INTRODUCTION
Owing to their known vector status, the distribution and geographical expansion of British ticks and the prevalence of pathogens they transmit are important to public and veterinary health. One particularly important tick species is Ixodes ricinus, the most commonly reported species to feed on humans in Britain [Reference Jameson and Medlock1]. I. ricinus is an important vector of Borrelia burgdorferi sensu lato (s.l.), the aetiological agent of Lyme borreliosis, which is suggested to have been present within the British tick population for over 100 years [Reference Hubbard, Baker and Cann2]. Although there are about 1000 confirmed cases of Lyme borreliosis each year in England and Wales, it is assumed that as many as 3000 cases occur annually, with case numbers steadily increasing since 2001 [Reference Dubrey3]. Three pathogenic genospecies, B. burgdorferi sensu stricto (s.s.), B. afzelii and B. garinii are generally recognized as a cause of Lyme borreliosis worldwide, and all three occur in Britain [Reference Dubrey3]. B. garinii has been associated with neuroborreliosis and B. afzelii and B. burdgorferi s.s. have been associated with skin manifestations [Reference Stanek4].
Other tick-borne pathogens have also been detected in British tick species, including Anaplasma phagocytophilum, Babesia spp. [Reference Smith and Wall5] and Rickettsia spp. [Reference Tijsse-Klasen6]. The former, also transmitted by I. ricinus, is the causative agent of human granulocytic anaplasmosis. It can also cause disease in ruminants and companion animals, and like Borrelia bacteria, the prevalence of infection in questing ticks varies between and within countries across Europe [Reference Stuen, Granquist and Silaghi7]. A. phagocytophilum is suggested to be widespread within the tick population across the UK causing infection in domestic animals and livestock [Reference Ogden8]; however, human cases are rarely reported. Neoehrlichia mikurensis has recently been recognized as a cause of tick-borne disease in humans in Europe, although so far it has not been detected in British ticks [Reference Jahfari9]. Both of these pathogens are suggested to have the potential to interact with B. burgdorferi s.l. and through co-infection may affect transmission cycles within nature, and also affect immune responses to infection, resulting in altered disease presentation [Reference Andersson, Scherman and Råberg10, Reference Holden11].
More recently however, B. miyamotoi has been identified as a spirochaete that can cause relapsing fever in humans. It is related to, but distinct from B. burgdorferi s.l., being phylogenetically more similar to relapsing fever-like Borrelia such as B. lonestari and B. theileri [Reference Fraenkel, Garpmo and Berglund12, Reference Geller13]. It was first isolated from ticks in 1995 in I. persulcatus from Japan [Reference Fukunaga14] and then later in other Ixodid tick species in North America [Reference Ullmann15], Canada [Reference Ogden16] and Europe. B. miyamotoi bacteria have since been found in I. ricinus in at least eight different European countries [Reference Geller13, Reference Richter17–Reference Wilhelmsson22] where prevalence rates have varied between 0·5% and 5%, and tend to be tenfold less than that of B. burgdorferi s.l. [Reference Barbour23, Reference Chowdri24].
B. miyamotoi has only recently been recognized as a cause of disease in humans, with cases reported from Europe [Reference Hovius19], the USA [Reference Chowdri24–Reference Krause26] and Russia [Reference Platonov27]; the latter estimating that 1000 cases per year may occur. A potentially severe complication of B. miyamotoi infection is meningoencephalitis. Two different cases have been reported in immunocompromised individuals but not yet in immunocompetent individuals [Reference Hovius19, Reference Gugliotta25]. Immunocompetent patients have a varied clinical presentation, but how this compares with Lyme borreliosis or other tick-borne bacterial infections requires further investigation [Reference Chowdri24].
