Introduction
The application of highly active anti-retroviral therapy (HAART) has decreased human immunodeficiency virus (HIV)-caused mortality rates for a long period. However, as the HIV-1 virus is highly variable, the high-frequency mutations introduced during its replication could induce the development of HIV quasi-species in the body. Therefore, resulting mutations of proteins that are targeted by antiretroviral (ARV) drugs could cause drug resistance, and thus, induce antiretroviral treatment (ART) failure [Reference Frentz, Boucher and van de Vijver1].
In recent years, ‘primary drug resistance’ or ‘transmitted drug resistance’ (TDR) has become more and more prevalent [Reference Ma2, Reference Shao, Li and Ly3]. TDR is a global problem. Previous studies have shown that in developed countries, the incidence of primary drug resistance is about 10–20% [Reference Frentz, Boucher and van de Vijver1]. TDR may induce treatment failure and compromise treatment efficacy. Therefore, to improve treatment outcomes, both the Department of Health and Human Services (DHHS) and the European AIDS Clinical Society (EACS) guidelines recommend resistance testing for naive patients at entry into HIV care and before initiation of ART. The present study was performed to investigate the prevalence of HIV TDR in Beijing (China), according to the methods of HIV drug resistance threshold survey issued by the World Health Organization (WHO).
Materials and methods
Ethical approval
This study was approved by the Beijing Center for Diseases Prevention and Control. All procedures performed involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Patients
From April 2014 to February 2015, 1241 newly diagnosed HIV-1/AIDS patients from Beijing were included. All of them were ART-naive patients. The baseline data including age, sex, ethnicity and transmission routes were collected.
Sample collection and virus gene amplification
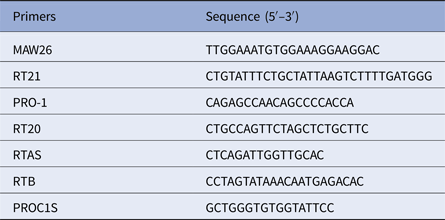
Venous blood was collected from all the patients, and the viral RNA was isolated with the viral RNA Mini Kit (Qiagen, Germany). RT-PCR was used to amplify the full-length pol-protease and the first 300 amino acids of reverse transcriptase. The primers are listed in Table 1. MAW26 and RT21 were used as the outer primer pair for the first cycle in the nested PCR approach while PRO-1 and RT20 were used as the inner primer pair for the second cycle. The reaction system and amplification conditions were as described previously [Reference Han4]. The PCR products were resolved by 1% agarose gel electrophoresis and identified in reference to the molecular weight standards. The specific bands were excised from the gel, purified by gel extraction kit (Qiagen), and sequenced on an ABI 377 sequencer (USA).
Table 1. Primers for amplification and sequence analysis of HIV-1 pol gene
Phylogenetic analysis
Sequencher 5.1 was used for splicing of all the sequences, followed by alignment using BioEdit 7.1. In order to identify the subtype of the virus gene, all the subtyping reference sequences were downloaded from the Los Alamos HIV database. Finally, manual corrections were performed as required. Mega 5.0 software was used to establish the neighbour-jointing phylogenetic tree, which was used for the identification of gene subtypes, with the support by Simplot 3.1.
Drug resistance analysis
Among 1241 patients, 932 patients’ pol regions sequences were amplified and analysed. The obtained sequences were submitted to the Stanford HIV Drug Resistance Database (http://hivdb.stanford.edu). The drug resistance sequence analysis provided by HIVdb software was used to identify the drug-related mutations. Thus, conclusions about the resistance to different drugs, as well as the degree of drug resistance (mild, moderate and high) for each sample could be obtained. The existence of surveillance drug resistance mutation (SDRM) was decided according to the drug resistance mutations described in the 2009WHO SDRM list [Reference Duong5].
Statistical analysis
SPSS 19.0 software (IBM, Beijing, China) was used for the statistical analysis. The data in this study were categorical variables and were described with frequencies and percentages. The χ 2 test or Fisher's exact test, as appropriate, were used for the analysis of the baseline data, gene subtypes and drug resistance. For the analysis of TDR-related risk factors, drug resistance was used as the dependent variable. In addition, independent variables such as sex, ethnicity, route of transmission, CD4 cell count and gene subtype were included in the binary logistic regression to investigate whether these variables were risk factors of HIV-1 drug resistance. Factors that showed a frequency of zero in the univariate regression analysis were analysed with Cornfield estimates of odds ratio and 95% confidence interval using Stata 14 (StataCorp LLC, Texas, USA). P < 0.05 was considered statistically significant.
