INTRODUCTION
An operation is a physical trauma to the patient often accompanied by physical and psychological stress. In addition, certain surgical procedures, especially neurosurgery and some ear, nose and throat (ENT) surgeries penetrate or involve the risk of penetrating the dura mater, and therefore a potential introduction of bacteria or a fistula of the meninges which may be a direct route for infection. The risk of acquiring meningitis after surgical procedures varies with the type of surgery but has not been examined on a nationwide population-based cohort before.
Bacterial meningitis is a rapid developing condition that may lead to severe morbidity (e.g. sensorineural hearing loss, seizures, motor problems, hydrocephalus, mental retardation, neuropsychological sequelae) or death [Reference de Jonge1]. The incidence of bacterial meningitis in Denmark (population: 5·5 million) was 3·2/100 000 persons in 2009 [Reference St-Martin2]. The introduction of Haemophilus influenza type b (Hib) vaccination in the childhood vaccination programme in 1993 in Denmark almost eliminated Hib as a cause of invasive disease. From October 2007 the 7-valent conjugated pneumococcal vaccine was included in a 2 + 1 schedule in the Danish Childhood Vaccination Programme and in 2010 substituted with the 13-valent pneumococcal vaccine; this has reduced the incidence of invasive pneumococcal disease, including meningitis in the vaccinated population [Reference Harboe3].
In the literature we found sound evidence for the association between cochlear implants and bacterial meningitis, in addition we encountered a few retrospective institution-based analyses on certain surgical procedures and several case reports on post-operative meningitis [Reference van Aken4–Reference Isaacson and Parke16].
In this article we present a retrospective nationwide registry-based cohort study over 14 years to estimate the risk of acquiring bacterial meningitis following surgery.
MATERIAL AND METHODS
To estimate the risk of acquiring bacterial meningitis after surgery we conducted a two-step analysis. First, we retrieved all cases of bacterial meningitis that had undergone a surgical procedure up to 3 months prior to the meningitis incident. Second, we retrieved all bacterial meningitis cases which the treating clinician had reported were probably due to prior surgery. Both studies are retrospective register-based cohort studies.
National notification system of bacterial meningitis
Bacterial meningitis cases were retrieved from the national notification system of bacterial meningitis, which has been a mandatory notifiable disease since 1 January 1980. The definition of a bacterial meningitis case is a clinical diagnosis of meningitis accompanied in most cases by a positive culture or a positive polymerase chain reaction, meningococcal antibody test or microscopy of diplococci in the cerebrospinal fluid (CSF). In a few cases in the reporting system the diagnosis is only clinical. On the registration form clinicians are asked for a possible transmission path. The notification system holds information on 96% (95% CI 95–98) of meningitis cases due to Neisseria meningitidis and >80% due to Streptococcus pneumoniae [Reference Howitz17–Reference Meyer19]. For other pathogens we are aware of underreporting [Reference Howitz17].
National Patient Registry (NPR)
In the first study, the surgical procedure(s) and dates are retrieved from the NPR which was introduced in Denmark on 1 January 1977. The registry holds information on the dates for admission, operation and discharge, the hospital and hospital department, the primary and secondary diagnoses, which since 1994 have been coded according to the 10th edition of the International Classification of Diseases, as well as surgical procedures, which since January 1996 have been coded according to the Danish version of Nomescos Classification of Surgical Procedures (NCSP-D). Since 1 January 1995 outpatient clinics have been included in the registry. We restricted our study period to 1 January 1996–31 December 2009.
Data on overall number of surgical procedures in the medical specialities were gathered from the NPR, available at the Statens Serum Institute homepage [20].
In study 1, in order to differentiate between the increased risk of bacterial meningitis due to the set-up around the operation or the surgical procedure itself, we defined a probable meningitis case due to surgery as the occurrence of meningitis within the first 10 days following surgery, from days 11–90 meningitis cases were considered as possible due to surgery. In the analyses we defined the shortest time period between surgery and meningitis episode as the incident. Only the first incident of bacterial meningitis after surgery for each patient was considered as a case. Episodes of bacterial meningitis which occurred >90 days after surgery were considered not to have a causal relationship with the surgical procedure.
In study 2, all notifications of bacterial meningitis where the clinician had reported prior surgery to be the likely transmission path without a time limitation were reviewed independently.
Statistical analyses
Data were extracted from registries using SAS software (SAS Institute Inc., USA) in study 1 and Access (Microsoft, USA) in study 2 and the unique personal identification number assigned to all Danes and persons treated at hospitals in Denmark were used for data linkage. Statistical analyses were conducted in Excel (Microsoft) and Stata v. 7.0 (StataCorp., USA).