Although previous studies have detected the presence of B. burgdorferi s.l. and A. phagocytophilum in I. ricinus from the UK, none have so far detected B. miyamotoi and few studies have assessed the prevalence of A. phagocytophilum or N. mikurensis in questing I. ricinus. As part of ongoing risk assessments for the implications of emerging tick-borne pathogens, it is necessary to conduct field and laboratory studies to detect novel and emerging pathogens in British ticks. Using qPCR and DNA sequencing, this study tested I. ricinus ticks from a number of locations across southern England for the detection of B. miyamotoi. Ticks were collected at different times of year to maximize the detection of B. miyamotoi, taking into account possible seasonal variation. Stored ticks were also tested from a previous year to test whether the pathogen may be endemic. Ticks were also screened for B. burgdorferi s.l., A. phagocytophilum and N. mikurensis. Although it was not an intention of this study to assess prevalence rates for any of these pathogens, some initial assessment of infection rates were determined to guide further field studies.
METHODS
Study areas and tick collection
Questing I. ricinus ticks were collected by dragging a 1 × 1 m cloth over vegetation at various locations across southern England. A sample of 110–240 I. ricinus ticks were collected from seven regions (total ~950) including: (a) woodland edge and grassland sites in the urban fringe of the city of Salisbury, Wiltshire (51·1° N, 1·8° W), (b) four woodland sites (Langley, Redlynch, Whiteparish, Hamptworth) in the northern part of the New Forest National Park (51·0° N, 1·7° W), (c) two woodland (Yarner, Dunsford) and two moorland (Shaugh Prior, Burrator near Yelverton) sites in east and west Dartmoor National Park, Devon (50·6° N, 3·7° W and 50·4° N, 4·0° W, respectively), (d) four woodland sites (Bentley, Pitton, Grovely, Sutton Mandeville) in south Wiltshire (51·1° N, 1·7° W and 51·1° N, 1·9° W), (e) woodland edge and bracken-dominated sites in Richmond Park, London (51·4° N, 0·3° W), (f) conifer plantations in Swinley Forest, Berkshire (51·4° N, 0·7° W) and (g) woodland/moorland edge sites near Luccombe in Exmoor National Park, Somerset (51·2° N, 3·6° W). In order to maximize the chances of detecting B. miyamotoi, I. ricinus ticks were collected from six of the regions during the active season from March to August 2013. In addition I. ricinus collected from Dartmoor during March–May 2009 were also tested, to give an indication of the presence of B. miyamotoi during the last 5 years. Focus was given to nymph and adult ticks as opposed to larvae, owing to the variability of infection rates in this stage [Reference Richter17]. The number of ticks collected by stage from each site is detailed in Table 1. Ticks were identified using a published taxonomic key [Reference Hillyard28] and were stored at −80°C prior to transportation to RIVM.
Table 1. Detection of Borrelia miyamotoi (Bm), B. burgdorferi s.l. (Bb) and Anaplasma phagocytophilum (Ap) by tick stage (adults/nymphs), location and sampling period.

* Two larvae were also tested but were negative.
n, ♀, ♂ = nymph, female, male, respectively.
Exact binomial 95% confidence intervals (CIs) for nymphs infected with Borrelia burgdorferi s.l. (Bb), B. miyamotoi (Bm) and Anaplasma phagocytophilum (Ap) are displayed in parentheses for each location and also the total nymphs collected.
DNA extraction and PCR amplification
All questing ticks (111 adults, 841 nymphs and two larvae) collected were analysed individually. DNA extraction was carried out on unfed ticks using ammonium hydroxide (NH4OH) [Reference Jahfari9]. Next, 100 μl of NH4OH (1 m) was added to each tick which was then boiled in a heating block at 100°C for 20–30 min. Tubes were then centrifuged and heated again at 100°C with the lids off to evaporate the ammonium. The remaining 50 μl lysates were then stored at 4°C.