Results
Baseline characteristics of the patients
The blood samples from all 1241 treatment-naive HIV/AIDS patients from Beijing were collected for amplification and sequencing. Finally, sequences of the pol regions could be analysed in 932 samples, including 902 males (96.78%). The mean age of the patients was 34 years (17–81 years). Eight hundred and ninety-four patients (95.92%) were Chinese Han origin, and 855 (91.74%) were men who have sex with men. Seven hundred and ninety (84.75%) patients’ baseline CD4 cell count was above 100 cells/μL. The most common HIV-1 gene subtype was CRF01-AE (n = 526, 56.44%). The total number of the treatment-naive patients with drug resistance was 63 (6.76%). The TDR rates to the three major types of drugs in treatment-naive patients in Beijing area were significantly different (P = 0.044) (Table 2).
Table 2. Baseline characteristics of HIV/AIDS patients
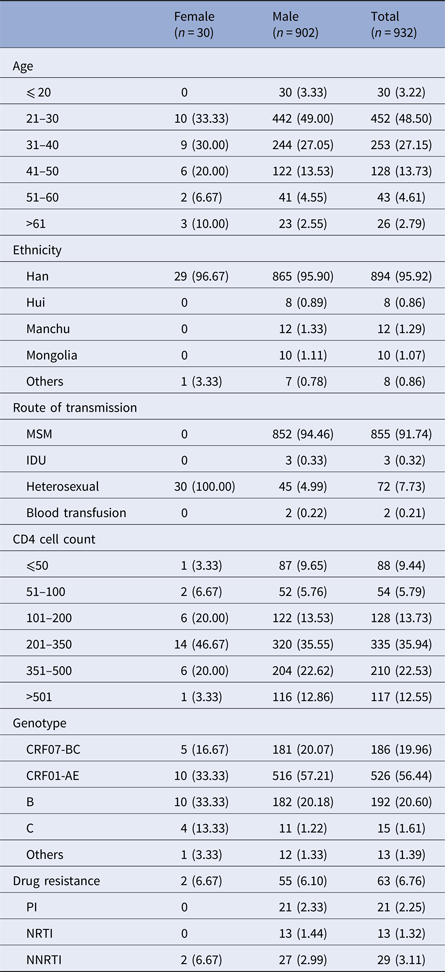
Data is shown as n (%).
MSM, men having sex with men; IDU, injection drug use; PI, protease inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor.
Resistance to ARV drugs
Protease inhibitors
Twenty-one patients were resistant to protease inhibitors (PIs), including one patient (0.11%) each was resistant to atazanavir, indinavir and saquinavir; four (0.43%) were resistant to tipranavir; and 17 (1.82%) patients to nelfinavir. However, no patient was found with drug resistance to darunavir or kaletra (LPV/r) (Table 3).
Table 3. Drug resistance to PIs in the patients
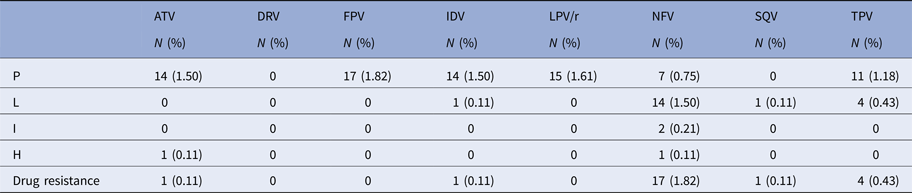
P, potential resistance; L, low resistance; I, intermediate resistance; H, high resistance.
ATV, atazanavir; DRV, darunavir; FPV, fosamprenavir; IDV, indinavir; LPV/r, kaletra; NFV, nelfinavir; SQV, saquinavir; TPV, tipranavir.
Drug resistance=L+I+H.