In study 1 ‘Endoscopy and endoscopic surgery’ was chosen as the reference group due to it being a minimal invasive procedure with little risk of causing bacterial meningitis as well as being a large group. The annual incidence rate was calculated as the cumulative incidence rate per 1-year exposure time, i.e. the 10-day window post-operatively multiplied by 10 days/365 days divided by the 14-year study period.
In study 2 the relative risk was calculated as the risk in operated individuals divided by the risk in the background population who had not undergone the operation type under study. In Tables 2–5 the age distribution in the operated group and the age in the background population are standardized to Danish population year 2000. For ENT surgery the majority of meningitis cases occurred within 10 days, but in order to avoid disregarding later cases we report both the 10-day and annual relative risks. For neurosurgery the date of operation was not reported for a few notifications and we have therefore chosen to report the annual relative risk. The population attributable risk is calculated as the cumulative incidence of bacterial meningitis in the operated group minus the cumulative incidence in the background population.
RESULTS
Study 1
In total, there were 378 incidents of bacterial meningitis registered following the 90-day post-surgery period from 1 January 1996 to 31 December 2009 in Denmark. Of these, 142 were first time registrations of bacterial meningitis after surgery, the remaining 236 were either duplicate registrations of the same meningitis episode or a new episode of bacterial meningitis following a surgical procedure. Of the 142 first-time cases of bacterial meningitis 41 cases occurred within 10 days following surgery; of these 20 (49%) were due to S. pneumoniae, 19 (46%) N. meningitidis and two (5%) cases due to Hib. In the 11–90 days period after surgery 52/101 (51%) cases were due to S. pneumoniae, 47 (47%) due to N. meningitidis and two (2%) due to Hib.
Of all first-time post-operative meningitis patients the median age was 63 years (range 0–99 years; 70 females, 72 males); in the meningitis cases occurring within 10 days of surgery the median age was 65 years (range 0–96 years; 20 females, 21 males).
In Table 1 the operations are divided into 14 surgical specialities, making it possible to extrapolate an average incidence rate per year. Overall the annual incidence of bacterial meningitis cases was 0·64/100 000 operations within the first 10 post-operative days and 0·20 cases/100 000 operations in the 11–90 days post-operative time period. The highest overall incidence was observed in patients undergoing ENT surgical procedures with a crude annual incidence rate of 4·98 meningitis cases/100 000 surgical procedures within 10 days of surgery, which was more than 11 times more frequent than the reference group (endoscopy and endoscopic surgery). The second and third highest annual incidence rates were in patients who had undergone neurosurgery and vascular surgery with, 3·02 and 1·62 cases/100 000 surgical procedures, respectively. No bacterial meningitis cases were registered within the first 10 days post-operatively from maxillofacial, mammary, obstetric and ophthalmological surgery.
Table 1. Number of bacterial meningitis cases in specialities within 0–10 days and 11–90 days after surgery, divided by aetiology in Denmark, 1996–2009

* The annual incidence rate is the crude incidence per one person-year at risk.
As presented in Table 1, seven patients became ill with bacterial meningitis within 10 days following endoscopy and/or endoscopic surgery and nine patients after a minor surgical procedure. In these patients 4/7 endoscopic procedures were preceded by gastroscopy and 5/9 and 3/9 minor surgeries were preceded by lumbar puncture and bone marrow aspiration/biopsy, respectively.
Study 2
Since ENT surgery and neurosurgery have the highest incidence rates of bacterial meningitis of the specialities, we scrutinized the notifications of bacterial meningitis from the national notification system for the period 1996–2009.
ENT surgery
In total, 14 bacterial meningitis cases were reported by clinicians to be due to ENT surgery with a median age of 58 years (range 2–73 years). Of these 14 cases, eight became infected with bacterial meningitis within 10 days of the surgical procedure, the remaining six cases were infected >10 days after the operation; one case was observed 12 days after surgery, two cases 20 days after surgery, one case was operated on for an adenocarcinoma in the nasal cavity 10 months previously, and later received radiation treatment, one case had had six episodes of bacterial meningitis over several years after surgery and one case underwent three operations including mastoidectomy, excision of a cholesteatoma and tympanoplasty, the latest 3 years previously. If we include all 14 cases and compare them to the age-standardized annual incidence of bacterial meningitis in the background population the relative risk is 0·88 [95% confidence interval (CI) 0·50–1·42; Table 2]. However, if we constrain the observation period to just the first 10 days following the surgical procedure and therefore only eight cases, the age-adjusted 10-day risk following surgery is 15·31 (95% CI 7·29–32·13). In the following results we include all 14 cases.
