Traditionally scientific authors generate, analyse and have access to raw data and prepare an article that disinterested observers would accept reflects an appropriate interpretation of those data. Authorship has been changing, however, and journals now accept that articles may be authored by individuals who have made a substantial contribution to the conception and design or the acquisition of data or analysis and interpretation of data in a study, or who have drafted or critically revised the intellectual content of an article and who have approved the final version of the published article (International Committee of Medical Journal Editors, 2000; Reference Rennie, Flanagin and YankRennie et al, 2002). This new authorship matrix is consistent with many articles being ghostwritten (Reference Davidoff, DeAngelis and DrazenDavidoff et al, 2001).
Unacknowledged editorial or writing assistants to academic authors – so-called ghostwriters – are often employed by medical communication agencies working for pharmaceutical companies. Efforts have been made to quantify the extent to which ghostwriting is happening, with Flanagin et al (Reference Flanagin, Carey and Fontanarosa1998) reporting that up to 11% of articles published in six peer-reviewed journals in 1996 involved the use of ghostwriters.
There are a number of delicate issues that need to be teased out in this area, ranging from the practicalities of regulating authorship to the more profound questions of whether ghostwriting is an unfortunate accidental development in the scientific enterprise or whether it reflects some fundamental aspect of the way modern science is conducted. There can be few if any of these issues or questions, however, that would not benefit from some quantification of what is happening. Against this background we have sought to quantify the literature profile of articles on one drug, sertraline, that were in production in 1998.
METHOD
This article distinguishes between traditional and non-traditional authorship on the basis of a judgement as to whether the authors are free in a traditional manner to share with others the raw data from studies they author. We have assumed that authors working on company-sponsored articles are, in general, not at liberty to share proprietary raw data and are even less likely to do so if they have not seen the raw data in the first instance. By raw data here is meant untabulated data; tabulation is arguably a primary and key act of authorship. In pharmaceutical-company-sponsored clinical trials, this initial tabulation is invariably performed either within the company or within a contract research organisation that passes on tabulated data and trial reports to medical writing agencies. This practice, almost by definition, gives rise to a non-traditional form of authorship. In contrast, we have assumed that individuals who conduct studies of their own design, regardless of funding source, can share raw data, if necessary.
We have used two data sources: Medline and EMBASE literature retrieval services searching for the word sertraline in the titles of articles from 1998, which were scrutinised for articles referring to the therapeutic uses of sertraline; and a document prepared for Pfizer Pharmaceuticals by Current Medical Directions (CMD) on 29 January 1999, which gives a worldwide status update for 85 articles on Pfizer's antidepressant sertraline, some of which had been published in 1998 and others subsequently in 1999, 2000 or early 2001. The CMD document was made available to us on a non-confidential basis in the course of legal proceedings.
Current Medical Directions is a medical information company, based in New York and set up in 1990 to deliver scientifically accurate information strategically developed for specific target audiences (http://www.cmdconnect.com). This agency writes up studies, review articles, abstracts, journal supplements, product monographs, expert commentaries and textbook chapters. It conducts meta-analyses and organises journal supplements, satellite symposia and consensus conferences, as well as advisory boards for its clients.
The CMD document indicates that CMD was coordinating articles on sertraline. These articles appear to involve proprietary data in almost all instances. There were a number of publications that the document suggests originated within communication agencies, with the first draft of articles already written and the authors’ names listed as ‘to be determined’. In the case of subsequently published articles in this series, the authors’ names are available. A further series of articles had very similar academic and company authors, already published or with authors’ names designated. Finally, there were articles that do not appear to have been written within a communication agency and do not have a Pfizer name on them, but they acknowledge Pfizer funding or support. Some of these articles involve economic models, constructed on the basis of tabulated rather than raw data. Others are review articles. Three involved clinical trials.
The Medline and EMBASE articles on sertraline include articles listed in the CMD series and some not listed in that series, henceforth called the non-CMD series. In the non-CMD series, the majority are reports of studies not supported by Pfizer and only one appears to have involved the generation of proprietary data; accordingly we have assumed that these authors are in a position to share raw data if requested.
