Introduction
Metabolic syndrome (MS), also named syndrome X or metabolic syndrome X, is defined by the National Cholesterol Education Program–Adult Treatment Panel III as the presence of at least 3 cardiovascular disease (CVD) risk factors from a list of the following 5: abdominal obesity (waist circumference >102 cm for males or >88 cm for females), impaired fasting glucose ( ≥ 6·1 mmol/L), elevated fasting triacylglycerides (TAG ≥ 1·7 mmol/L), decreased high-density lipoprotein cholesterol (HDL-C, < 1 mmol/l for males and < 1·3 mmol/L for females), and high blood pressure (BP ≥ 130/85 mm Hg)(1). Over the last two decades, the number of people with MS has increased considerably. This increase is directly associated with the global epidemic of obesity and diabetes(Reference Zimmet, Alberti and Shaw2). MS affects approximately 10–25 % of adults worldwide, in some countries and age groups the percentage can be as high as 50–60 %(Reference Eckel, Grundy and Zimmet3). MS increases the risk of suffering from type 2 diabetes and CVD by 5- and 2-fold, respectively(Reference Grundy, Cleeman and Daniels4). There is a wealth of evidence from epidemiologic and clinical studies suggesting that modifications in dietary fat composition affect the risk of CVD. Although diet is not specifically identified as a risk factor for MS, there is good evidence to suggest that hypercaloric-hyperlipidic diets, particularly rich in SFA promote obesity, insulin resistance and MS(Reference Roche5–Reference Warensjo, Riserus and Vessby7). Very long chain n-3 polyunsaturated fatty acids (VLC n3 PUFA) in the diet (namely EPA and DHA) have a positive effect on blood lipid levels. The pleiotropic, cardioprotective effects VLC n3 PUFA have been extensively reported. They specifically reduce plasma TAG levels and thereby the risk of CVD. Other potential mechanisms of cardiovascular protection may include lowering of BP, reduced thrombotic tendency, antiinflammatory and antiarrhythmic effects, improved vascular endothelial function, increased plaque stability, increased paraoxonase levels and improved insulin sensitivity(Reference Hooper, Thompson, Harrison and Summerbell8).
As a consequence, health authorities and scientific associations have made recommendations of VLC n3 PUFA intake, like the European Food Safety Authority (EFSA) that recommends 250 mg/day(9) for the general population. Only recently, WHO has established new intake recommendations for adults of 250 mg of EPA+DHA per day and 300 mg of EPA+DHA for pregnant or lactating women (of which 200 mg should be DHA)(10). For hypertriglyceridemic subjects, the American Heart Association (AHA) recommends: 2–4 g EPA+DHA/day(Reference Kris-Etherton, Harris and Appel11), and for patients with CVD, the AHA recommends 1 g/day(Reference Kris-Etherton, Harris and Appel11) to maintain cardiac function, reduce TAG levels and the risk of CHD.
Therapeutic lifestyle modification to manage risk factors is the first-line therapy for MS patients who are not at high risk of suffering a CVD event(Reference Eckel, Grundy and Zimmet3). However, from a clinical perspective, it is difficult to define the optimum diet with which to treat MS. The use of VLC n3 PUFA could potentially benefit MS by reducing risk factors. However, not many studies have investigated the effects of EPA+DHA in this type of patients and there are no recommendations available. To better understand the possible VLC n3 PUFA benefits, the literature was systematically reviewed for randomised controlled trials (RCT) that published effects of VLC n3 PUFA in MS patients.
Materials and Methods
Literature search
The research question applied to the systematic review was “what are the effects of VLC n3 PUFA in MS patients?”. The VLC n3 PUFA of particular interest with respect to MS included EPA and DHA from fish oils or their purified forms. The review included English, Spanish, French, Italian, Portuguese and German articles, without limits on time frame or country, published before April 2011. We searched for publications using the electronic databases MEDLINE and ISI Web of Science. The MeSH terms used in the general search and the search strings used were “metabolic syndrome X” AND “metabolic syndrome” AND “fatty acids, omega-3” NOT “alpha-linolenic acid”. The search was then limited to “RCT” and “humans” only.
Elegibility criteria
To qualify, RCT could only include subjects diagnosed with MS who had three, four or five characteristics of the MS, without history of CVD. RCT reporting chronic effects of EPA+DHA (as either supplements or dietary components) and postprandial studies were considered. The studies had to quantify the EPA+DHA supplementation. Studies reporting effects of alpha-linolenic acid in MS patients were excluded. Also, in vitro, animal studies and non-RCT were excluded. 138 articles were firstly identified in the search, 92 original articles, 26 reviews, 12 proceedings papers, 7 meeting abstracts and 1 editorial material. When we limited for RCT and humans (as mentioned above), the number of publications was reduced to 14. The titles and abstracts were analysed by 2 independent scientists of the field. Three studies were excluded as they did not meet the search criteria: an animal study(Reference Yamada, Yoshida and Nakano12), a RCT using alpha-linolenic acid as omega 3 source instead of EPA+DHA(Reference Esposito, Marfella and Ciotola13), and a study describing and evaluating a food-exchange model in MS patients for further research trials(Reference Shaw, Tierney and McCarthy14). The 11 remaining trials were relevant to the research question and were included(Reference Brady, Williams and Lovegrove15–Reference Simão, Godeny and Lozovoy25). The references sections of those 11 studies revealed 6 additional RCT with MS patients that met the search criteria, and were also included(Reference Satoh, Shimatsu and Kotani26–Reference Hartwich, Leszczynska-Golabek and Kiec-Wilk31).
