all Uyghurs who differ from the previous survey population (Han nationality), which may contribute to ethnic differences in the survey results. Last, we acknowledge the contribution of iodised salt consumption in reducing the prevalence of IDD in China(Reference Zhao, Han and Shi45). Lipiodol capsules were administered as a supplement to iodised salt. Therefore, we cannot solely attribute the improved iodine nutrition status and reduced prevalence of endemic goitre found in this study to the effect of Lipiodol capsules. It was methodologically too complex to distinguish which of the gains made in the control of IDD resulted from salt iodisation and which were from iodine supplementation with Lipiodol capsules.
Conclusion
The policy of taking Lipiodol capsules as a supplement to iodised salt has improved the iodine nutrition status of women in iodine-deficient areas of Xinjiang since 2006. The external environments of the survey areas in Xinjiang are in a state of iodine deficiency, and thus, there remains a risk for new cretinism. Generally speaking, the rural network of iodised salt supply in Xinjiang is sound, but the quality of iodised salt is not very stable. Since iodine deficiency and iodine excess can both have adverse effects on the human body, it is important to note that this study found that iodised salt alone was adequate for women who were not pregnant or lactating. Therefore, this suggests no need for supplementation with Lipiodol capsules in women who are not pregnant or lactating. In order to avoid the effects of iodine excess, we suggest a gradual discontinuation of Lipiodol capsules in women with special needs, based on the existing iodine nutrition level of local women aged 20–50 years. In addition, while actively taking comprehensive prevention and control measures, it is necessary to improve monitoring of iodine deficiency disorders and iodine nutrition, especially the monitoring of pregnant women, children and other key groups to allow for timely intervention.
Acknowledgements
We express our sincere thanks to the Xinjiang Uighur Autonomous Region Centre for Disease Control and Prevention for their kind assistance in samples collection.
This study was supported by the Central Transfer Payments for Prevention and Treatment of Endemic Disease Project (2016) and by the National Natural Science Foundation of China (grant nos. 81872561 and 81703175).
The authors’ contributions to the manuscript were as follows – H. S. and L. Z. designed the research, wrote the statistical analysis plan and had primary responsibility for final content; L. L., P. L., Q. L., X. S., J. H., F. M., L. F., J. L., W. C. and L. Z. conducted the research and collected the data; L. L. and S. W. analysed the data; L. L., H. S. and S. W. wrote the first draft of the manuscript; L. L. and H. S. interpreted the data and all authors contributed to writing and editing the manuscript and read and approved the final manuscript.
There are no conflicts of interest to declare.










