Introduction
Degenerative diseases may share some common pathological mechanisms such as oxidative stress including brain and eye diseases. In this paper, the common microRNA (miRNA) mechanisms for some oxidative stress-related degenerative brain and eye diseases are discussed including Parkinson's disease (PD), Alzheimer's disease (AD), glaucoma and age-related macular degeneration (AMD).
A free radical is any molecular species capable of independent existence that contains unpaired electrons in atomic orbitals. Free radicals are highly reactive and behave as oxidants or reductants because they can either donate an electron to or accept an electron from other molecules (Ref. Reference Lobo1). There are many types of free radicals including oxygen- and nitrogen-based species. Reactive oxygen and nitrogen species (RONS) contributed to the development of various diseases; however, intracellular RONS could also be an important component of intracellular signalling cascades (Ref. Reference Weidinger and Kozlov2). Reactive oxygen species (ROS) are by-products derived from cellular oxidative metabolism. The intrinsic biochemical properties of ROS play an essential role in regulating various functions of living organisms, contributing to the development of living organisms. They are involved in many important cellular activities such as gene transcription, signalling transduction and immune response. However, ROS overproduction can lead to oxidative stress, a phenomenon caused by an imbalance between ROS production in cells and tissues and the ability of a biological system to detoxify these reactive products (Ref. Reference Pizzino3). Oxidative stress is associated with a variety of diseases. Excess ROS can eventually lead to cell death.
The brain consumes more energy than any other tissue and is a major metaboliser of oxygen. The brain relies heavily on mitochondria to produce energy. During ageing, damaged mitochondria produced less adenosine triphosphate, and more ROS accumulated. ROS caused oxidative stress that triggered neurodegenerative diseases (Ref. Reference Stefanatos and Sanz4). Neurodegenerative diseases are caused by excessive and pathological loss of neurons, leading to dementia, cognitive impairment and so on. Microglial activation and oxidative stress are hallmarks of neurodegenerative disease (Ref. Reference Simpson and Oliver5). Oxidative stress is related to all major neurodegenerative diseases and is associated with neuronal injury and pathological progress. As a result, oxidative stress is widely recognised as a potential target for protective therapies (Ref. Reference Konovalova6).
Of these four oxidative stress-related degenerative diseases discussed in this paper, two of them (PD and AD) are brain diseases, and the other two (AMD and glaucoma) are eye diseases. Ocular measurements have recently been suggested as potential sources of biomarkers for the early detection of neurodegenerative diseases (Ref. Reference Guidoboni7). The optic nerve is the most accessible part of the central nervous system (CNS), so there might be a strong connection between optic neuritis and CNS disease (Ref. Reference Jenkins and Toosy8). Amyloid-beta (Aβ), p-tau, chronic inflammation and iron dyshomoeostasis might be common pathogenic mechanisms linking AD, glaucoma and AMD, and iron chelation is a common therapeutic option for these disorders (Ref. Reference Ashok9). Ocular disorders presented characteristics of neurodegenerative diseases and, on the other hand, AD and PD showed peculiar alterations at the ocular level (Ref. Reference Marchesi10). Despite the possible link between eye and brain diseases, both may not have a very strong association because patients with brain diseases do not always have eye diseases, and vice versa. However, because ocular conditions are easier to detect and diagnose than brain conditions, we may be interested in whether ocular conditions can be prognostic biomarkers in patients with brain diseases (Fig. 1).
Figure 1. Ocular conditions might be prognostic biomarkers in patients with brain diseases.