Quality and applicability assessment
All RCT were assessed for both study quality and applicability (Tables 1 and 2). Methodological quality refers to the design, conduct, and reporting of the clinical study. Because studies with a variety of design types were evaluated, a 3-level classification of study quality was used(Reference Balk, Lichtenstein, Chung, Kupelnick, Chew and Lau32), as follows: A) least bias, when the study mostly adhered to the commonly held concepts of good quality, including formal randomized study, clear description of the population, setting, interventions and comparison groups, appropriate measurement of outcomes, appropriate statistical and analytic methods and reporting, no reporting errors, < 20 % dropout, clear reporting of dropouts, and no obvious bias. B) Susceptible to some bias. The study had some deficiencies but none likely to cause major bias or may be missing information making assessment of the limitations and potential problems difficult. C) significant bias. Study has serious errors in design, analysis, or reporting or may have large amount of missing information or discrepancies in reporting. Regarding applicability, a 3-level classification was also used. I: if the sample was representative of the target population sufficiently large to cover both sexes, a wide age range, and where dietary intake was controlled. II: if the sample was representative of a relevant sub-group of the target population and food intake was not controlled but the subjects received dietary advice; III: if sample was representative of a narrow subgroup of subjects only, not well applicable to other subgroups and/or where dietary intake was not controlled at all.
Table 1 RCT describing chronic effects produced by the supplementation with EPA+DHA in MS patients(1)

(1) Abbreviations: Atherogenic index, log (TAG/HDL-C); BP, blood pressure; CAVI, cardio-ankle vascular index; CETP, cholesteryl ester transfer protein; CRP, C-reactive protein; CV, cardiovascular; HDL-C, high-density lipoprotein cholesterol; Hsp27, heat shock protein 27; VLC n3 PUFA, long chain n-3 polyunsaturated fatty acids; LDL-C, low-density lipoprotein cholesterol; MS, metabolic syndrome; n, number of subjects in the study; NEFA, non-esterified fatty acid; PWV, pulse wave velocity; TC, total cholesterol; RCT, Randomized Controlled Trial; SAA-LDL, Serum Amyloid A–LDL; sdLDL, small dense LDL.
(2) The criteria used to assess quality and applicability of the studies in the Materials and Methods section.
Table 2 RCT describing postprandial effects produced by the supplementation with EPA+DHA in MS patients(1)

(1) Abbreviations: AUC, area under the curve; CRP, C-reactive protein; IMA, Ischemia Modified Albumin; VLC n3 PUFA, long chain n-3 polyunsaturated fatty acids; LDL-C, low-density lipoprotein cholesterol; MS, metabolic syndrome; PAI-1, plasminogen activator inhibitor-1; SFA, saturated fatty acids; sICAM-1 soluble form of intercellular cell adhesion molecule-1; t-PA, tissue plasminogen activator; TRL, triacylglycerol rich lipoproteins; RCT, Randomized Controlled Trial.
(2) The criteria used to assess quality and applicability of the studies in the Materials and Methods section.
Results
The studies that fulfilled the inclusion criteria and were analysed for relevance to the research question were 17 in total(Reference Brady, Williams and Lovegrove15–Reference Hartwich, Leszczynska-Golabek and Kiec-Wilk31). Information on each study is summarised in Tables 1 and 2. Twelve publications showed results from European countries (Spain, Norway, Ireland, France, Poland, The Nederlands, Sweden, Denmark, United Kingdom), two from Canada, three from Japan, one from Brazil and one from Iran. Seven European publications were results of the LIPGENE project, a large scale European Union Project conducted by 25 research centres across Europe focused on diet, genomics and the MS (http://www.ucd.ie/lipgene/). The number of participants varied from 8(Reference Tulk and Robinson19, Reference Montegaard, Tulk and Lauritzen21) to 957(Reference Saito, Yokoyama and Origasa17 )in the selected studies. Eleven studies reported chronic effects of VLC n3 PUFA by administering n3 rich diets or supplements for a period of time and analysed parameters of MS patients in fasting conditions, compared with controls (Table 1). Six RCT published recently have evaluated the postprandial response of MS patients to fat loads enriched with VLC omega 3 PUFA(Reference Tulk and Robinson19, Reference Montegaard, Tulk and Lauritzen21, Reference Jiménez-Gómez, Marín and Pérez-Martínez23, Reference Perez-Martinez, Moreno-Conde and Cruz-Teno29–Reference Hartwich, Leszczynska-Golabek and Kiec-Wilk31), the most recent ones are results from the LIPGENE project (Table 2). The results of the trials analysing chronic effects and biomarkers in fasting conditions are presented first.