We have attempted to estimate the impact of these two different groups of articles as follows. The impact factor for each journal was established using Journal Citation Report for 1999 from the Institute for Scientific Information Inc. For one journal in the CMD series and for four journals in the non-CMD series it was not possible to obtain impact factors and so these have not been included. Using Medline, we systematically searched for the number of Medline listings for each author in both the CMD and non-CMD author series. This permits us to offer three estimates of literature impact. First, we have estimated the mean number of Medline listings for a CMD author versus a non-CMD author, giving an estimate of author impact. Second, we have assumed the total number of Medline listings for all authors of an article and multiplied by the journal impact factor; taking a mean of these values for all articles in a respective series gives a literature profile per article from each series. Third, we have multiplied the mean literature profile per article, as calculated above, by the number of articles in each series to give an annual literature impact factor for both the CMD and non-CMD series.
The impact factor of a journal and the Medline listings linked to academic authors give an estimate of the potential impact of an article, and as such can be expected to guide the considerations of a company such as CMD, which ‘strives to exceed the expectations’ of their clients and ‘assist them in achieving their strategic objectives’ (http://www.cmdconnect.com). The actual impact of an article may, however, differ significantly from its apparent potential. We have therefore also established the actual citation rates for the articles in both series using the Institute for Science Information Web of Science database. For citation rates we have restricted the comparison to articles from both series that were published in 1998.
In this study, the appearance of an individual's name on an article is designated as an authorship. An individual author, therefore, may have several authorships.
RESULTS
Using Medline with sertraline as a keyword and searching article titles we found 59 distinct articles in 1998 with sertraline in the title. Altogether for 1998, 1999 and 2000 Medline listed 81% of the CMD articles published in 1998, 1999 and 2000 (excluding supplement and health economics articles). In 1998, 12 of the 20 articles appearing in the CMD document appeared in the Medline search. Of the eight not appearing in Medline, five came from the only supplement in the CMD series and three from health economics journals; none of these eight articles had sertraline in their title.
Non-CMD articles
Excluding from the Medline series those articles listed by CMD leaves 47 papers that included sertraline in the title. We have excluded a further ten papers as follows. One was a Pfizer-funded large multi-centre study that outlined therapeutic advantages for sertraline in depression. The nature of this paper and its funding suggest an overlap with the CMD series of articles laid out below, in that the data are proprietary. This article has not been counted in either series. Eight papers that deal with animal, healthy volunteer or non-therapeutic metabolic research were excluded, as was one letter that offered a comment on the methodology of a sertraline trial.
This leaves 37 papers dealing with the therapeutic effects of sertraline. In addition to those articles retrieved by Medline, we have included a further four EMBASE-listed papers not found in the CMD document. EMBASE also retrieved a further seven papers on toxicology and three on biochemical studies, which are not considered further.
Thus, in total, there are 41 non-CMD articles on therapeutics with sertraline: 19 are categorised by the Medline retrieval process as journal articles or clinical trials; 16 are categorised as letters; and 6 are categorised as randomised controlled trials. Of the 41 articles, 3 report ambiguous findings for sertraline, 20 report negative findings and 18 report positive findings, including positive results for depression (3), for premature ejaculation (2) and for dialysis hypotension (1). Of the 41 non-CMD studies, three received support from Pfizer but the authors appeared likely to be in possession of the raw data.
Of the 20 papers offering negative findings for sertraline, 16 detail adverse effects, including serotonin syndrome, hypomania, hyponatraemia, suicide attempts, extrapyramidal problems, urinary retention and priapism. There were, in addition, one review paper on extrapyramidal problems associated with sertaline use and one negative trial on the use of sertraline in chronic pain.
Five of the 41 papers appeared in the Journal of Clinical Psychopharmacology, three in the Journal of Clinical Psychiatry, two in Psychosomatics, one in the American Journal of Psychiatry and the rest in a number of lower-impact journals, including some non-English-language journals. There were 121 authorships from 120 individual authors and an average of 2.95 authors per article. These articles were 3.4 pages in length on average.