To understand more mechanisms linking brain oxidative stress-related diseases and eye stress-related diseases, in this study, common miRNA biomarkers for brain diseases and eye diseases are reviewed. A miRNA is a small non-coding RNA that plays an important role in many biological functions including gene regulation. The first miRNA was discovered in the early 1990s when studying the nematode Caenorhabditis elegans regarding the gene lin-14 (Ref. Reference Lee, Feinbaum and Ambros11). Since then, many miRNAs have been discovered for different species, and they were shown to be highly conserved across species (Ref. Reference O'Brien12). In the canonical miRNA biogenesis pathway, primary miRNAs are transcribed and then processed into precursor miRNA (pre-miRNAs) that produce functional mature miRNAs, the −3p single-stranded miRNA and the −5p single-stranded miRNA. miRNAs have been used as biomarkers for many diseases such as coronavirus disease 2019 (COVID-19) and neurological diseases (Refs Reference Wang, Taguchi and Liu13, Reference Wang14). The association between diseases can be explored using miRNA biomarkers (Refs Reference Wang15, Reference Wang and Ho16, Reference Wang17). miRNA biomarkers were used to explore the comorbidities of COVID-19 (Ref. Reference Wang18). The serum concentration levels of miR-499, miR-21, miR-155 and miR-208a were significantly increased in COVID-19 patients compared with the healthy controls (Ref. Reference Garg19). miRNAs in serum, cerebrospinal fluid and brain tissue have been investigated in AD as novel markers for treatment and diagnosis (Ref. Reference Liu20). miR-146a, miR-335-3p and miR-335-5p were found to be downregulated in PD patients compared with controls (Ref. Reference Oliveira21).
miRNAs are closely related to ROS, which is fine-tuned by dysregulated miRNAs, and vice versa (Ref. Reference Zhang22). Oxidative stress affects the expression levels of miRNAs and miRNAs regulate many genes involved in oxidative stress response (Ref. Reference Konovalova6). miRNAs can be oxidised, leading to the misidentification of target mRNAs. Oxidative stress and miRNAs are closely related during neurodegenerative processes such as mitochondrial dysfunction, deregulation of proteostasis and neuroinflammation. Mitochondrial dysfunction could damage by-products of respiration, and mitochondrial ROS were involved in cell signalling (Ref. Reference Brookes23). This paper discusses common miRNA biomarkers of oxidative stress-related eye and brain diseases via pre-miRNAs. For more details on the specific mature miRNA biomarkers, readers can refer to the cited references.
Oxidative stress-related eye and brain diseases
The oxidative stress-related diseases, glaucoma, AMD, PD and AD, are reviewed in this section.
Glaucoma
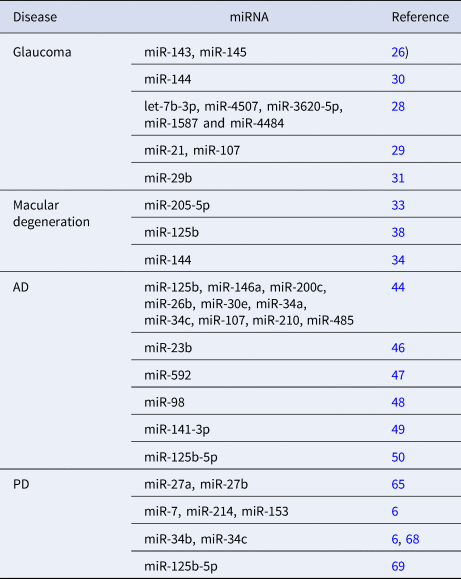
Glaucoma is a disease with characteristic optic neuropathy and vision loss, and primary open-angle glaucoma (POAG) is the most common type of glaucoma worldwide. POAG is a chronic neurodegenerative disease of optic nerve damage associated with an open anterior chamber angle and elevated intraocular pressure (IOP). POAG can induce retinal ganglion cell apoptosis and degenerate the optic nerve head (ONH). ROS plays a key role in the pathogenesis of POAG. Certain miRNAs were involved in the delicate balance of extracellular matrix synthesis and deposition regulated by chronic oxidative stress in POAG-associated tissues (Ref. Reference Tabak, Schreiber-Avissar and Beit-Yannai24). Various miRNAs are abundantly expressed in the eyes. The miRNA expressions in the normal human ciliary body, cornea and trabecular meshwork were studied to better understand miRNA function and disease involvement in these tissues (Ref. Reference Drewry25). Many miRNAs were identified in ocular tissue.