Studies reporting chronic effects of VLC-n3 PUFA
The first RCT in MS patients reporting effects of VLC n3 PUFA was published in 2004 by Brady et al. This study examined whether a higher intake of n-6 PUFA in combination with a low intake of VLC n3 PUFA played a role in markers of MS in Indian Asians(Reference Brady, Williams and Lovegrove15). The results demonstrated that supplementation with VLC n3 PUFA had no impact on insulin action but showed significant reductions in fasting TAG and a reduction in the percentage of denser, proatherogenic LDL particles. Another RCT with MS patients investigated the effects of an EPA+DHA and oleic acid enriched milk compared with a control milk(Reference Benito, Caballero and Moreno16). At the end of the 3-month study, MS patients of the enriched milk group improved fasting glucose levels and significantly reduced BP, TAG, total and LDL-cholesterol(Reference Vessby, Unsitupa and Hermansen33). In a study carried out in Japan(Reference Satoh, Shimatsu and Kotani26), the supplementation with 1·8 of EPA for 3 months showed reductions in the TAG of the remnant lipoprotein particles, cholesteryl ester transfer protein (CETP) activity (that may reduce the atherogenic process34), the proportion of small dense LDL particles (sdLDL) and C-reactive protein (CRP). The JELIS study was a large-scale clinical trial examining the effects of EPA on coronary artery disease (CAD) (n = 14,981)(Reference Saito, Yokoyama and Origasa17). The patients were assigned to an EPA+statin group, administering 1·8 g of EPA/d, or a control group that received only the statin, for a period of 5 years. EPA did not affect TC, LDL-C or HDL-C, but significantly reduced TAG. The EPA treatment lowered the risk for major coronary events of the high-risk group (high TAG/low HDL-C) by 53 % compared with the control, even in a Japanese population with high fish intake(35) (100 g/day on average or 0·1–2 g/day of EPA+DHA depending on the fish source).
Ebrahimi et al. (Reference Ebrahimi, Ghayour-Mobarhan and Rezaiean18) investigated the effects produced by the administration of 1 g of fish oil per day to a group of MS patients. The intervention group significantly reduced weight, systolic BP, TC, LDL-C, TAG, high sensitivity CRP and heat shock protein 27 (Hsp27) antibody titres, a protein associated with CVD risk factors(Reference Pockley, Georgiades and Thulin36), at the end of the 6 months period. A recent RCT investigated the anti-atherogenic mechanisms of EPA in MS patients(Reference Satoh, Shimatsu and Kotani20). The EPA treatment (1·8 g/day of purified EPA for 3 month), significantly reduced the levels of TAG, Serum Amyloid A–LDL (SAA-LDL, a novel oxLDL(Reference Ogasawara, Mashiba and Wada37)) and CRP. Adiponectin, an antiinflamatory marker related with MS(Reference Matsuzawa, Funahashi and Kihara38) increased by 8 %. Pulse wave velocity (PWV) and cardio-ankle vascular index (CAVI) that estimate arterial stiffness were also reduced.
Four RCT from the LIPGENE project investigated effects of VLC n3 PUFA on 1) LDL phenotype(Reference Hartwich, Malec and Partyka22), 2) BP(Reference Gulseth, Gjelstad and Tierney24), 3) insulin sensitivity(Reference Tierney, McMonagle and Shaw27), and 4) oxidative stress and inflammatory markers(Reference Petersson, Risérus and McMonagle28). In these four studies, the group of MS patients were divided into 4 study groups (A-D) that received one of the following four isocaloric diets (LIPGENE diets): (A) High SFA diet; (B) High MUFA diet; (C) low-fat diet with a daily supplement of 1·24 g/d high oleic sunflower oil; and (D): low-fat with a supplement of 1·24 g/d VLC n3 PUFA (n3 diet). The diet and supplements were administered for 12 weeks and the biomarkers were analysed at the beginning and at the end of the study period.
In the first study(Reference Hartwich, Malec and Partyka22), the n3 diet resulted in favorable alteration of LDL phenotype from small, dense proatherogenic to large LDL particles and also decreased LDL density. In the second study(Reference Gulseth, Gjelstad and Tierney24), no significant differences on systolic or diastolic BP were found in any of the study groups. The authors discussed that the low dose of omega 3 used in the study (1·2 g/day) could have explained the lack of effect as only supplements of high doses (>3 g/d) have been reported to lower BP in hypertensive patients(Reference Mansia, De Backer and Dominiczak39). In the third study(Reference Tierney, McMonagle and Shaw27), the LIPGENE diets showed no effect in insulin sensitivity, TC, LDL-C, inflammation or BP in the entire cohort. Regarding oxidative stress and inflammation, the results obtained by Peterson et al. (Reference Petersson, Risérus and McMonagle28) showed no effects of the LIPGENE diets on markers of oxidative stress or inflammation. This lack of effects was explained by the low dose of omega 3 compared with previous studies(Reference Vessby, Unsitupa and Hermansen33 )or the endogenous levels of n3 fatty acids at baseline being too high to be modified by the diets administered.