The CMD articles
The CMD's document outlined 85 papers in the production process during 1998: two appeared in 1998 but have a 1997 date (these are excluded from the analysis); 20 appeared in 1998; 18 appeared in 1999; and 17 appeared in 2000 or early 2001. We have used the results for all these articles published from 1998 to 2000 to generate mean impact factors for authors, journals and articles, but have generated the overall annual literature impact for the CMD series from the articles published in 1998 alone.
The 85 articles cover depression (14), seasonal affective disorders (1), dysthymia (7), panic disorder (8), post-traumatic stress disorder (2), general anxiety (2), obsessive–compulsive disorder (1), differentiation between selective serotonin reuptake inhibitors (17), what is termed ‘outcomes research’ (largely pharmaco-economic articles) (10), the use of sertraline in the elderly (10), the use of sertraline in children (6), the use of sertraline in women (4), sertraline pharmacokinetics (2) and sertraline in paedophilia (1).
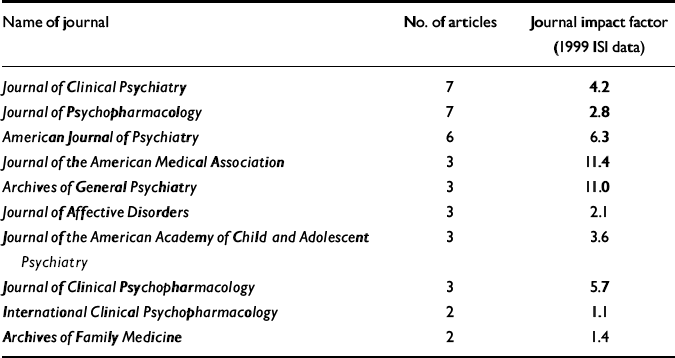
The 55 published articles that form the basis of this analysis have a mean length of 10.7 pages, with 365 authorships drawn from a total of 207 individual authors, giving a mean of 6.6 authors per article. Of these, there are 182 academic and 25 company authors. Two of these articles follow current journal guidelines and acknowledge writing support from individuals not listed as authors. These 55 articles offer the results of 25 clinical trials from a number of different therapeutic areas, including areas in which Pfizer were seeking licenses at that time for sertraline, in addition to eight review articles and six articles offering economic models based on Pfizer trial data. All of the clinical trial results were favourable to Pfizer, as were the economic analyses. One of the review articles, from a Pfizer author, offered a frank acknowledgement of the capacity of sertraline to induce agitation/akathisia and the links between this and treatment-induced suicidality (Reference LaneLane, 1998). (This may be because the intention of Lane's paper was to place the clinical problem in context rather than to identify specific problems.) The 55 papers appeared in the journals listed in Table 1. The British Medical Journal, European Psychiatry, the British Journal of Psychiatry, the American Heart Journal, Pharmacoeconomics and 13 other journals published a single article each.
Table 1 Journals taking articles on sertraline linked with Current Medical Directions
| Name of journal | No. of articles | Journal impact factor (1999 ISI data) |
|---|---|---|
| Journal of Clinical Psychiatry | 7 | 4.2 |
| Journal of Psychopharmacology | 7 | 2.8 |
| American Journal of Psychiatry | 6 | 6.3 |
| Journal of the American Medical Association | 3 | 11.4 |
| Archives of General Psychiatry | 3 | 11.0 |
| Journal of Affective Disorders | 3 | 2.1 |
| Journal of the American Academy of Child and Adolescent Psychiatry | 3 | 3.6 |
| Journal of Clinical Psychopharmacology | 3 | 5.7 |
| International Clinical Psychopharmacology | 2 | 1.1 |
| Archives of Family Medicine | 2 | 1.4 |
Of the 85 articles, 23 are listed as possibly originating within communications agencies. Of the 55 published articles, the names of several academic authors appear on more than one article, with one individual being named as a co-author on 12 of these articles. Of the published articles, 13 of the 55 do not appear to have a company author or to have been through an agency. Four of these 13 articles involve economic models based on data provided by Pfizer, and it is assumed that these authors do not have access to raw data. Five of the 13 are review articles appearing in a company-sponsored symposium supplement. The remaining four articles acknowledge support funding, of which three involve clinical trials. Of these three trials, Pfizer personnel are listed as having reviewed draft articles in two, but the authors appear to hold the data.