Various miRNAs could be used as biomarkers to assist in the early diagnosis of POAG. IOP is the major primary risk factor for blindness in glaucoma patients. The expression of miR-143 and miR-145 is enriched in the smooth muscle and trabecular meshwork of the eye. Targeted deletion of miR-143/145 in mice results in a significant reduction in IOP (Ref. Reference Li26). Aqueous humour (AH) is a dynamic intraocular fluid that supports the vitality of tissues that regulate IOP. AH is the liquid inside the front part of the eye. The eye constantly produces a small amount of AH, and an equal amount of AH flows out through the trabecular meshwork of the drainage angle. An imbalance in AH production and drainage can lead to IOP. Exosomes are a major constituent of AH (Ref. Reference Perkumas27). The expression profiles of miRNAs in the AH of glaucoma patients and the control group were compared (Ref. Reference Tanaka28). Fifty-seven miRNAs showed a statistically significant difference in expression levels between the control group and the glaucoma group. Among them, let-7b-3p, miR-4507, miR-3620-5p, miR-1587 and miR-4484 were most significantly different. Trabecular meshwork cells damaged by oxidative stress released extracellular miRNAs, including miR-21 and miR-107, as established in vitro and glaucoma AH (Ref. Reference Izzotti29). The over-expression of miR-144-3p promoted proliferation and invasion of human trabecular meshwork cells by inhibiting the expression of fibronectin 1 in oxidative stress human trabecular meshwork cells, and thus miR-144-3p could be a potential target for glaucoma treatment (Ref. Reference Yin and Chen30). Silencing of miR-29b-3p could protect human trabecular meshwork cells against oxidative injury by upregulation of RNF138 to activate the extracellular signal-regulated kinase pathway (Ref. Reference Liu31).
Macular degeneration
Both AMD and diabetic retinopathy (DR) are typically associated with oxidative stress. The use of antioxidant agents could be used as a co-adjuvant therapy for these diseases. miRNAs are involved in the regulation of angiogenesis, oxidative stress, immune response and inflammation in AMD and DR (Ref. Reference Gemayel, Bhatwadekar and Ciulla32). miR-205-5p was modulated by oxidative stress and regulates vascular endothelial growth factor A (VEGFA)-angiogenesis (Ref. Reference Oltra33). Hence, miR-205-5p is proposed as a candidate against eye-related proliferative diseases (Ref. Reference Oltra33).
The retinal pigment epithelium (RPE) is usually exposed to high levels of pro-oxidative stimuli. Inhibition of miR-144 could enhance nuclear factor erythroid-2-related factor 2 (Nrf2)-dependent antioxidant signalling in RPE and prevent oxidative stress-induced AMD (Ref. Reference Jadeja34). VEGFA enhancement and neovascular overgrowth are the clinical hallmarks of AMD (Refs Reference Marneros35, Reference Penn36). VEGFA was produced by retinal cells, including the RPE (Ref. Reference Deissler37). Activation of the Nrf2 signal pathway could protect RPE cells from oxidative damage, and miR-125b could target the Nrf2/hypoxia-inducible factor-1α signal pathway to protect RPE from oxidative damage (Ref. Reference Liu38).
Alzheimer's disease
AD is an irreversible neurodegenerative disorder affecting both cognition and emotional behaviour (Ref. Reference Tramutola39). Extracellular accumulation of Aβ peptide and the flame-shaped neurofibrillary tangles of the microtubule-binding protein tau are two major hallmarks required for a diagnosis of AD (Ref. Reference Murphy and LeVine40). miRNA contributes to the development of AD by regulating the accumulation of Aβ peptides and tau phosphorylation (Refs Reference Wang41, Reference Nakano42, Reference Swarbrick43). In addition, oxidative stress is one of the major pathomechanisms of AD, as well as other key events such as mitochondrial dysfunction, inflammation, metal dysregulation and protein misfolding.
