Circadian rhythms
Mammals have developed an endogenous circadian clock located in the brain suprachiasmatic nuclei (SCN) of the anterior hypothalamus that responds to the environmental light–dark cycle (Fig. 1). Light is absorbed through the retina and this information is transmitted to the SCN, which in turn relays the information via neuronal connections or circulating humoral factors to peripheral clocks, such as the liver, heart and lungs, regulating cellular and physiological functions(Reference Reppert and Weaver1–Reference Schibler, Ripperger and Brown3). The clock mechanism in both SCN neurons and peripheral tissues consists of CLOCK and BMAL1 (brain-muscle-Arnt-like 1) proteins that heterodimerise and bind to E-box sequences to mediate transcription of tissue-specific genes, including Periods (Per1, Per2, Per3) and Cryptochromes (Cry1, Cry2). PER and CRY constitute part of the negative feedback loop, which inhibits CLOCK:BMAL1-mediated transcription(Reference Reppert and Weaver1, Reference Froy, Chang and Reppert4).
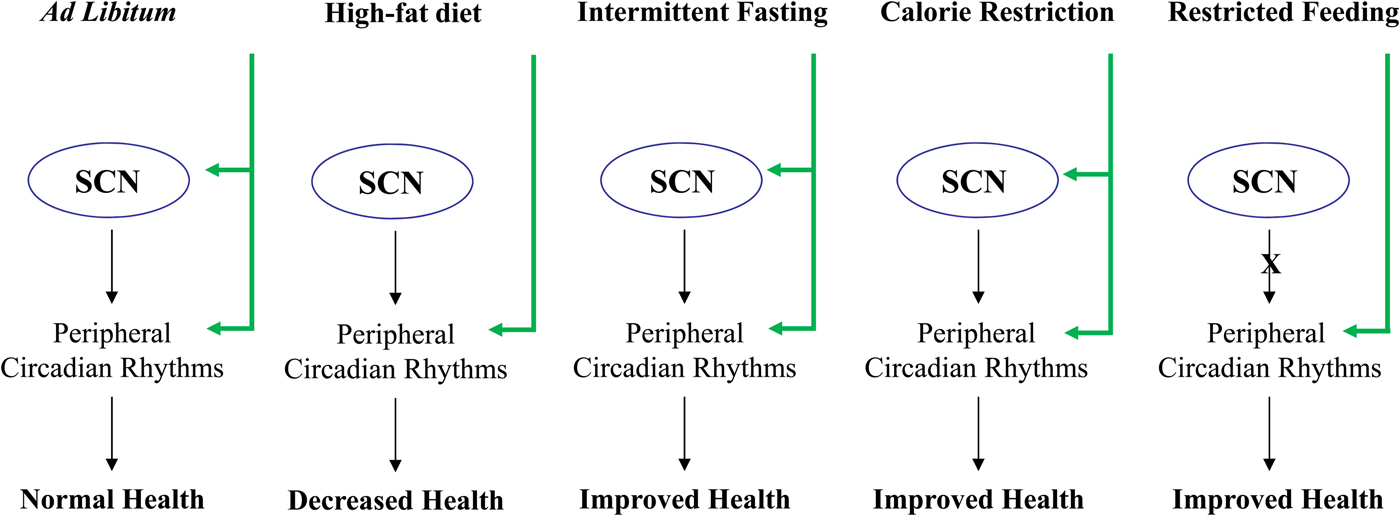
Fig. 1. Effect of feeding diet regimens on circadian rhythms and health. SCN, suprachiasmatic nuclei.
Chronodisruption and ageing
Disruption of the coordination between the endogenous clock and the environment leads to symptoms of fatigue, disorientation and insomnia. Night-shift workers have disrupted circadian rhythms and they exhibit metabolic disorders, hormone imbalance(Reference Davis and Mirick5), psychological and sleep disorders(Reference Qureshi and Mehler6), and increased incidence of cancer and malignant growth(Reference Davis and Mirick5). Longevity in hamsters is decreased with disruption of rhythmicity and is increased in older animals given fetal SCN implants that restore high-amplitude rhythms(Reference Hurd and Ralph7). Even chronic reversal of the external light–dark cycle at weekly intervals results in a significant decrease in the survival time of cardiomyopathic hamsters(Reference Penev, Kolker and Zee8).