Comparison of CMD and non–CMD articles
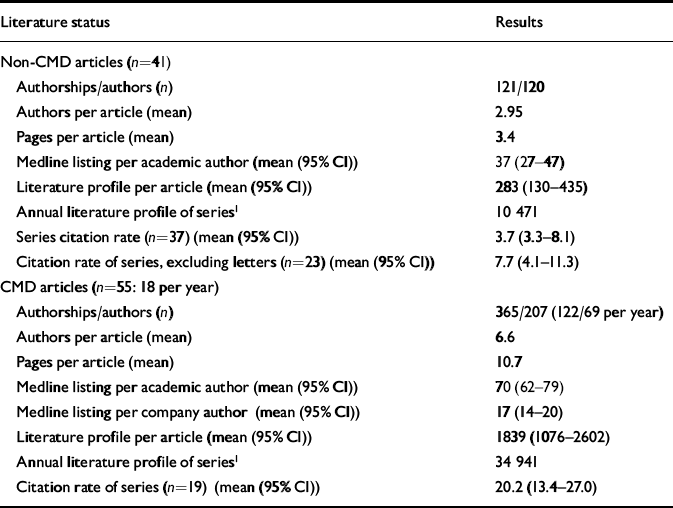
In Table 2 we list the mean number of authors per article, the mean number of pages per article and the mean number of Medline listings per author for each series. There are statistically significant differences between the two series of articles on each of these features. In addition, as outlined we list the mean literature profile per article in each series. Finally, we constructed an annual literature profile for each series by multiplying the mean literature profile per article by the number of articles from the series that year. Using a paired sample t-test, the two series of articles differed significantly in terms of Medline listing per authorship (P≤0.001; 95% CI for the difference between the series was 11.2–42.4) and in terms of the literature profile per article (P≤0.002; 95% CI for the difference between the series was 623–2570).
Table 2 The literature profile of Current Medical Directions (CMD) and non-CMD articles
| Literature status | Results |
|---|---|
| Non-CMD articles (n=41) | |
| Authorships/authors (n) | 121/120 |
| Authors per article (mean) | 2.95 |
| Pages per article (mean) | 3.4 |
| Medline listing per academic author (mean (95% CI)) | 37 (27-47) |
| Literature profile per article (mean (95% CI)) | 283 (130-435) |
| Annual literature profile of series1 | 10 471 |
| Series citation rate (n=37) (mean (95% CI)) | 3.7 (3.3-8.1) |
| Citation rate of series, excluding letters (n=23) (mean (95% CI)) | 7.7 (4.1-11.3) |
| CMD articles (n=55: 18 per year) | |
| Authorships/authors (n) | 365/207 (122/69 per year) |
| Authors per article (mean) | 6.6 |
| Pages per article (mean) | 10.7 |
| Medline listing per academic author (mean (95% CI)) | 70 (62-79) |
| Medline listing per company author (mean (95% CI)) | 17 (14-20) |
| Literature profile per article (mean (95% CI)) | 1839 (1076-2602) |
| Annual literature profile of series1 | 34 941 |
| Citation rate of series (n=19) (mean (95% CI)) | 20.2 (13.4-27.0) |
In addition to the above, we have determined the citation rates for the CMD and non-CMD series of articles. We have compared the CMD articles both with the mean citation rates for the whole non-CMD series (n=37) and for the non-CMD series with the letters excluded (n=23). In each case, there is a significant difference between the data-sets. Using a paired t-test, the difference between the two series was statistically significant at P≤0.001 (95% CI for the difference between the series was 9.6–22.7). Comparing the CMD series with the non-CMD series excluding letters gives a result of P≤0.001 (95% CI for the difference between the series was 7.1–20.8).
In addition, in the non-CMD series there was a mean journal impact factor of 3.0 (95% CI 2–4) for articles reporting beneficial effects of sertraline, versus 1.78 (95% CI 1–2.5) for those reporting negative effects. The mean literature profile for favourable articles was 351 (95% CI 59–643), versus 172 (95% CI 1.7–337) for negative articles.