The oxidative stress-associated miRNAs including seven upregulated miRNAs (miR-125b, miR-146a, miR-200c, miR-26b, miR-30e, miR-34a, miR-34c) and three downregulated miRNAs (miR-107, miR-210, miR-485) were found in vulnerable brain regions of AD at the prodromal stage (Ref. Reference Nunomura and Perry44). N-Acetylglucosaminyltransferase III (GnT-III) is a glycosyltransferase responsible for synthesising a bisecting N-acetylglucosamine residue. The mRNA levels of GnT-III were found highly expressed in the brains of AD patients and GnT-III was expressed strongly in AD model mice (Ref. Reference Wang45). A study showed that GnT-III might be targeted by miR-23b, and activation of the Akt/GSK-3β signalling pathway could contribute to tau-lesion inhibition by miR-23b (Ref. Reference Pan46). In addition, miR-23b could inhibit oxidative stress by altering Aβ-precursor protein processing. This might conclude that overexpression of miR-23b could interrupt the pathogenesis of AD (Ref. Reference Pan46). The mechanism of miR-592, KIAA0319 and the Keap1/Nrf2/ARE signalling pathway in AD was examined (Ref. Reference Wu47). Downregulation of miR-592 could inhibit oxidative stress injury of astrocytes in rat models of AD by upregulating KIAA0319 through the activation of the Keap1/Nrf2/ARE signalling pathway.
Hairy and enhancer of split-related with YRPW motif protein 2 (HEY2) is a hairy-related transcription factor family of Notch-downstream transcriptional repressors. miR-98 could target HEY2 to inhibit the activity of the Notch pathway, contributing to the inhibition of the production of Aβ and the improvement of oxidative stress and mitochondrial dysfunction in AD mice (Ref. Reference Chen, Zhao and Chen48). Exosomes are extracellular vesicles that can carry miRNAs and establish intercellular communication in neurons. Exosomal miRNAs can modulate the activity of multiple physiological pathways in neurodegenerative diseases, including oxidative stress responses. miR-141-3p was a potential serum biomarker for AD, that was observed with low concentrations in the plasma exosomes of AD patients (Ref. Reference Lugli49). miR-125b-5p was upregulated in cerebrospinal fluid-derived exosomes of patients with AD compared with healthy controls (Ref. Reference McKeever50). Inhibition of miR-125b-5p reduced ROS levels and lowered mitochondrial membrane potential, thereby demonstrating neuroprotective effects against oxidative stress (Ref. Reference Shen51).
Parkinson's disease
PD is a chronic neurodegenerative disease named after James Parkinson, who reported this clinical syndrome in 1817 (Ref. Reference Chia, Tan and Chao52). The PD has motor and non-motor symptoms including tremors, slowed movement, rigid muscles, impaired posture and balance, speech changes, writing changes, sleep disorders, depression, cognitive changes, illusions and delusions (Ref. Reference Wang53). PD was demonstrated to be associated with several genes including α-synuclein (SNCA); parkin (PARK2); PTEN-induced putative kinase 1 (PINK1); DJ-1 (PARK7); leucine-rich repeat kinase 2 (LRRK2); DnaJ (Hsp40) homologue, subfamily C, member 13 (DNAJC13), coiled-coil-helix-coiled-coil-helix domain containing 2 (CHCHD2), transmembrane protein 230 (TMEM230) and resistance to inhibitors of cholinesterase 3 (RIC3) (Refs Reference Gasser54, Reference Stefanis55, Reference Zimprich56, Reference Dawson and Dawson57, Reference Puschmann58).
The stimulation of oxidative stress is critical for the evolution of metabolic syndrome and PD (Ref. Reference Whaley-Connell, McCullough and Sowers59). In the 1-methyl- 4-phenyl- 1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD, oxidative stress might be an early event that directly killed some of the dopaminergic (DA) neurons (Ref. Reference Zhou, Huang and Przedborski60). PINK1 and parkin were involved in mitochondria-associated autophagy, and the loss of function of these proteins leads to the accumulation of damaged mitochondria (Refs Reference Lazarou61, Reference Pickrell and Youle62). In the pathogenesis of PD, mitochondria dysfunction is closely related to ROS (Ref. Reference Puspita, Chung and Shim63). PD might be more relevant to oxidative stress than AD (Ref. Reference Wang64).