It has been shown that circadian rhythms change with normal ageing, including a shift in the phase and decrease in amplitude(Reference Hofman and Swaab9, Reference Froy and Miskin10). Deficiency of the CLOCK protein significantly affects longevity, as the average lifespan of Clock−/− mice was reduced by 15% compared with wild-type mice, while maximum life span was reduced by more than 20%. CLOCK deficiency also resulted in the development of cataracts and dermatitis, two age-specific pathologies(Reference Seyfarth, Schliemann and Antonov11, Reference Iroku-Malize and Kirsch12), at a much higher rate than in wild-type mice(Reference Dubrovsky, Samsa and Kondratov13). In addition, Bmal1−/− knockout mice have reduced life span and they display various symptoms of premature ageing, including cataracts and organ shrinkage(Reference Kondratov, Kondratova and Gorbacheva14). Per1,2 −/− mice are morphologically indistinguishable from wild-type animals at birth, but as early as 12–14 months of age they start to develop features of premature ageing, such as a faster decline in fertility, loss of soft tissues and kyphosis(Reference Lee15, Reference Froy16).
It has been reported that old mice are approximately 20 times less sensitive to the synchronising effect of light compared with young animals(Reference Zhang, Brainard and Zee17). When the SCN becomes less sensitive, the endogenous period (τ) becomes extremely important. A positive link between τ close to 24 h and survival has been previously suggested(Reference Hurd and Ralph7, Reference Wyse, Coogan and Selman18). According to this suggestion, τ longer or shorter than 24 h necessitates a daily synchronisation to external time cues (i.e. light–dark cycles) with a physiological cost proportional to the deviation. This cost might affect survival. We have recently shown that a long-lived transgenic mouse has a τ of 24 h at a young and old age compared with its short-lived genetic background whose τ is 23·5 h at young age and 25 h at old age(Reference Gutman, Genzer and Chapnik19).
Circadian rhythms in metabolism
Obesity has become a serious and growing public health problem(Reference Wyatt, Winters and Dubbert20). Attempts to understand the causes of obesity and develop new therapeutic strategies have mostly focused on the imbalance between energy expenditure and energy intake. However, studies in the last decade link energy regulation to the circadian clock at the behavioural, physiological and molecular levels(Reference Oishi, Shirai and Ishida21–Reference Froy24), emphasising that the timing of food intake itself may play a significant role in weight gain(Reference Arble, Bass and Laposky25). Obesity, which is characterised by the excess of fat accumulation in white adipose tissue, has been related to irregular sleep–wake schedules, high snacking frequency or social jet lag known to disrupt the circadian clock(Reference McHill and Wright26).