Detection of B. burgdorferi s.l. and B. miyamotoi was carried out using multiplex qPCR in the IQ Multiplex Powermix with a final volume of 20 μl, containing iTaq DNA polymerase (Bio-Rad Laboratories, USA), 200 nm each primer, and 5 μl template DNA [Reference Heylen29]. For B. burgdorferi s.l. two targets were used, namely the outer surface protein A gene (OspA) (forward primer: 5′-AAT ATT TAT TGG GAA TAG GTC TAA-3′; reverse primer: 5′-CTT TGT CTT TTT CTT TRC TTA CA-3′ and probe: 5′-Atto520-AAG CAA AAT GTT AGC AGC CTT GA-BHQ1-3′) and the Borrelia flagellin gene (flaB) (forward primer: 5′-CAG AIA GAG GTT CTA TAC AIA TTG AIA TAG A-3′; reverse primer: 5′-GTG CAT TTG GTT AIA TTG YGC-3′ and probe: 5′-Atto425-CAA CTI ACA GAI GAA AXT AAI AGA ATT GCT GAI CA-Pho-3′, where X stands for an internal BHQ-1 quencher attached to thymine). A specific part of the flaB gene was used for the detection of B. miyamotoi, (forward primer: 5′-AGA AGG TGC TCA AGC AG-3′; reverse primer: 5′-TCG ATC TTT GAA AGT GAC ATA T-3′ and probe: 5′-Atto647N-AGC ACA ACA GGA GGG AGT TCA AGC-BHQ2-3′). The multiplex qPCR cycling program (using a light cycler 480 real-time PCR system, Hoffmann–La Roche, Switzerland) was performed using a two-step PCR program: Taq activation for 5 min at 95°C followed by 60 cycles of 5 s at 94°C and 35 s at 60°C involving a single point measurement at 60°C with corresponding filters, finishing with one cycle of 20 s at 37°C for cooling the plate. Detection of A. phagocytophilum and N. mikurensis was carried out in a duplex qPCR exactly as described previously [Reference Jahfari9, Reference Coipan30].
Validation of runs included checking and verifying controls, amplification curves and fluorescence scale and analysis was performed using a second derivative calculation. DNA extraction, PCR master mix preparation, sample addition and PCR amplification were all performed in separate assigned laboratories to minimize cross contamination. Positive and negative controls were also included in each PCR cycle to highlight potential contamination and false positives.
In order to confirm presence and to carry out genospecies analysis, a further PCR was performed on positive tick lysates targeting the glpQ (forward primer: 5′-ATG GGT TCA AAC AAA AAG TCA CC-3′; reverse primer: 5′-CCA GGG TCC AAT TCC ATC AGA ATA TTG TGC AAC-3) and p66 (forward primer: 5′-GAT ACT AAA TTA TTA AAT CCA AAA TCG-3; reverse primer 5′-GGA AAT GAG TAC CTA CAT ATG G-3) genes for B. miyamotoi and the 5S-23S intergenic spacer region for B. burgdorferi s.l. (forward primer: 5′-GAGTTCGCGGGAGAGTAGGTTATTGCC-3′; reverse primer: 5′-TCAGGGTACTTAGATGGTTCACTTCC-3′), respectively.
Both PCRs for Borrelia detection were conducted using Hot Star Taq Master Mix kit (Qiagen, The Netherlands.) The PCR program for B. miyamotoi glpQ and p66 was as follows: Taq polymerase activation for 15 min at 95°C followed by 10 cycles of 30 s at 94°C, 30 s at 62°C (annealing temperature, reducing by 1°C each cycle) and 60 s at 72°C; then 40 cycles at an annealing temperature of 53°C; finishing with 10 min at 72°C. For amplifying the 5S-23S DNA of B. burgdorferi s.l. the following program was used: Taq polymerase activation for 15 min at 94°C followed by 10 cycles of 20 s at 94 °C, 30 s at 70°C (annealing temperature, reducing by 1°C each cycle) and 30 s at 72°C; then 40 cycles at an annealing temperature of 60°C; finishing with 7 min at 72°C. PCR product was then visualized on agarose gel (1·5%).