Finally, one study carried out in Brazil(Reference Simão, Godeny and Lozovoy25) investigated the effects produced by the administration of 1·8 g of EPA +1·2 g DHA per day (10 g of fish oil) to a group of MS patients. The fish oil group decreased TAG but increased total cholesterol, LDL-C and the antioxidant capacity of plasma. In addition, the fish oil group worsened the glucose control in line with other previous studies(Reference Mori, Burke and Puddey40, Reference Woodman, Mori and Burke41).
Postprandial studies with VLC-omega 3 PUFA
Elevated postprandial lipemia has recently emerged as a risk factor for CVD, indicating that this disease is in part a postprandial fenomenon(Reference Zilversmit42, Reference Kolovou, Anagnostopoulou and Daskalopoulou43). Postprandial hyperlipidemia is characteristic of MS patients, with increased postprandial TAG-rich lipoprotein (TRL) concentrations(Reference Ruotolo and Howard44, Reference Giugliano, Ceriello and Esposito45).
Tulk and Robinson(Reference Tulk and Robinson19) showed that increasing the VLC n3 PUFA content of a high-SFA oral fat load did not change postprandial TAG or inflammatory responses. Using a similar design, the same research group showed that a high n3 fat load increased PAI-1 reduced t-PA activity compared with the low n-3 fat load. The authors concluded that in the context of a high SFA intake, a lower capacity for fibrinolysis was produced by the high VLC n3 PUFA fat load.
From the LIPGENE project, four RCT have evaluated postprandial effects of VLC n3 PUFA, all of them using a similar design, as follows. MS patients were divided into 4 groups that received the LIPGENE diets A-D (see above) for 12 weeks. A fat challenge with the same fat composition as the LIPGENE diets, but administering 0·7 g of fat load/kg of body weight, was conducted pre- and postintervention. Jimenez-Gómez et al. (Reference Jiménez-Gómez, Marín and Pérez-Martínez23) examined the postprandial effects of the fat loads in the lipoprotein profile of 117 MS patients. The n3 diet group had a lower postprandial TAG concentration than the other diet groups. In contrast, long-term ingestion of the n3 diet did not have any effect on postprandial TAG and TRL. The same research group reported no effects of the n3 diet on the endothelium-dependent vasomotor function and plasma levels of cellular adhesion molecules in 75 MS patients(Reference Perez-Martinez, Moreno-Conde and Cruz-Teno29). Another study reported that the n3 diet group could favourably modify the LDL particle profile and plasma Ischemia Modified Albumin (IMA)(Reference Hartwich, Leszczynska-Golabek and Kiec-Wilk31). Finally, the administration of the LIPGENE fat loads did not affect forearm muscle FA handling in men with MS, but the VLC n3 PUFA group decreased their postprandial TAG concentrations(Reference van Hees, Saris and Hul30).
Discussion
Biomarkers of intake
The use of biomarkers of intake is essential to show compliance with the intake of the supplement or test food of the study and to correlate effects produced by the dietary fat. However, there is little consensus about which biomarker of dietary fat modification should be used in RCT. Of the studies showing chronic effects of VLC n3 PUFA, only 7 studies reported biomarkers of intake in blood, including plasma concentration of EPA/DHA fatty acids(Reference Satoh, Shimatsu and Kotani26–Reference Petersson, Risérus and McMonagle28, Reference Saito, Yokoyama and Origasa17), increases in the n3/n6 ratio of plasma FA(Reference Hartwich, Malec and Partyka22), percentage of n3 FA in serum phospholipids(Reference Benito, Caballero and Moreno16) and the ratio of n6:n3 platelet membrane phospholipids(Reference Brady, Williams and Lovegrove15). All the VLC n3 PUFA supplements used in the trials were either fish oils high in EPA (as triglycerides) or purified EPA (as ethyl esters) with the exception of one study(Reference Benito, Caballero and Moreno16) using DHA-rich oil (from unknown origin). The intervention periods ranged from 6 weeks(Reference Benito, Caballero and Moreno16) to 5 years(Reference Saito, Yokoyama and Origasa17) and the amounts of VLC n-3 PUFA administered ranged from 186 mg/day(Reference Benito, Caballero and Moreno16) to 3 g/d(Reference Simão, Godeny and Lozovoy25). The increases in plasma EPA or n3/n6 ratio ranged 60 %-120 % and were reported after at least 6 weeks of intake. The available RCT showed that in order to observe a significant increase in plasma EPA in MS patients, a minimum amount of 1·2 g or 4 g of fish oil has to be administered for at least 3 months or 6 weeks, respectively. The study using the low dose (186 mg/day) of DHA oil for 2 months did not show any significant increase in plasma EPA or DHA. The use of one standardised biomarker of dietary fat modification would be desirable for this type of interventions. In this sense, erythrocyte EPA+DHA could be an option as it has been suggested to be a useful biomarker for CHD that may also predict the risk of cardiac events(Reference Harris46).