Table 2. Total number and relative risk of bacterial meningitis following any ear, nose and throat (ENT) surgical procedure, Denmark, 1996–2009
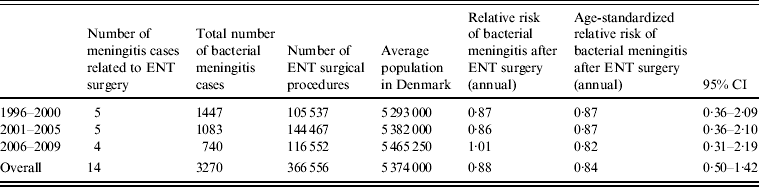
CI, Confidence interval.
Six cases occurred after middle or inner ear surgery. Of these, two were operated on due to vestibular schwannomas; one following excision of a cholesteatoma, one after a cochlear implant, one after stapedectomy and one after a mastoidectomy due to mastoiditis. For middle and inner ear surgery, compared to the annual incidence of bacterial meningitis in the background population, the relative risk was 1·04 (95% CI 0·47–2·32; Table 3).
Table 3. Total number and relative risk of bacterial meningitis following middle and inner ear surgical procedures, Denmark, 1996–2009
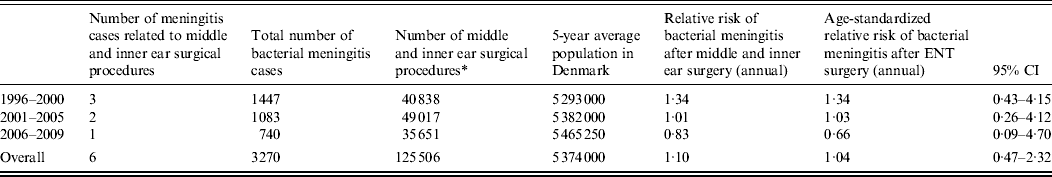
ENT, Ear, nose and throat; CI, confidence interval.
* Included NCSP-D letter codes: DCA, DCB, DCC, DCD, DCW, DDA, DDB, DDC, DDD, DDW, DEA, DEB, DEC, DED, DEE, DEW, DFA, DFB, DFC, DFD, DFE, DFW, DGA, DGB, DGW.
Seven bacterial meningitis cases presented following surgery in the nasal sinuses. Of these, two occurred after polypectomy, two following functional endoscopic sinus surgery (FESS), one after reducing an inverted papilloma, one after unspecified sinus surgery 1 month earlier and one after surgery and irradiation of an adenocarcinoma in the nose, resulting in a large nasal cavity. We found a significant increased risk for bacterial meningitis following surgery in the nasal sinuses compared to the annual age-adjusted incidence in the background population [relative risk (RR) 4·56, 95% CI 2·17–9·57] (see Table 4). The risk was present during all the three calendar periods studied, especially the latter two, i.e. years 2001–2009. The population attributable risk over the 14-year study period was 1·6/10 000 operations of the nasal sinuses.
Table 4. Total number and relative risk of bacterial meningitis following surgical procedures of the nasal sinuses, Denmark, 1996–2009

ENT, Ear, nose and throat; CI, confidence interval.
* Included NCSP-D letter codes: DMA, DMB, DMC, DMW, DNA, DNB, DNC, DNW, DPA, DPB, DPC, DPW.
One patient became infected with bacterial meningitis due to sepsis, which followed a retropharyngeal abscess developed after uvulopalatopharyngoplasty (UPPP).
The pathogens were present in eight cases with S. pneumoniae of which five were following surgery in the nasal sinuses and three following ear surgery, two Hib of which one was after ear surgery and one following nose surgery and of the remaining four one was with a mixed infection from the above-mentioned retropharyngeal abscess, one with Klebsiella oxytoca after ear surgery and two where the cultivations were negative following one ear and one nose operation.
Neurosurgery
In total there were 23 bacterial meningitis cases notified from clinicians due to previous neurosurgery, the median age was 45 years (range 0–78 years). No specific surgical procedures appeared to be predominate, the most common being post-operative cerebral shunt or fistula. We found an overall significant increased annual age-standardized risk of acquiring bacterial meningitis after neurosurgery (RR 1·97, 95% CI 1·31–2·97) for the entire study period compared to the incidence of bacterial meningitis in the background population (Table 5).