DNA sequencing
Both strands of the PCR amplicons of B. miyamotoi and B. burgdorferi s.l. were sequenced on the Applied Biosystems 3730 DNA Analyzer (Applied Biosystems, USA) using Big Dye Terminator v. 3.1 Cycle Sequencing kit (PerkinElmer, Applied Biosystems). The performed sequences were analysed and compared with sequences available from Genbank with the use of Bionumerics software (Applied Maths NV, Belgium). A. phagocytophilum was not sequenced.
Statistics
Minitab v. 16 (State College, PA, USA) was used to generate exact binomial 95% confidence intervals (CIs) for pathogen prevalence rates and to perform a two-tailed Fisher's exact test to compare B. burgdorferi s.l. and B. miyamotoi prevalence.
RESULTS
B. miyamotoi was detected in three of the 954 ticks tested (0·3%, 95% CI 0·06–0·92). All positive ticks were nymphal I. ricinus and were collected from three geographically distinct locations during April 2013 in the New Forest, May 2013 in urban Salisbury and April 2009 in west Dartmoor, suggesting a wide geographical distribution (Fig. 1). DNA amplification with conventional PCR of one of the three B. miyamotoi isolates was successful. Analysis of both glpQ and p66 genes showed clustering with other isolates from Europe (Fig. 2).
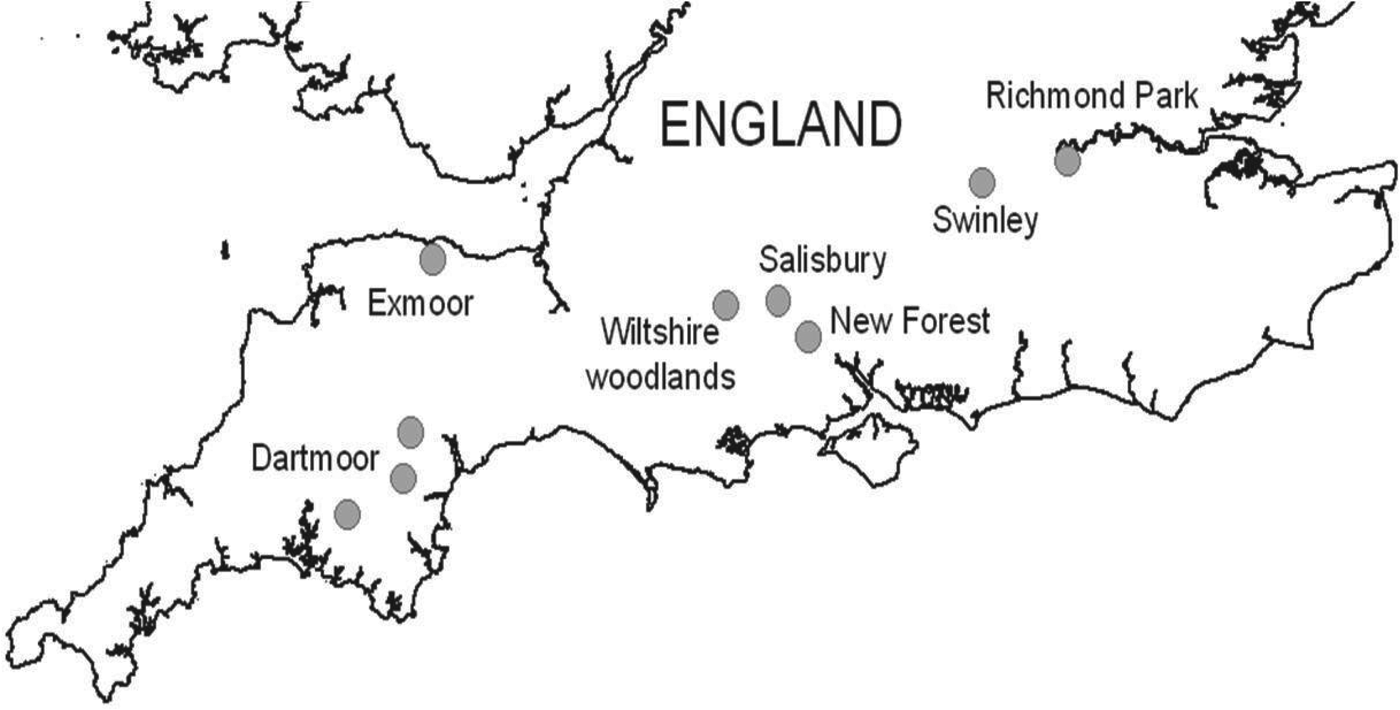
Fig. 1. Locations of field collections of questing Ixodes ricinus ticks in southern England.