miR-27a and miR-27b suppressed the expression of PINK1, contributing to inducing oxidative stress (Ref. Reference Kim65). SNCA could induce oxidative stress and increase ROS levels (Refs Reference Perfeito, Ribeiro and Rego66, Reference Zhang67), and the downregulation of miR-7, miR-214, miR-153 and miR-34b/c might contribute to SNCA-mediated neurotoxicity in PD (Refs Reference Konovalova6, Reference Kabaria68). miR-125b-5p was downregulated in MPTP-induced PD mouse models and MPP+-induced PD cell models (Ref. Reference Xiao69).
Table 1 summarises some miRNAs related to oxidative stress mechanisms in glaucoma, AMD, PD and AD.
Table 1. miRNAs related to the four oxidative stress-related diseases
Common miRNA biomarkers
Common miRNA biomarkers for all of the four diseases or some of the four diseases are reviewed in this section. The PubMed and Google Scholar databases were used to find relevant papers by performing a systematic search using the following terms ‘miRNA, Glaucoma’, ‘miRNA, macular degeneration’, ‘miRNA, Parkinson’ and ‘miRNA, Alzheimer’. Table 2 summarises some of the miRNA biomarkers that were indicated as such in at least two references. The tissues in which the miRNAs were detected are also provided in Table 2 if they were mentioned in the reference papers.
Table 2. Common miRNA biomarkers of glaucoma, AMD, PD or AD
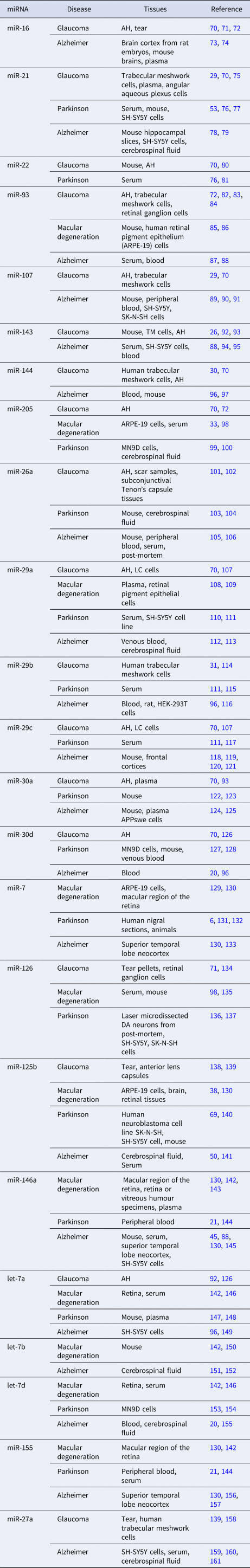
The miRNAs in Table 2 involved in these diseases are reviewed as follows. Tears are a biological fluid with a potential diagnostic value for ophthalmic diseases. POAG-patient tear pellets showed different expressions of miR-16 and miR-126 in comparison with pellets obtained from healthy persons (Ref. Reference Tamkovich71). miR-16-5p was one of the most abundant miRNAs detected in AH (Ref. Reference Wecker70). The other miRNAs in Table 2 detected in AH included miR-21-5p, miR-22-3p, miR-144-3p, miR-205-5p, miR-29a-3p, miR-29c-5p, miR-30a-5p and miR-30d-5p (Ref. Reference Wecker70). The use of polydopamine-polyethylenimine nanoparticles (PDA/PEI NPs) as miRNA carriers in the treatment of ocular hypertension and glaucoma was investigated (Ref. Reference Tan75). PDA/PEI NPs/miR-21-5p has been demonstrated as a promising anti-glaucoma drug for treating POAG. Tetrahedral frame nucleic acids (tFNAs) can be used as miRNA carriers in retinal neurons. tFNAs could transfer miR-22 into damaged retinal neurons that had a neuroprotective effect on glaucoma (Ref. Reference Li80). Up-regulation of miR-93-5p, binding with phosphatase and tensin homologue, suppressed the autophagy of retinal ganglion cells through the AKT/MTOR pathway in N-methyl-d-aspartate-induced glaucoma (Ref. Reference Li84).