The circadian clock regulates metabolism and energy homeostasis in peripheral tissues(Reference Froy24, Reference Garaulet and Madrid27, Reference Kuehn28). This is achieved by mediating the expression and/or activity of certain metabolic enzymes and transport systems(Reference Hirota and Fukada29, Reference Kohsaka and Bass30) involved in cholesterol metabolism, amino acid regulation, drug and toxin metabolism, the citric acid cycle, and glycogen and glucose metabolism(Reference Froy24, Reference Garaulet and Madrid27, Reference La Fleur, Kalsbeek and Wortel31–Reference Ramsey, Marcheva and Kohsaka34). Moreover, lesions of rat central clock in the SCN abolishes diurnal variations in whole body glucose homeostasis(Reference Cailotto, La Fleur and Van Heijningen35), altering not only rhythms in glucose utilisation rates but also endogenous hepatic glucose production. Indeed, the SCN projects to the pre-autonomic paraventricular nucleus neurons to control hepatic glucose production(Reference Kalsbeek, Ruiter and La Fleur36). Similarly, glucose uptake and the concentration of the primary cellular metabolic currency ATP in the brain and peripheral tissues have been found to fluctuate around the circadian cycle(Reference La Fleur32, Reference Kalsbeek, Ruiter and La Fleur36, Reference Yamazaki, Ishida and Inouye37). In addition, many hormones involved in metabolism, such as insulin (Reference La Fleur, Kalsbeek and Wortel31), glucagon(Reference Ruiter, La Fleur and van Heijningen38), adiponectin(Reference Ando, Yanagihara and Hayashi39), corticosterone(Reference De Boer and Van der Gugten40), leptin and ghrelin(Reference Ahima, Prabakaran and Flier41, Reference Bodosi, Gardi and Hajdu42), have been shown to exhibit circadian oscillation.
However, the most compelling connection between the circadian clock and metabolism is achieved by genetic knockout or mutated clock genes. Homozygous Clock mutant mice have a greatly attenuated diurnal feeding rhythm, are hyperphagic and obese, and develop a metabolic syndrome of hyperleptinaemia, hyperlipidaemias, hepatic steatosis and hyperglycaemia(Reference Turek, Joshu and Kohsaka22). Combination of this mutation with the leptin knockout (ob/ob) resulted in significantly heavier mice than the ob/ob phenotype(Reference Oishi, Ohkura and Wakabayashi43), emphasising the inter-relations between leptin and the circadian clock(Reference Froy24, Reference Garaulet and Madrid27, Reference Green, Takahashi and Bass44). In addition, Bmal1−/− knockout mice, similarly to Clock mutant mice, exhibit suppressed diurnal variations in glucose and TAG as well as abolished gluconeogenesis(Reference Rudic, McNamara and Curtis45).
Moreover, several key metabolic factors have been shown to participate in the core clock mechanism. REV-ERBα, the negative regulator of Bmal1 (Reference Preitner, Damiola and Lopez-Molina46), is induced during normal adipogenesis(Reference Chawla and Lazar47). The positive regulators of Bmal1 expression, retinoid-related orphan receptor α and PPARα, regulate lipid metabolism(Reference Sato, Panda and Miraglia48, Reference Canaple, Rambaud and Dkhissi-Benyahya49). In turn, CLOCK:BMAL1 heterodimer regulates the expression of Rev-erbα, Pparα and Rora (retinoid-related orphan receptor α)(Reference Oishi, Shirai and Ishida21, Reference Preitner, Damiola and Lopez-Molina46, Reference Sato, Panda and Miraglia48–Reference Inoue, Shinoda and Ikeda51). PPARγ co-activator-1α, a PPARγ transcriptional co-activator that regulates energy metabolism, stimulates the expression of the clock genes, Bmal1 and Rev-erbα, through co-activation of the retinoid-related orphan receptors; mice lacking PPARγ co-activator-1α show abnormal diurnal rhythms of activity, body temperature and metabolic rate(Reference Liu, Li and Liu52). AMP-activated protein kinase, a sensitive sensor of low energy and nutrient state in the cell, leads to the degradation of PER and CRY proteins(Reference Eide, Woolf and Kang53, Reference Lamia, Sachdeva and DiTacchio54). Degradation of the negative feedback loop leads to a phase advance in the circadian expression pattern of clock genes in mice(Reference Um, Yang and Yamazaki55, Reference Barnea, Madar and Froy56). Mammalian target of rapamycin, which functions as a sensor of cellular nutrient and energy levels, is regulated by light in the SCN(Reference Cao, Lee and Cho57). One of the key factors in the mammalian target of rapamycin pathway, protein 70 S6 kinase 1, rhythmically phosphorylates BMAL1 allowing it to both associate with the translational machinery and stimulate circadian oscillations of protein synthesis(Reference Lipton, Yuan and Boyle58). SIRT1, a key factor involved in metabolism and life span, interacts directly with CLOCK and deacetylates BMAL1 and PER2(Reference Asher, Gatfield and Stratmann59–Reference Nakahata, Kaluzova and Grimaldi61).