Fig. 2. Phylogenetic tree of UK Borrelia miyamotoi isolate and several reference sequences from Genbank.
B. burgdorferi s.l. was detected in 40/954 ticks (4·2%, 95% CI 3·01–5·7) tested, with at least one positive tick collected from all locations surveyed, except Richmond Park, London. Infected ticks were collected during March, April and May in 2009 and during April, May, July and August in 2013. B. burgdorferi s.l. was detected in 33/841 nymphs tested (4%, 95% CI 2·7–5·5), 6/90 females (6·6%, 95% CI 2·5–14·0) and 1/21 males (5%, 95% CI 0·12–23·8). The highest numbers of infected ticks were found in Exmoor (10%, 95% CI 5·3–16·8), Salisbury (8·3%, 95% CI 4·1–14·8) and Dartmoor (7·5%, 95% CI 3·5–13·8) (Table 1).
Twenty-four ticks yielded sequence products corresponding to B. burgdorferi s.l. Twelve sequences were matched to B. garinii isolates, seven to B. afzelii and five to B. valaisiana (Table 2). All potential pathogenic isolates from Exmoor and Salisbury were B. garinii and all potential pathogenic isolates from Wiltshire woodlands and Surrey were B. afzelii. Both pathogenic genospecies were isolated from ticks from Dartmoor.
Table 2. Summary of genospecies of Borrelia DNA sequencing by location
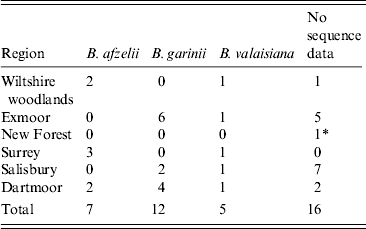
* B. miyamotoi was sequenced from an isolate in the New Forest.
A. phagocytophilum was detected in 22/954 ticks (2·3%, 95% CI 1·5–3·5) collected from five of the regions surveyed (Table 1). Infected ticks were collected during March and April in 2009 and April, May, June, July and August during 2013. A. phagocytophilum was detected in 19/841 nymphs tested (2·3%, 95% CI 1·4–3·5) and 3/90 females (3·3%, 95% CI 0·69–9·43), with the highest numbers of infected ticks found in Dartmoor (8·3%, 95% CI 4·1–14·8) (Table 1). One co-infection of B. afzelii and A. phagocytophilum was detected in a female I. ricinus in a Wiltshire woodland (Bentley) during May 2013. No other co-infections were detected, despite the presence of at least two different pathogens within ticks collected from five of the seven regions surveyed. DNA of N. mikurensis was not detected in any of the 954 tick lysates.
DISCUSSION
The detection of potential human pathogens in ticks and the need to understand their ecological drivers is essential for helping to protect public health from emerging tick-borne pathogens; particularly those that may cause similar symptoms to endemic diseases [Reference Branda and Rosenberg31]. This is the first detection of B. miyamotoi in I. ricinus ticks in the UK. There is no evidence so far to suggest that this pathogen causes human clinical disease in the UK, however it highlights that a number of non-Lyme tick-borne potential pathogens occur in UK ticks. There is sufficient evidence from other countries to suggest that B. miyamotoi may cause human disease, and further investigations of this bacterium as a potential cause of clinical disease in the UK are recommended.