Effects of VLC n3 PUFA on MS
a) VLC n3 PUFA and blood lipids
Elevated fasting TAG increases the risk of CVD(1). The TAG lowering effects of VLC n3 PUFA have been extensively described(47). High doses (2–4 g/d) of EPA+DHA decrease serum triglycerides in both normo- and hyperlipidaemic individuals but low doses have also reported reductions when substitute SFA(Reference Baro, Fonollá and Peña48, Reference Carrero, Baró and Fonollá49). The magnitude of the effects is related both to the dose of EPA+DHA and to the baseline concentrations of triglycerides(Reference Jacobsen50). Harris(Reference Harris51) observed a mean reduction of 35 % in subjects with hypertriglyceridaemia and of 24 % in those with serum triglycerides < 2 mmol/L. In the meta-analysis by Balk et al. (Reference Balk, Lichtenstein, Chung, Kupelnick, Chew and Lau32), a mean reduction of 27 % in serum triglyceride concentrations was observed. In the diet and lifestyle recommendations given by the AHA, 2–4 g EPA+DHA per day provided in capsules under physician's supervision are recommended for individuals with hyper-triglyceridaemia to lower TAG 20–40 %(Reference Kris-Etherton, Harris and Appel11).
All RCT reporting blood lipid effects upon supplementation with VLC n-3 PUFA showed fasting TAG reductions, ranging from 7 % to 25 % (Fig. 1). One study also reported 40 % reductions of TAG in remnant lipoprotein particles(Reference Satoh, Shimatsu and Kotani26). The mechanisms that explain the TAG lowering effect include inhibition of triglyceride synthesis, stimulation of fatty acid beta-oxidation, and increased lipoprotein lipase-mediated clearance of VLDL triglycerides(Reference Jacobsen50). The reported reductions in apo CIII levels(Reference Tierney, McMonagle and Shaw27) would facilitate TRL catabolism and apo-E dependent hepatic uptake of TRL remnants. Increased postprandial lipemia has recently emerged as a CVD risk factor, indicating that this disease is, at least in part, a postprandial phenomenon. Patients with MS usually have increased postprandial lipemia. However, the 5 postprandial studies administering VLC n3 PUFA showed little improvements in the postprandial TAG response. Only one study concluded that the administration of VLC n-3 PUFA could reduce the adverse postprandial TAG-raising effects of long-term LFHCC diets (23).
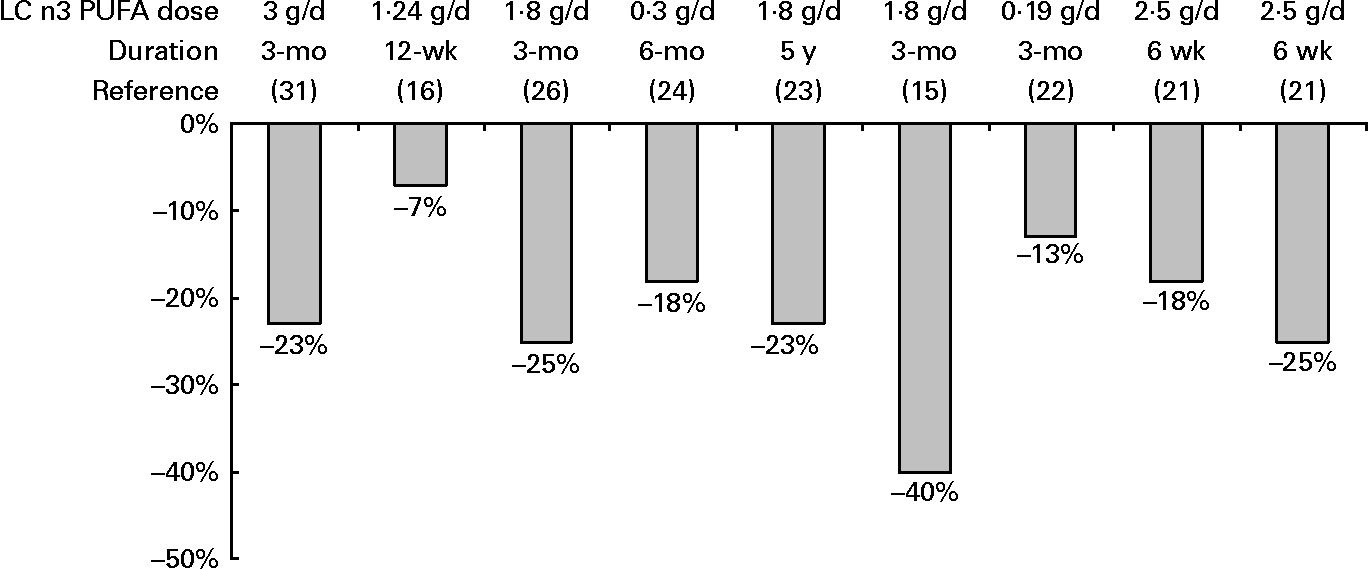
Fig. 1 TAG reductions(1,2) reported in RCT with MS patients upon VLC n3 PUFA supplementation. (1) Expressed as percentages calculated from baseline values. (2) All reductions were statistically significant (P < 0·05).