Table 5. Total number and relative risk of bacterial meningitis following neurosurgery, Denmark, 1996–2009

CI, Confidence interval.
The pathogens were present in 15 cases with S. pneumoniae, one case with Hib and seven cases with other pathogens consisting of two Pseudomonas aeruginosa, one Escherichia coli, one K. pneumoniae and three with unknown or negative cultivation.
DISCUSSION
The overall risk in <1 case/100 000 operations for acquiring bacterial meningitis after surgery is low; however, in study 1 neurosurgery and ENT surgery represent a 7–11 times increased risk compared to the reference group undergoing endoscopic surgical procedures. Surprisingly, more cases of nosocomial bacterial meningitis were reported in the first 10 days after ENT surgery than after neurosurgery. Of ENT surgical procedures in study 2, surgery in the nasal sinuses had a fourfold increased risk compared to the background population; however, the number of cases are few and we did not find an overall increased risk for ENT surgery in study 2. ENT surgery is performed most often via non-sterile anatomical sites compared to neurosurgery which predominantly is performed via sterile surroundings.
The average incubation time for S. pneumoniae is 1–3 days and for N. meningitidis 3–4 days, stretching from 1 to 10 days [Reference Heymann21]. In case of a nosocomial infection due to surgical trauma to the dura mater and following direct transmission of bacteria to the central nervous system the incubation time will be shorter.
In a retrospective study of 228 trans-sphenoidal operations at University Hospital Rotterdam, The Netherlands, van Aken et al. found an incidence of meningitis of 3·1% and post-operative CSF leakage was found to be an independent risk factor [Reference van Aken4]. Kono et al. performed a retrospective analysis of the first 1000 endoscopic skull-base surgical procedures from Pittsburgh Medical Center and found an incidence of meningitis of 1·8%, which is comparable to open craniotomy. Similarly, post-operative CSF leak was found to be the most important risk factor [Reference Kono5]. Of 156 patients who underwent intradural tumour resection at one hospital in Israel, 5·7% developed post-operative meningitis and CSF leak was similarly a significant risk factor [Reference Horowitz6].
Newer surgical equipment, like computer-aided surgery (CAS) in nasal sinus and skull base surgery, enables the surgeon to have three two-dimensional images of the patient's anatomical structures in opposing planes during surgery; however, the images are stationary pre-operative CT scans which are not updated during the operation. Conducting an operation in such settings may be beneficial; however, it also enables the surgeon to operate closer to the eyes and brain. In Denmark CAS-FESS has until recently been centralized at one hospital and at the present, we have not observed any bacterial meningitis following CAS-FESS at Rigshospitalet, Copenhagen.
In vestibular schwannoma surgery the patient faces a similar risk of CSF leak and the retrosigmoid approach has shown an incidence rate of post-operative meningitis of 2–30% [Reference Charalampakis7, Reference Fishman8]. In our study we found two patients who contracted bacterial meningitis after vestibular schwannoma surgery, one due to K. oxytoca and one with an unknown pathogen.
Persons who have received a cochlear implantation are at higher risk of becoming ill with meningitis; however, both congenital inner ear malformations and the implantation of the cochlear implant can facilitate the entry of bacteria into the meninges [Reference Wei9, Reference Reefhuis10, Reference Parner22]. We found one patient with a cochlear implant infected with non-encapsulated H. influenzae. Patients who receive a cochlear implant are recommended pre-surgical vaccination against Hib and S. pneumoniae. If a device-related post-operative communication between the CSF and the environment is left in neurosurgery, this may function as an entry port for pathogens and is a risk factor for developing meningitis [Reference Kourbeti11].
Streharova et al. [Reference Streharova12] compared 171 cases of bacterial meningitis acquired after surgery with 201 community-acquired bacterial meningitis cases and found that the pathogens in meningitis cases after surgery were more often negative staphylococci, Enterobacteriaceae and Acinetobacter baumannii, while community-acquired meningitis cases were caused by S. pneumoniae, N. meningitidis and Hib.
Papadakis et al. reported on a 7-year-old girl who developed meningitis 2 days after tonsillectomy while still under preventive antibiotic treatment [Reference Papadakis13]. The blood and CSF culture failed to grow any bacteria. Further, case reports of bacterial meningitis following adenotonsillectomy have been reported, but the complication appears to be rare [Reference Guirguis and Berkowitz14, Reference Pancharoen15]. Isaacson & Parke [Reference Isaacson and Parke16] suggested that it is probably a retrograde flow of bacteria via an anastomotic network of veins that causes occasional bacterial meningitis cases [Reference Isaacson and Parke16].