Postoperative filtering tract scarring is one of the main reasons for the failure of glaucoma filtration surgery. miR-26a played an important role in the formation of filtering tract scar and functioned as a potential drug target (Ref. Reference Wang, Deng and He101). miR-30a-3p and miR-143-3p were upregulated in the AH of POAG patients compared with controls (Ref. Reference Hubens93). miR-30d-5p was significantly upregulated in pseudoexfoliation (PEX) glaucoma patients compared with the control (Ref. Reference Cho126). miR-126 facilitated the apoptosis of retinal ganglion cells in glaucoma rats by promoting the VEGF–Notch signalling pathway (Ref. Reference Wang134). The level of miR-125b expression was increased in POAG patients and PEX syndrome glaucoma patients compared with cataracts alone patients (Ref. Reference Tomczyk-Socha138). Intracameral delivery of miR-146a can long-term reduce IOP in rats. This miR-146 effect observed in rats could provide the development of effective gene therapy for human glaucoma (Ref. Reference Luna162).
The ONH is the site of initial optic nerve damage in glaucoma. ONH-derived lamina cribrosa (LC) cells are adversely affected in glaucoma and cause deleterious changes in ONH. miR-29a-3p and miR-29c-3p were downregulated in POAG LC cells compared with non-glaucomatous LC cells (Ref. Reference Lopez107). let-7a-5p and miR-143-3p were found to be significantly upregulated in the normal-tension glaucoma (NTG) patients compared with the controls (Ref. Reference Seong92). miRNA profiles of patients with PEX glaucoma or NTG compared with normal controls using individual AH samples were studied in Korea (Ref. Reference Cho126). In NTG patients, let-7a-5p and let-7b-3p were significantly upregulated compared with controls. Salidroside (Sal) had a protective effect on H2O2-injured human trabecular meshwork cells. miR-27a was upregulated by Sal, and miR-27a suppression could reverse the protective effect of Sal on H2O2-injured human trabecular meshwork cells (Ref. Reference Zhao158). This result might provide a therapeutic strategy for the remedy of glaucoma.
The role of miR-93 and miR-126 in AMD was investigated using a laser-induced choroidal neovascularisation mouse model, and miR-93 and miR-126 were suggested as putative therapeutic targets for AMD in humans (Refs Reference Wang85, Reference Wang135). miR-29a-3p was expressed in the patient group (Ref. Reference Ertekin108). MEG3 was demonstrated to play a protective role against AMD by maintaining RPE differentiation via the miR-7-5p/Pax6 axis (Ref. Reference Sun129). miR-146a-5p has a high-affinity target in the complement factor H, the most strongly and consistently advanced AMD-associated gene. It suggested that miR-146a-5p could be a biomarker for advanced AMD (Ref. Reference SanGiovanni142). miR-155-5p, let-7a-5p, let-7b-5p and let-7d-5p significantly elevated in advanced AMD retina (Ref. Reference SanGiovanni142).
Three exosomal miRNAs, miR-21-3p, miR-22-3p and miR-223-5p, could significantly discriminate PD from healthy controls (Ref. Reference Manna76). Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) has been reported to be upregulated in PD. The MALAT1/miR-205-5p axis could regulate the apoptosis of MN9D cells by directly targeting LRRK2, which was involved in the molecular pathogenesis of PD (Ref. Reference Chen, Huang and Li99). Two miR-24 and miR-205 in cerebrospinal fluid could distinguish PD from controls (Ref. Reference Marques100). miR-26a/death-associated protein kinase 1 signalling induced synucleinopathy and DA neuron degeneration in PD (Ref. Reference Su103). Circular RNA circTLK1 regulated DA neuron injury during PD by targeting miR-26a-5p/DAPK1 (Ref. Reference Chen104). miRNA-155-5p was upregulated in PD patients compared with healthy controls whereas miRNA-146a-5p was downregulated in PD patients in comparison with healthy controls (Ref. Reference Caggiu144). Inhibiting GSK3β by 7-BIO alleviated the 1-methyl-4-phenylpyridinium-4-methyl-1 (MPP+) induced neurotoxicity by regulating miR-29a-3p expressions in PD model SH-SY5Y cells (Ref. Reference Ahmadzadeh-Darinsoo110). Serum miR-29a and miR-29c levels were downregulated in PD patients compared with healthy controls (Ref. Reference Bai111). miR-29b levels were shown to be associated with different subsets of PD cognition and could accurately discriminate PD patients with dementia (PDD) from non-PDD (Ref. Reference Han115). GLT-1 was a critical factor in the development of PD and miR-30a-5p could regulate GLT-1 expression and function by ubiquitination of these glutamate transporters through the PKCα pathway in vitro and in vivo (Ref. Reference Meng122).