Finally, when the CMD articles were considered on their own, there was a statistically significant correlation between journal impact factors and citation rates (r=0.67; P≤0.01). When both CMD and non-CMD articles for 1998 were considered, there was a significant correlation between journal impact factor and citation rates (r=0.71; P≤0.01), which increased further if the letters in the non-CMD series were excluded (r=0.74; P≤0.01). There were comparable statistically significant correlations between citation rates and the composite potential literature profile measures that we constructed, as well as between journal impact factors and the composite potential literature profile measures.
DISCUSSION
These data address two issues in the scientific literature. First, they offer a further quantification of the number and impact of articles based on proprietary data, and the possible extent of ghostwriting based on a single drug. Second, they offer a first set of figures on the likely impact of a series of articles prior to publication and the subsequent citation rates of those articles.
Literature impact
In the debate on how to evaluate the scientific literature, Seglen (Reference Seglen1992) has argued that citation rates rather than journal impact factors should be used. We have used both measures and, in addition, a composite literature impact measure. The debate on how to evaluate articles has hitherto focused on the extent to which scientific articles may or may not have moved a scientific field forward. Citation rates arguably reflect the true scientific worth of an article better than the impact factor of a journal. However, in the field of therapeutics, pharmaceutical companies may be more interested in short-term gains with major purchasers than in developing the science base of the field. To the extent that this is happening, the prestige of journals and their apparent authors will be of greater importance to them than the actual citation rates of articles. Indeed, in a mirror image of Seglen's arguments for other scientific domains, citation rates seem at risk of being artificially boosted by ghostwriters for companies in a way that is less likely to happen for journal impact factors. The findings reported here, however, appear unaffected by the method used to evaluate the respective literatures.
The profile of the articles reported here suggests that the background of certain authors may have increased the possibility of the company's publications appearing in the most prestigious journals. Specific journals seem to have been targeted. The combination of distinguished journal, distinguished author, an efficient distribution system and sponsored platforms appears to have led to an impact on the therapeutics domain greatly in excess of 50% of the impact of the rest of the literature on sertraline. The impact of this literature on third-party payers and other interested parties is at present unquantifiable. The question of literature impact would seem to be tied closely to the nature of ghostwriting. Authorship lines from perceived opinion-leaders with minimal company representation and non-declaration of other non-academic authorship inputs increase the likelihood that these articles will be influential with prescribers and purchasers.
Effect of ghostwriting on academia
One of the expressed concerns about ghostwriting has been the way in which this process leads to a lack of recognition for the people who actually write the articles. The converse of this point is that academics become opinion leaders in a therapeutics field because they appear to have their names on a larger proportion of the literature appearing in the most prestigious journals than do others and because they get asked to national and international meetings to present data with which they may not have first-hand acquaintance. Whether or not the academic authors in this series saw the raw data from the studies the CMD articles are based on, these authors cannot share proprietary raw data with colleagues in the way that has been traditional in the scientific domain. This, allied to the volume of industry-linked authorship, indicates a process of changing scientific authorship that could conceivably culminate in a situation in which the dominant figures in therapeutics actually have comparatively little first-hand research experience and few raw data that they can share with others.
It should be acknowledged that there are a number of good aspects to the ghostwriting process. First, authorship by a communications agency or within a company makes it more likely that at least some of the results of research will enter the public domain than if the production of articles were left to the senior clinicians involved in clinical trials. Second, the quality of the writing is probably consistently superior as a consequence. Third, there is every reason to believe that at least some communications agencies will take the efforts by journal editors to encourage disclosure of interests more seriously than many academic investigators will. Fourth, there are data to indicate that the reporting of adverse events in company-sponsored and monitored clinical trials is more comprehensive than the reporting of adverse events in government-sponsored or other independent studies (Reference ShamooShamoo, 2001).
However, analyses of published results on antidepressant studies in recent years have made it clear that a considerable proportion of negative results are not published, to the extent that the sponsorship of a published study is now a demonstrable predictor of the findings of that study (Reference FreemantleFreemantle et al, 2000; Reference Gilbody and SongGilbody & Song, 2000). There is, however, little reason to believe that this bias does not affect the entire domain of therapeutics, including psychotherapy, whether supported by pharmaceutical companies or not. The tensions involved show in the figures reported here. On the one hand, the CMD-linked articles report universally positive results. On the other hand, the CMD-linked articles contain a much higher proportion of randomised controlled trials, conventionally seen as offering a superior calibre of data, than do the non-CMD articles.