None of the considered studies reported modifications of HDL-C by administration of VLC n3 PUFA. One study showed an activity reduction of CETP, but without effect of HDL-C(Reference Satoh, Shimatsu and Kotani26). Regarding TC and LDL-C, the RCT supplementing VLC n3 PUFA to MS patients showed conflicting results: an increase in TC and LDL cholesterol was reported when high doses (3 g/d) were used for 3 months(Reference Simão, Godeny and Lozovoy25), whilst administration of 1 g of fish oil containing 300 mg EPA+DHA for 6 months resulted in TC and LDL-C reductions(Reference Ebrahimi, Ghayour-Mobarhan and Rezaiean18). In this latter study, the volunteers were not instructed to maintain their diet and lifestyle, the dietary intake was not controlled and a 3·8 % reduction of body weight was obtained at the end of the study, which could have played a part in the reductions observed. Benito et al. (Reference Benito, Caballero and Moreno16) also described reductions in TC and LDL-C upon 3-month consumption of a dairy drink where SFA were substituted by EPA+DHA (186 mg/day) and oleic acid. EPA plus DHA at high doses (2–4 g/d) have multiple effects on blood lipids(Reference Jacobsen50). TC and LDL-C concentrations, are generally not affected by this VLC n3 PUFA supplementation, but in subjects with hypertriglyceridaemia, LDL-cholesterol concentrations may be increased by 5–10 %(Reference Balk, Lichtenstein, Chung, Kupelnick, Chew and Lau32, Reference Harris51, 52). Whilst the TAG reduction produced by VLC n3 PUFA produces clear CV benefits, the concomitant LDL-C increases reported at moderate to high doses represent a risk to these patients. The daily dose of EPA+DHA and length of time needed to produce a relevant TAG reduction without increasing LDL-C or TC is yet to be determined for MS patients. More studies are required to clarify this point.
b) VLC n3 PUFA and LDL phenotype
LDL particle size is an important predictor of CV events and progression of CHD(Reference Austin, Breslow and Hennekens53, Reference Rizzo and Berneis54). The sdLDL pro-atherogenic particles have been associated with obesity, insulin resistance, high blood TAG and oxidative stress(Reference Kwiterovich55). Supplementation of MS patients with VLC n3 PUFA consistently reduce the percentage of sdLDL particles in favour of the large, buoyant, less atherogenic LDL phenotype(Reference Brady, Williams and Lovegrove15, Reference Hartwich, Malec and Partyka22, Reference Perez-Martinez, Moreno-Conde and Cruz-Teno29) which potentially reduces the CV risk. sdLDL concentrations are very much influenced by plasma TAG(Reference Griffin56). In MS, slow clearance of TAG from TRL lipoproteins allows for longer periods of circulating TAG, which can directly affect the composition of LDL. Increased transfer of TAG to LDL via CETP during neutral-lipid exchange and subsequent hydrolysis of LDL-TAG by lipases results in a preponderance of sdLDL(Reference Griffin56). Suppression of TAG production in the liver by EPA and reduction of CETP activity may explain the beneficial phenotype shift.
c) VLC n-3 PUFA and BP
Data from the available studies indicate that supplementation of MS patients with VLC n3 PUFA does not produce significant effects on BP with the doses used. The large and well designed trial by Gulseth et al. (Reference Gulseth, Gjelstad and Tierney24) from the LIPGENE project addressed the effects of VLC n3 PUFA on the BP of MS patients. This trial showed no effects on BP of a dose of 1·24 g/day for three months. In line with this, a trial by Murphy et al. (Reference Murphy, Meyer and Mori57), 86 overweight subjects with high serum TAG were randomised to 1 g/day of EPA+DHA (from enriched foods) or placebo, for 6 months, obtained no significant effects on BP. However, other two RCT trials described above(Reference Benito, Caballero and Moreno16, Reference Ebrahimi, Ghayour-Mobarhan and Rezaiean18) reported significant decreases of BP using much lower amounts (300 mg and 186 mg/day). In those trials however, the patients from the intervention group lost a significant amount of weight during the study period which may indicate that the BP effect may not be derived from the n3 supplementation.