Effect of restricted feeding on circadian rhythms
Limiting the time and duration of food availability with no energy reduction is termed restricted feeding (RF)(Reference Schibler, Ripperger and Brown3, Reference Hirota and Fukada29, Reference Stephan62, Reference Cassone and Stephan63). Animals which receive food ad libitum every day at the same time for only a few hours, adjust to the feeding period and consume their daily food intake during that limited time(Reference Honma, Honma and Hiroshige64–Reference Froy, Chapnik and Miskin66). Restricting food to a particular time of day has profound effects on the behaviour and physiology of animals. Two to four hours before the meal, the animals display food anticipatory behaviour, which is demonstrated by an increase in locomotor activity, body temperature, corticosterone secretion, gastrointestinal motility and activity of digestive enzymes(Reference Stephan62, Reference Honma, Honma and Hiroshige64, Reference Saito, Murakami and Suda67, Reference Comperatore and Stephan68), all are known output systems of the circadian clock. RF is dominant over the SCN and drives rhythms in arrhythmic and clock mutant mice and animals with lesioned SCN, regardless of the lighting conditions(Reference Stephan62, Reference Stephan, Swann and Sisk69–Reference Horikawa, Minami and Iijima73). In most incidents, RF affects circadian oscillators in peripheral tissues, with no effect on the central pacemaker in the SCN(Reference Schibler, Ripperger and Brown3, Reference Hirota and Fukada29, Reference Cassone and Stephan63, Reference Hara, Wan and Wakamatsu71, Reference Oishi, Miyazaki and Ishida72, Reference Damiola, Le Minh and Preitner74, Reference Stokkan, Yamazaki and Tei75). Thus, RF uncouples the SCN from the periphery(Reference Lin, Liu and Li76). We have shown that long-term daytime RF can increase the amplitude of clock gene expression, increase expression of catabolic factors and reduce the levels of disease markers leading to better health(Reference Sherman, Frumin and Gutman77) (Fig. 1). RF diet regimen resembles the month of Ramadan, as Muslims abstain from eating and drinking during the activity period. The average low levels of cholesterol and TAG found during RF are in agreement with those found during Ramadan(Reference Ibrahim, Habib and Jarrar78, Reference Salehi and Neghab79). Aksungar et al. (Reference Aksungar, Topkaya and Akyildiz80) demonstrated that Ramadan fasting has some positive effects on the inflammatory state and on risk factors for CVD, such as C reactive protein and homocysteine.
Effect of energy restriction on circadian rhythms
Calorie restriction (CR) refers to a dietary regimen low in energy without malnutrition. CR restricts the amount of energy to 60–75% of ad libitum-fed animals(Reference Masoro, Shimokawa and Higami81). It has been documented that CR significantly extends the life span of rodents by up to 50%(Reference Koubova and Guarente82, Reference Masoro83). In addition to the increase in life span, CR also delays the occurrence of age-related diseases, such as cancer, diabetes and cataracts(Reference Masoro83–Reference Roth, Mattison and Ottinger86). Theories on how CR modulates ageing and longevity abound, but the exact mechanism is still unknown(Reference Masoro83). The reduction of energy intake, and, as a result, in oxidative stress, is considered the critical beneficial factor in the CR diet regimen(Reference Masoro83). It has been argued that in mice, the oxidative stress theory can account for age-related diseases, such as cancer, but not for longevity per se (Reference Muller, Lustgarten and Jang87).