FTY720-Mitoxy, a derivative of a PD's drug FTY720, could significantly increase the miR-30d-5p level (Ref. Reference Vargas-Medrano127). miR-30d-5p was upregulated in AD patients and let-7a-5p, miR-29b-3p and miR-144-5p were downregulated in AD patients compared with healthy controls (Ref. Reference Satoh, Kino and Niida96). Let-7a suppresses SNCA-induced microglial inflammation by targeting STAT3 in PD (Ref. Reference Zhang147). Let-7d was downregulated in a 6-OHDA-induced cellular model of PD, and let-7d played an important role in DA neuronal cell injury (Refs Reference Li153, Reference Li154). miR-7 in brain areas associated with DA neurodegeneration significantly decreased in PD patients and parkinsonian MPTP-induced animals (Ref. Reference Titze-de-Almeida and Titze-de-Almeida131). Elevated levels of miR-126 might play a functional role in DA neurons and PD pathogenesis by downregulating IGF-1/PI3K/AKT signalling (Ref. Reference Kim136).
miR-93 was identified as a key node in the miRNA–mRNA network by topological analysis for AD. Long noncoding RNAs (lncRNAs) might play an important role in the development and treatment of AD. lncRNA NEAT1 aggravated Aβ-induced neuronal damage by sponging miR-107, suggesting a novel approach to the treatment of AD (Ref. Reference Ke91). miR-143-3p inhibition promoted neuronal survival in a vitro cellular model by targeting NRG1, and the miR-143-3p/NRG1 axis is a potential therapeutic target for AD treatment (Ref. Reference Sun94). A panel of miRNAs including miR-143-3p is a promising substitute for the traditional measurement of p-tau/Aβ-42 in cerebrospinal fluid as an effective biomarker of AD (Ref. Reference Jia95). Overexpression of miR-26a-5p suppressed tau phosphorylation and Aβ accumulation in the AD mice by targeting DYRK1A (Ref. Reference Liu105). The protective effects of klotho and linagliptin treatment on human peripheral blood mononuclear cells (PBMCs) of AD patients and healthy controls were studied. Klotho induced miR-29a expression in the PBMCs of healthy controls, whereas miR-29a expression was induced in the AD group by klotho and linagliptin (Ref. Reference Sedighi112).
A low miR-29c-3p level was detected in the brain tissue of AD animal models (Ref. Reference Cao118). Dysregulation of the miR-30a-5p/ADAM10/SIRT1 pathway was a key mediator of AD pathogenesis (Ref. Reference Sun124). miR-7-5p expression was significantly increased in LPS + Aβ-42-stimulated PBMCs of AD patients (Ref. Reference La Rosa133). miR-125b was downregulated in the serum of AD patients (Ref. Reference Tan141). Cerebrospinal fluid from AD patients contained higher amounts of let-7b compared with healthy controls (Ref. Reference Derkow152). The expression level of let-7d-5p was significantly increased in the AD patients compared with control individuals (Ref. Reference Poursaei155). Control of miR-155 might be a promising approach for AD treatment (Ref. Reference Song and Lee157). lncRNA NEAT1 regulated the development of AD by downregulating miR-27a-3p (Ref. Reference Dong159).