Indeed, EFSA, after a thorough revision of the scientific evidence, indicated that much higher doses of EPA + DHA ( ≥ 3 grams/day) are needed to have a short-term effect on systolic BP in subjects with untreated hypertension(52, Reference Mancia, De Backer and Dominiczak58) (~3–5 mmHg decrease in systolic BP), which may also have smaller effects in normotensives (~1 mmHg decrease in systolic BP). Studies with MS patients using those high doses of EPA+DHA are currently lacking. Interestingly, the recent study by Satoh et al. (Reference Satoh, Shimatsu and Kotani20) in MS patients showed that n3 supplementation of 1·8 g/day for 3 months improved pulse wave velocity and cardio-ankle vascular index, two biomarkers of arterial stiffness, the latter being less influenced by BP changes. Perhaps the use of these new biomarkers would be useful to better characterise the magnitude of the effects. It should be stated here that the above RCT did not differentiate between MS patients with normal or high BP at baseline, and antihypertensive medication was sometimes allowed(Reference Gulseth, Gjelstad and Tierney24). These factors make more difficult the interpretation of the data. Whether MS patients, all suffering from high BP as risk factor, may benefit from VLC n3 PUFA supplementation remains unanswered.
d) VLC n-3 PUFA and glucose control
Epidemiologic studies have reported a lower prevalence of impaired glucose tolerance and type-2 diabetes in populations consuming large amounts of fish(Reference Kromhout, Bosschieter and de Lezenne Coulander59). VLC n3 PUFA may decrease insulin resistance through a number of mechanisms including decrease in circulating TG and small dense LDL particles, increasing membrane fluidity and signal transduction. Further, substituting saturated fat with unsaturated fat, such as n-3 PUFAs, may have beneficial effects on insulin sensitivity(Reference Galgani, Uauy and Aguirre60). This change in dietary fat may reduce the risk of progression to type-2 diabetes from impaired glucose tolerance by improving hemostasis, albuminuria, subclinical inflammation, oxidative stress and slowing progression of artery narrowing(Reference Nettleton and Katz61). Clinical studies have shown that consumption of n-3 PUFAs has cardioprotective effects in persons with type-2 diabetes without adverse effects on glucose control and insulin activity. 3 different meta-analysis concluded that the use of moderate to high amounts of fish oil in diabetics had no effect on glycemic control(Reference Balk, Lichtenstein, Chung, Kupelnick, Chew and Lau32, Reference Friedberg, Janssen and Heine62, Reference Montori, Farmer and Wollan63). More recently published reviews also failed to show effects of VLC n-3 PUFA on the glucose control of type-2 diabetes patients(Reference Hartweg, Perera and Montori64–Reference Mostad, Bjerve and Basu66).
In MS patients, effects of fasting glucose, insulin and insulin resistance (shown as HOMA) were reported in 5 RCT using different doses of n-3 PUFA. 3 g/day of EPA+DHA for 3 months increased blood glucose and insulin resistance by 4 % and 24 %, respectively(Reference Simão, Godeny and Lozovoy25). Three studies administering daily doses of 2·5 g for 6 weeks, 1·8 g and 1·2 g both for 3 months did not show any effects(Reference Brady, Williams and Lovegrove15, Reference Satoh, Shimatsu and Kotani26, Reference Tierney, McMonagle and Shaw27). No changes in glycosilated haemoglobin were reported(Reference Satoh, Shimatsu and Kotani26). The 3-month study with the low dose of EPA+DHA (186 mg/day) administered in a dairy drink showed that fasting glucose was reduced by 5·3 % in the n3 group but the effect of the oleic acid and the weight reduction observed cannot be ruled out. Therefore, there is no evidence of beneficial effects of VLC n3 PUFA on the blood glucose control of MS patients and may even be harmful at high doses. Future studies with different EPA+DHA doses are needed to determine if increased consumption of n-3 PUFA will delay the conversion of insulin-resistant subjects (MS) to diabetics.
e) VLC n-3 PUFA and inflammation
Several features of MS are associated with increased inflammatory and oxidative stress markers(Reference Pickup67–Reference Basu69). For example, MS patients usually have high CRP as a manifestation of proinflammatory status. In theory, supplementation with EPA+DHA should originate production of less-potent eicosanoids with anti-inflammatory and antithrombotic effects (compared with the n6 araquidonic acid) but the reports available are controversial. The LIPGENE intervention studies showed no significant effect of dietary fat modification (quantity and quality) and VLC n3 PUFA on several markers of inflammation (CRP, 15-keto-13,14-dihydro-PGF2α, IL-6, TNFalpha, sICAM, sVCAM, resistin, adiponectin, leptin) and oxidative stress (8-iso-PGF2α)(Reference Tierney, McMonagle and Shaw27, Reference Petersson, Risérus and McMonagle28). The postprandial studies only showed modest effects: higher endothelial cell function, decrease of sICAM-1(Reference Perez-Martinez, Moreno-Conde and Cruz-Teno29) and reduced postprandial elevation of the IMA which is related to oxidative stress(Reference Hartwich, Leszczynska-Golabek and Kiec-Wilk31). In contrast, other three RCT administering daily doses of 1·8 g, 0·3 g and 0·186 g of EPA+DHA showed significant reductions of CRP and of the inflammatory status(Reference Satoh, Shimatsu and Kotani26, Reference Benito, Caballero and Moreno16, Reference Ebrahimi, Ghayour-Mobarhan and Rezaiean18). Satoh et al. (Reference Satoh, Shimatsu and Kotani20) also reported an increase in adiponectin, an anti-atherogenic adipocytokine, and reduced serum amyloid A-LDL complex, an oxidised form of LDL generated under inflammatory conditions. In the LIPGENE trials, the n3 PUFA supplementation was carried out in the context of a low fat high complex carbohydrate diet, where other factors may play a role. The study by Benito et al. (Reference Benito, Caballero and Moreno16) and Ebrahimi et al. (Reference Ebrahimi, Ghayour-Mobarhan and Rezaiean18) were poorly controlled and produced BP and body weight reductions. The only controlled study with weight stable MS patients in which both groups received the same diet showed CRP reductions in the n3 supplemented group(Reference Satoh, Shimatsu and Kotani26) but was performed with a low number of participants (n = 44, 22 per group). More controlled studies with different doses of VLC n3 PUFA and higher number of participants are necessary to test the effects of VLC n3 PUFA on the inflammation associated with MS.