Problems with ghostwriting
If the methods employed in industry-linked authorship make the publication process both more efficient and more effective, then what, if any, are the problems linked to new styles of authorship? In addition to having the potential to produce a set of authorities on therapeutics with little clinical experience, we list two other issues. First, most studies are now sponsored, designed and analysed, in addition to being efficiently written, by pharmaceutical companies. This is a process that in psychopharmacology picked up pace from 1980 (Reference HealyHealy, 2002a ). As long as the greatest proportion of studies are both undertaken by and published by pharmaceutical companies, the primary questions being asked in the therapeutics domain may well relate to the marketing interests of those companies rather than to unanswered scientific questions, as the CMD series of articles outlined here demonstrates. Recent efforts to encourage pharmaceutical companies to publish the results of all of their studies imply that therapeutics will become scientific if all studies are published. Complete publication of studies would, in fact, only bring the field of therapeutics up to an acceptable business ethics standard. A field is only scientific if scientific questions are addressed.
The second issue relates to the correspondence between published articles and raw data. The current CMD series throws up issues of concern in this area. First, one study in this series had one patient on sertraline who committed suicide, and three others on sertraline who reported increasing suicidal ideation necessitating treatment discontinuation, in contrast to just one case of emergent suicidality on a comparable drug and no problems on placebo. There is no reference to these data in the final published article. Second, of the six published paediatric psychopharmacology CMD articles, only one article mentions one suicidal act. There were in fact six suicidal acts on sertraline and three further cases of suicidality in the subject group from which these articles come, including four suicidal acts in 44 patients with depression given sertraline, which is a rate of 9% (Pfizer Expert Report, 1997). The effects of sertraline in paediatric depression were outlined by Alderman et al (Reference Alderman, Wolkow and Chung1998), who reported only the adverse events that occurred in more than 10% of patients.
Attention has previously been drawn to possible incongruities in the reporting of suicidal acts on recent antidepressants in a meta-analysis by Khan et al (Reference Khan, Warner and Brown2000) (see Reference HealyHealy, 2002b ). Importantly, the categorisation of suicidal acts on placebo from these trials reported by Khan et al is also reported in a number of other articles, suggesting that these academic authors may all be using data previously tabulated by the respective companies. This has clear implications for any assessment of the hazards of these drugs, and for the confidence that can be placed in the process by which these articles were written.
A possible solution
Problems of this sort could be overcome if, in addition to making available the gross details of negative studies, as some companies do, companies also made the raw data or primary data tables from therapeutic trials available. This may be seen as a counsel of perfection, but if pharmacotherapy is to be a scientific business rather than just a business adopting the appearances of science, no less than this is needed. It should be remembered that the capitalisation of the industry depends entirely on the voluntary participation of health care consumers in studies of the kind reviewed here. If ghostwriting is an inevitable feature of modern scientific writing, the potential availability of the raw data would do more to ensure a correspondence between those data and a published end result than could be achieved by any other mechanism.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ This paper makes it clear that an increasing proportion of the clinical trial literature in pharmacotherapeutics is managed through medical writing agencies.
-
▪ The articles reported here contain significant discrepancies between published data and the raw data from the actual clinical trials.
-
▪ In addition to already recognised implications for ghostwriters, the new style of authorship has implications for academic authors.
LIMITATIONS
-
▪ From the available material it is not possible to know what data the apparent authors of articles were privy to.
-
▪ In the absence of clear agreement on how to assess the impact of academic articles, it is not possible to know for certain the relative longer-term impact of company-supported articles versus independent articles.
-
▪ It is unclear what proportion of studies or data on sertraline for the relevant period have been published.
Acknowledgement
The authors acknowledge input from Professor E. Young of Ann Arbor, Michigan, USA, to the drafting of this article.





eLetters
No eLetters have been published for this article.