Ticks infected with B. miyamotoi were from three geographically distinct areas of southern England; in a rural wooded habitat in the New Forest, in an urban woodland habitat in Salisbury, and from a moorland habitat in Dartmoor. This suggests a possible widespread distribution of B. miyamotoi infected ticks across southern England and that a range of ecologically diverse habitats may support the transmission of this pathogen. Positive ticks were collected during 2009 and 2013 suggesting some degree of endemicity. It is feasible that I. ricinus collected before 2009 may test positive for B. miyamotoi, considering that the pathogen has been found previously in I. ricinus ticks elsewhere in Europe, perhaps as early as 1986 [Reference Stanek32]. Future studies that broaden the geographical range, and focus on positive sites will provide further information to better assess the distribution and prevalence rates of B. miyamotoi.
B. burgdorferi s.l. infection rates in ticks was significantly higher compared to B. miyamotoi (Fisher's exact, P = 0·0001), and this mimics other studies where a tenfold higher rate of B. burgdorferi s.l. has been reported [Reference Barbour23, Reference Branda and Rosenberg31]. Forty ticks tested positive for B. burgdorferi s.l. (4·2%, 95% CI 3·01–5·7), which is similar to overall prevalence rates found in other studies in the UK [Reference Bettridge33–Reference James35] which reported 3·3%, 5–7·7% and 5·6%, respectively. However, direct comparisons between prevalence rates need to be made with caution due to differences in sample sizes, stages of ticks tested and molecular methods used.
Twenty-four of the 40 positive samples were successfully genotyped, with B. garinii being the most commonly reported (50%), followed by B. afzelii (29%) and B. valaisiana (20%) (Table 2). Although low numbers of infected ticks were sequenced (but within the expected levels for the molecular method used), this finding is in contrast to a previous study in northern England where B. valaisiana (58%) and B. garinii (33%) were reported as the most commonly detected genotypes [Reference Bettridge33] and also to a study in Scotland which reported B. afzelii (48%) and B. garinii (36%) as the most common [Reference James35]. B. burgdorferi s.l.-infected ticks were found in all survey sites except Richmond Park; however, infected ticks have been reported here previously [Reference Guy and Farquhar36].
Although a range of habitats suitable for ticks were surveyed, the number of infected ticks between sites varied, with sites supporting high tick densities not necessarily being supportive of high numbers of ticks infected with B. burgdorferi s.l. This may be explained by the complex ecology of B. burgdorferi s.l., but further work is required here. A number of studies from Europe [Reference Junttila37, Reference Reis38] have reported high B. burgdorferi s.l. infection rates in ticks in urban areas and associated this with an underestimated public health risk [Reference Corrain39]. This may also be the case for ticks in urban Salisbury where 8·3% (95% CI 4·1–14·8) of ticks were found to be infected. However, further field data is required from Salisbury and from additional urban sites to investigate the significance of such habitats with regards to Lyme borreliosis risk. Interestingly this urban site was also one of the three sites harbouring B. miyamotoi-infected ticks.
It is noteworthy that in four of five regions with sequence data, only one pathogenic genospecies of B. burgdorferi s.l. was detected in each, suggesting, albeit with low sample sizes, a dominance of different genotypes within different habitats. This again may be explained by the ecology of the pathogen, and further studies that investigate the spatial heterogeneity of genospecies infection rates may help to guide the understanding of the clinical disease in humans.
A. phagocytophilum was detected in 2% (95% CI 1·4–3·5) of nymphs and 3% (95% CI 6·9–9·4) of females, and in accordance with B. burgdorferi s.l. and B. miyamotoi, ticks infected with A. phagocytophilum were found across all regions except Surrey and Salisbury. The detection of A. phagocytophilum and B. afzelii co-infection in one of 90 females tested is also worth noting. Although this was a rare occurrence in our study, the cross-over of the distribution of B. burgdorferi s.l. and A. phagocytophilum infected ticks in five of the seven regions surveyed, highlights the opportunity for co-infection in humans. This is particularly important as co-infection in humans is known to affect human susceptibility to infection with B. burgdorferi s.l. [Reference Andersson, Scherman and Råberg10] with both pathogens acting synergistically to avoid host immune responses. This can result in mixed clinical manifestations (making diagnosis more difficult) and increased disease severity [Reference Holden11].