f) VLC n-3 PUFA and CHD risk
One study addressed this point(Reference Saito, Yokoyama and Origasa17) by analysing the high TAG/low HDL-C subgroup of patients (administered with 1·8 g EPA/day +a statin) of the JELIS primary prevention trial. The results showed that in the high TAG/low HDL-C group, the risk of major coronary events was particularly high, and EPA was shown to potently reduce the risk by 53 %. In the high TAG/low HDL-C group, level of plasma EPA at the time of registration was low, but EPA administration increased plasma levels, suggesting some correlation between plasma EPA level and major coronary events risk. Fish intake has been associated with decreased risk of CVD(Reference Kris-Etherton, Harris and Appel70). A few epidemiological studies have reported that men who ate at least some fish weekly had a lower coronary heart disease (CHD) mortality rate than that of men who ate none (Reference Kromhout, Bosschieter and de Lezenne Coulander59, Reference Kromhout, Feskens and Bowles71, Reference Dolecek and Granditis72). A 30-year follow-up of the Chicago Western Electric Study showed that men who consumed 35 g or more of fish daily compared with those who consumed none had a relative risk of death from CHD of 0·62 and a relative risk of nonsudden death from MI of 0·33(Reference Daviglus, Stamler and Orencia73). For these reasons and on the basis of the available data, the regular intake of fish, at least twice a week (preferably oily fish), is recommended to patients without documented CHD like the MS patients.
Concluding remarks
MS is characterised by a cluster of CVD risk factors. The available RCT on MS patients convincingly show that the administration of VLC n3 PUFA doses > 1 g for at least 3 months produced a significant reduction of triglycerides ranging from 7 % to 25 %. These results confirm the hypotriglyceridemic effect of VLC n3 PUFA in MS patients. The triglyceride lowering may produce further benefits on % sdLDL and perhaps ameliorate the inflammatory process associated with MS. No clear effects were found on other MS markers. High doses of VLC n3 PUFA ( ≥ 3 g/day) may produce further TAG reductions but could raise other risk factors such as LDL-C. The combination of VLC n3 PUFA plus a statin may be useful to prevent the occurrence of coronary events. More studies are needed using different amounts of VLC n3 PUFA, time lengths, dietary backgrounds and different profiles of MS patients before clear recommendations can be made
Although both EPA and DHA are part of the fat composition of seafood products, their percentages in the oils differ depending on the species and other factors. Whilst fish oils in general usually contain about 30 % of EPA+DHA, tuna oil can be as high as 25 % in DHA and herring oil usually contains 18 % of EPA. In recent years, differential effects on gene expression, cell function and physiology have been reported for EPA and DHA in their purified forms, reviewed in(Reference Gorjão, Azevedo-Martins and Rodrigues74). Indeed, purified EPA and DHA administered in capsules seem to have differential effects on serum lipids and lipoproteins, LDL particle size, glucose and insulin release(Reference Mori, Burke and Puddey40, Reference Buckley, Shewring and Turner75). In addition, DHA and EPA possess different affinity for organs. DHA generally exceeds EPA 5- to 30-fold in most organs(Reference Arterburn, Hall and Oken76). Future research should explore these differential effects in MS patients. In addition, nutrigenetics and nutrigenomics are exceptional tools to identify possible EPA+DHA gene targets related to MS risk factors like obesity and insulin resistance.
Finally, in the last decade we have witnessed an extraordinary increase in the number of functional foods targeted to the ever-growing health conscious population. Several categories among these foods can be found according to their health target, including the cardiovascular (CV) system. These foods, when they are consumed regularly and in the context of a healthy diet and lifestyle, intend to maintain or improve the CV health by reducing the levels of risk factors. The dietary supplementation of MS patients with EPA+DHA produces benefits. Apart from increasing the intake of fish, the use of functional foods enriched with EPA+DHA represents an alternative that may produce benefits in MS patients(Reference Benito, Caballero and Moreno16, Reference Lopez-Huertas77).
Conflict of interest
The author declares no conflict of interest.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The author would like to express his gratitude to Prof Carmina Wanden-Berghe of Cardenal Herrera University of Elche (Spain) and Prof Angel Gil of Granada University (Spain) for their guidance and help with the article search and screening of abstracts. The author would also like to thank Juan J Carrero of the Karolinska Institut at Stockholm (Sweden), for his help with the publication.