As opposed to RF, CR entrains the clock in the SCN(Reference Challet, Caldelas and Graff88–Reference Resuehr and Olcese91), indicating that energy reduction could affect the central oscillator. CR during the daytime affects the temporal organisation of the SCN clockwork and circadian outputs in mice under light–dark cycle. In addition, CR affects photic responses of the circadian system, indicating that energy metabolism modulates gating of photic inputs in mammals(Reference Mendoza, Drevet and Pevet92). These findings suggest that synchronisation of peripheral oscillators during CR could be achieved directly due to the temporal eating, as has been reported for RF(Reference Hara, Wan and Wakamatsu71, Reference Damiola, Le Minh and Preitner74, Reference Stokkan, Yamazaki and Tei75), or by synchronising the SCN(Reference Challet, Caldelas and Graff88–Reference Mendoza, Graff and Dardente90), which entrains the peripheral tissues(Reference Froy, Chapnik and Miskin93, Reference Froy and Miskin94) (Fig. 1).
Effect of intermittent fasting on circadian rhythms
Intermittent fasting (IF) allows food to be available ad libitum every other day. Similarly to energetically restricted animals, IF-fed animals exhibit increased life span as well as improved cardio- and neuro-protection and increased resistance to cancer(Reference Mattson95). One suggested mechanism for its beneficial effects is the stimulation of cellular stress pathways induced by the IF diet regimen(Reference Mattson95, Reference Anson, Guo and de Cabo96). IF alters circadian rhythms depending on the time of food introduction (Fig. 1). When food was introduced during the light period, mice exhibited almost arrhythmicity in clock gene expression in the liver. Unlike daytime feeding, night-time feeding yielded rhythms similar to those generated during ad libitum feeding(Reference Froy, Chapnik and Miskin97).
Effect of high-fat diet on circadian rhythms
Several studies have shown that a high-fat diet leads to disruptions in locomotor activity in total darkness and to elevated food intake during the rest phase under light–dark conditions(Reference Kohsaka, Laposky and Ramsey98). These changes were also manifested by disrupted clock gene expression in the hypothalamus, liver and adipose tissue as well as altered cycling of hormones in mice, rats and human subjects(Reference Barnea, Madar and Froy56, Reference Kohsaka, Laposky and Ramsey98–Reference Barnea, Madar and Froy102). In addition, a high-fat diet induced a phase delay in clock and clock-controlled genes(Reference Barnea, Madar and Froy56, Reference Barnea, Madar and Froy102) (Fig. 1). Combining high-fat diet with RF led to a leaner phenotype although the energy intake was the same as mice fed a low-fat diet(Reference Sherman, Genzer and Cohen103). Altogether, these studies demonstrate the importance of timing of feeding over its content.
Effect of breakfast on circadian metabolism
Breakfast has previously been demonstrated to be of major importance for the 24-h regulation of glucose(Reference Mekary, Giovannucci and Willett104). Indeed, skipping breakfast has been shown to be associated with weight gain and other adverse health outcomes, including insulin resistance and increased risk for developing type 2 diabetes. In contrast, consumption of a high-energy breakfast and a low-energy dinner resulted in a significant reduction of all-day postprandial glycaemia and body weight(Reference Jakubowicz, Wainstein and Ahren105–Reference Jakubowicz, Barnea and Wainstein107). The importance of breakfast has recently been demonstrated in type 2 diabetic patient who skipped breakfast and had increased postprandial hyperglycaemia after both lunch and dinner in association with impaired insulin response(Reference Jakubowicz, Wainstein and Ahren108).
Conclusions
Disruptions in clock genes and/or circadian rhythms promote ageing and shorten life span, whereas appropriate resetting of circadian rhythms leads to well-being and increased longevity. Life span extension has been a goal of research for several decades. CR, IF and RF reset circadian rhythms and promote better health (Fig. 1). In addition, breakfast consumption has been shown to affect all-day metabolism. Therefore, it is necessary to increase our understanding of circadian regulation over metabolism and longevity and to design new therapies based on this regulation.
Financial Support
None.
Conflicts of Interest
None.
Authorship
The author had sole responsibility for all aspects of preparation of this paper.