N. mikurensis is a potentially emerging tick-borne pathogen, having caused febrile illness in a number of immunocompromised humans since 2010 [Reference Jahfari9] and may also interact with other tick-borne pathogens such as B. burgdorferi s.l. [Reference Andersson, Scherman and Råberg10]. It was not detected in any of the 954 tick lysates tested in our study, suggesting, along with evidence from a previous study [Reference Jahfari9], that it may not be established within UK tick populations. The presence of this pathogen in I. ricinus in many European countries has been well documented [Reference Jahfari9, Reference Silaghi40–Reference Hornok42] making the apparent absence of this pathogen in UK ticks of interest to both UK and European researchers.
Unlike B. burgdorferi s.l., transovarial transmission is highly probable in B. miyamotoi, with some studies suggesting over 90% of larvae derived from B. miyamotoi-infected egg batches were infected [Reference Richter17]. Furthermore, the numbers of Borrelia bacteria are reported to be significantly higher in ticks infected with B. miyamotoi compared to B. burgdorferi s.l. [Reference Wilhelmsson22]. High transovarial transmission could result in more persistent infection of larvae in the environment, and this could pose a potential health risk should they bite people and transmit B. miyamotoi [Reference Rollend, Fish and Childs43]. This may be important as human bites by larval I. ricinus are currently considered less important in B. burgdorferi s.l. transmission. The role of wildlife in the transmission of B. miyamotoi is currently unknown and requires further investigation, as well as the role of other human-biting tick species, such as I. hexagonus; the second most common tick species reported to bite humans in the UK.
It is important to recognize that this study was purely to detect the presence of pathogens, specifically B. miyamotoi. Further studies are now required to assess the prevalence of this and other tick-borne pathogens in ticks in England. Furthermore, infection rates and tick activity vary spatially and temporally and therefore snapshot infection rates should be treated with caution. Nevertheless they are useful in guiding further field studies in Borrelia and Anaplasma endemic areas. They also improve our understanding of the distribution of pathogens as well as the possible infection rates of pathogens in ticks.
This study presents the first detection of B. miyamotoi in British ticks, with the spirochaete detected in ticks from a wide geographical area in both rural and urban locations in southern England. The public health significance of B. miyamotoi has been debated elsewhere [Reference Hovius19], [Reference Branda and Rosenberg31], but published evidence suggests that it does cause clinical disease, and further studies that investigate this in the UK are recommended. Moreover, there is uncertainty regarding the detection of B. miyamotoi infections using current diagnostic tests for Lyme borreliosis, therefore this requires further investigation [Reference Hovius19, Reference Chowdri24, Reference Gugliotta25, Reference Branda and Rosenberg31]. Further research which allows us to establish prevalence rates and potential reservoir hosts of all three pathogens within the surveyed regions, and elsewhere in the UK, should be prioritized in order to further understand the ecological drivers of the diseases they cause. Raising awareness of B. miyamotoi among public health professionals and researchers alike will help to define the clinical picture of disease caused by B. miyamotoi. It may also encourage the consideration of co-infections during differential diagnosis to support the further quantification of the risk of tick-borne disease to UK public health.
ACKNOWLEDGEMENTS
We thank Dartmoor Tick Watch, Royal Parks London, Exmoor National Park, Salisbury Council and Bentley Wood Trust for providing access to sites, or provision of samples. We thank Kristel van Rooijen for her excellent technical assistance with molecular testing. We also thank Alexander Vaux, Maaike Pietzsch and Steve Leach at Public Health England for assistance in the field and support to the project.
PHE's work described in this paper is a result of ongoing vector surveillance activities funded under a core grant in aid. RIVM's work described in this paper was done under the framework of EurNegVec Cost Action TD1303.
DECLARATION OF INTEREST
None.