Discussion
Table 2 lists 23 common miRNA biomarkers, which are related to at least one of the two eye diseases (glaucoma or AMD) and at least one of the brain diseases (PD or AD). These common miRNAs show that there might have common pathological mechanisms between these eye diseases and brain diseases. Among these miRNAs, 13 miRNAs are associated with three of these diseases. Seven miRNAs and three miRNAs are related to two and four diseases, respectively. Table 3 provides the numbers of the four diseases that are associated with these biomarkers. More than half of these 23 miRNAs are associated with at least three of these diseases. These miRNA biomarkers can be used to study common mechanisms among these diseases (Fig. 2).
Table 3. Numbers of the four diseases associated with these miRNA biomarkers
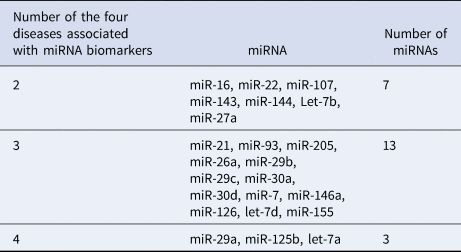

Figure 2. Common miRNA biomarkers for eye diseases (glaucoma or AMD) and brain diseases (PD or AD).
Recent articles have discussed miRNA-based therapeutic approaches for neurodegenerative diseases. Gene therapy methods for AD often involved targeting RNA through the use of synthetic antisense oligonucleotides (ASOs), small synthetic molecules designed to regulate protein translation (Ref. Reference Grabowska-Pyrzewicz163). miRNA-based ASOs might be more powerful therapeutics compared with traditional options. However, delivering miRNAs to the CNS for neurodegenerative disease therapy can be challenging because of the blood–brain barrier (BBB), which limits their transfection efficiency. To increase transfection efficiency and overcome the BBB, two strategies have been formulated: restoring suppressed miRNA levels using miRNA mimics (agonists) or inhibiting miRNA function using anti-miRs (antagonists) to repress overactive miRNA function (Ref. Reference Roy164). Additionally, miRNA expression may be influenced by sex, suggesting sex-specific therapeutic strategies to be implemented in disease treatment (Ref. Reference Paul165).
Although PD, AD, glaucoma and AMD share common miRNA pathological mechanisms, we cannot conclude that they have a very strong connection. Eye diseases might be triggered by other diseases such as metabolic disorders or caused by the overuse of electronic products for young patients. The eye disease may not be directly related to the onset of brain disease. However, for those with brain diseases, the eye condition may be a window into the brain condition. It is much easier to monitor eye conditions than brain conditions, and ocular conditions may be useful prognostic biomarkers for patients with brain diseases. In addition, antioxidants are a persuasive therapy against severe neuronal loss, that can prevent the development of these diseases. Diet is a major source of antioxidants. Antioxidants, such as glutathione, arginine, citrulline, taurine, creatine, selenium, zinc, vitamin E, vitamin C, vitamin A and tea polyphenols can help regulate ROS (Ref. Reference Uttara166). A balanced diet with various whole foods can provide natural sources of antioxidants to prevent these diseases.
Conclusions
The four oxidative stress-related ageing disorders, glaucoma, AMD, AD and PD, are discussed in this paper. The common miRNAs involved with these diseases are reviewed. Since these diseases share many common miRNA biomarkers, it may indicate that these diseases have some common pathological mechanisms. However, these common miRNA biomarkers are not sufficient to conclude the significant associations between these diseases. Several previous studies showed that the eye might be a window to the brain. Additionally, glaucoma and AMD share common miRNA biomarkers with PD and AD. This fact might indicate that the eye condition of PD or AD patients may be a prognostic biomarker for monitoring PD and AD course. It is easier to examine the eye condition than the brain condition. When a PD or AD patient's eye condition changes, this can be a warning of a change in PD or AD course.
Author contributions
H. W. conceived of the presented idea, collected the data and wrote the paper.
Financial support
This research was funded by the Ministry of Science and Technology 109-2118-M-009-005-MY2, Taiwan.
Competing interest
None.