Patients with immune deficiencies (acquired immune suppressions like nephritic syndrome, hypogammaglobulinaemia, splenectomized and HIV disease) have a higher risk of acquiring bacterial meningitis and it is a limitation of this study that we were not able to control for this. If patients had more than one episode of meningitis, even with different bacteria, we only considered the first episode, so as to not overestimate the incidence, since some patients may have an underlying suppressed immune system and have a higher background risk, e.g. one patient had 12 registered post-surgical episodes of bacterial meningitis. A selection bias may also occur if surgeons deselect risk patients for elective surgery; we do not know if this is the case.
The strength of this study is that it is a nationwide population-based study using validated registries over a 14-year period. Further, we have found no other papers analysing the risk of bacterial meningitis for different specialities and operations overall. One weakness is underreporting, which is why these estimates should be considered as minimum numbers; however, the severity of the disease combined with the extended surveillance system described in the Materials and Methods section supports the value of comprehensive registries. Still, post-operative clinical meningitis cases, where the clinician may initiate antibiotic treatment without a definitive diagnosis, will not appear in the registries unless the clinician reports the case as a clinical bacterial meningitis case, which is the exception. To obtain a view of the underreporting we performed two separate studies involving two independent registries instead of relying on one registry. Study 1 compares the risk of becoming ill with bacterial meningitis after an invasive procedure for different surgical specialities. In Table 1 we present the crude annual incidence rate; however, this rate should not be compared to the incidence rate of bacterial meningitis in the background population since data are not age-standardized. In study 2 (Tables 2–4), the results are age-standardized which allows a comparison between calendar periods and incidence rates in the background population. Most bacterial meningitis cases occur in the first days after an operation; however, some cases were seen >10 days after surgery and were reported by the clinician as a case due to surgery. This is probably due to a fistula or shunt and it is debatable whether it would be more correct to code these cases as long-term sequelae. For ENT we chose both to report the 10-day and annual age-adjusted relative risk. For neurosurgery we chose only the annual relative risk, since data in a few cases were limited to operation month and not the exact date. The absolute numbers are too small to stratify on bacteria; it should be noted that S. pneumoniae has the highest incidence in toddlers and those aged ⩾60 years, while N. meningitidis has the highest age-specific incidence in persons aged <20 years, with a peak in those aged <5 years [Reference St-Martin2]. The median age of ⩾60 years in these studies facilitates S. pneumoniae as the aetiology.
For surgery of the nasal sinuses we found a significant increased relative risk during the two later calendar periods from years 2001–2009. A population attributable risk of 1·6 cases/10 000 operations in the nasal sinuses compared to the background population, estimates that five cases could have been prevented in the study period 1996–2009 (34 563 operations; Table 4). We have refrained from conducting a cost-benefit analysis, since cases are few and the estimates would have large confidence intervals. We found, based on this study, no strong evidence to recommend a change in the existing vaccination policy. With vaccination of Danish children against Hib and virulent S. pneumoniae serotypes, an increasing number of younger patients are likely to be better protected before surgery. This is because the majority of bacteria causing meningitis in this study was S. pneumonia. In our view, it is essential that the surgeon and the post-operative healthcare professionals observing the patients react to symptoms resembling rhinorrhoea and/or otorrhoea, which could originate from a CSF leak in order to prevent retrograde spread of pathogens.
In conclusion, we found that surgery itself appears not to be a risk factor for bacterial meningitis, it is probably the penetration of the dura mater, intentional or unintentional, in neurosurgery and ENT surgery which carries the risk of introducing pathogens to the central nervous system. In our view, the rather low absolute numbers of bacterial meningitis following neurosurgery or ENT surgery compared to the number of operations conducted do not lend support for the vaccination of all patients undergoing surgery in or close to the brain. However, the existing recommendations of vaccinating patients before a planned organ transplantation, cochlear implantation, leakage of CSF, previous invasive pneumococcal disease and patients who are immunosuppressed should be borne in mind by physicians planning and/or following a patient before and after an operation [Reference Valentiner-Branth23]. Our finding of a significant increased risk of bacterial meningitis following neurosurgery and surgery of the nasal sinuses should be considered by the national vaccination committee and points to the importance of reporting meningitis cases to sustain a comprehensive surveillance system allowing interventions to be instituted if certain surgical procedures prove unfavourable or meningitis cases begin to cluster.
DECLARATION OF INTEREST
None.