Nucleoside reverse transcriptase inhibitors
Thirteen patients showed drug resistance to nucleoside reverse transcriptase inhibitors (NRTIs), including three (0.32%), three (0.32%), four (0.43%), six (0.64%), nine (0.97%), 10 (1.07%) and 10 (1.07%) patients who were resistant to zidovudine, tenofovir disoproxil fumarate, stavudine, didanosine, emtricitabine, lamivudine and abacavir, respectively (Table 4).
Table 4. Drug resistance to NRTIs in the patients
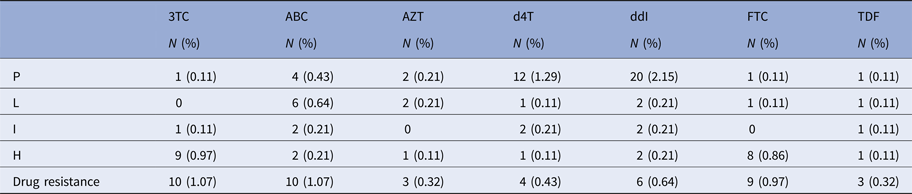
P, potential resistance; L, low resistance; I, intermediate resistance; H, high resistance.
3TC, lamivudine; ABC, abacavir; AZT, zidovudine; d4T, stavudine; ddI, didanosine; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate.
Drug resistance=L+I+H.
Non-nucleoside reverse transcriptase inhibitors
The number of the patients with drug resistance to non-nucleoside reverse transcriptase inhibitors (NNRTIs) was the highest (n = 29), of which 10 (1.07%), 23 (2.47%), 23 (2.47%) and 23 (2.47%) were resistant to etravirine, efavirenz (EFV), nevirapine (NVP) and rilpivirine (RPV), respectively (Table 5).
Table 5. Drug resistance to NNRTIs in the patients
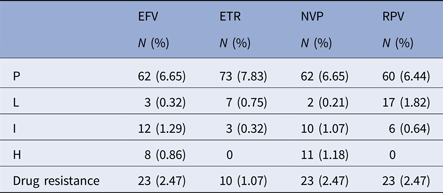
P, potential resistance; L, low resistance; I, intermediate resistance; H, high resistance.
EFV, efavirenz; ETR, etravirine; NVP, nevirapine; RPV, rilpivirine.
Drug resistance=L+I+H.
HIV-1 gene subtype and drug resistance
As shown in Table 6, the TDR rate was 4.84%, 6.65%, 5.21%, 33.33%, 30.77% in patients with CRF07-BC, CRF01-AE, B, C subtypes and other genotypes, respectively (χ 2 = 35.683, P < 0.001). Similar to overall drug resistance, the TDR to NNRTIs were also significantly different among the genotypes (χ 2 = 80.979, P < 0.001): the TDR rate in the C subtype was the highest (33.33%). Moreover, patients with C and other subtypes showed TDR only to NNRTIs. NRTIs and PIs resistance mutations mainly occur in genotypes CRF07-BC and CRF01-AE. While, there was no resistance to PIs in genotype B.
Table 6. HIV-1 genotype and drug resistance
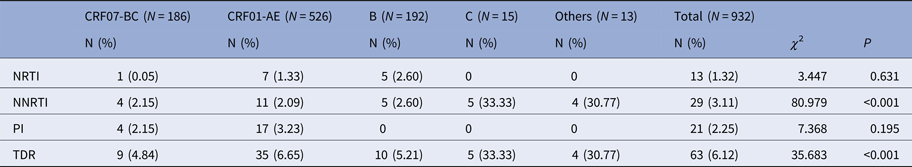
TDR, total drug resistance.
Risk factors of TDR by binary logistic regression
In order to further identify the independent risk factors of HIV-1 drug resistance, sex, age, ethnicity, route of transmission, baseline CD4 cell count and HIV-1 gene subtype were included as independent variables in the binary logistic regression. Result showed that the HIV-1 genotype was a potential factor associated with TDR. TDR rates in CRF01-AE, B subtypes and CRF07-BC were similar. However, comparing with the CRF07-BC, the odds ratio values of C and other subtypes were 14.416 (95% CI 3.582–58.020, P < 0.001) and 7.880 (95% CI 1.745–35.587, P = 0.007), respectively (Table 7).
Table 7. Univariate and multivariate logistic regression for risk factors of TDR
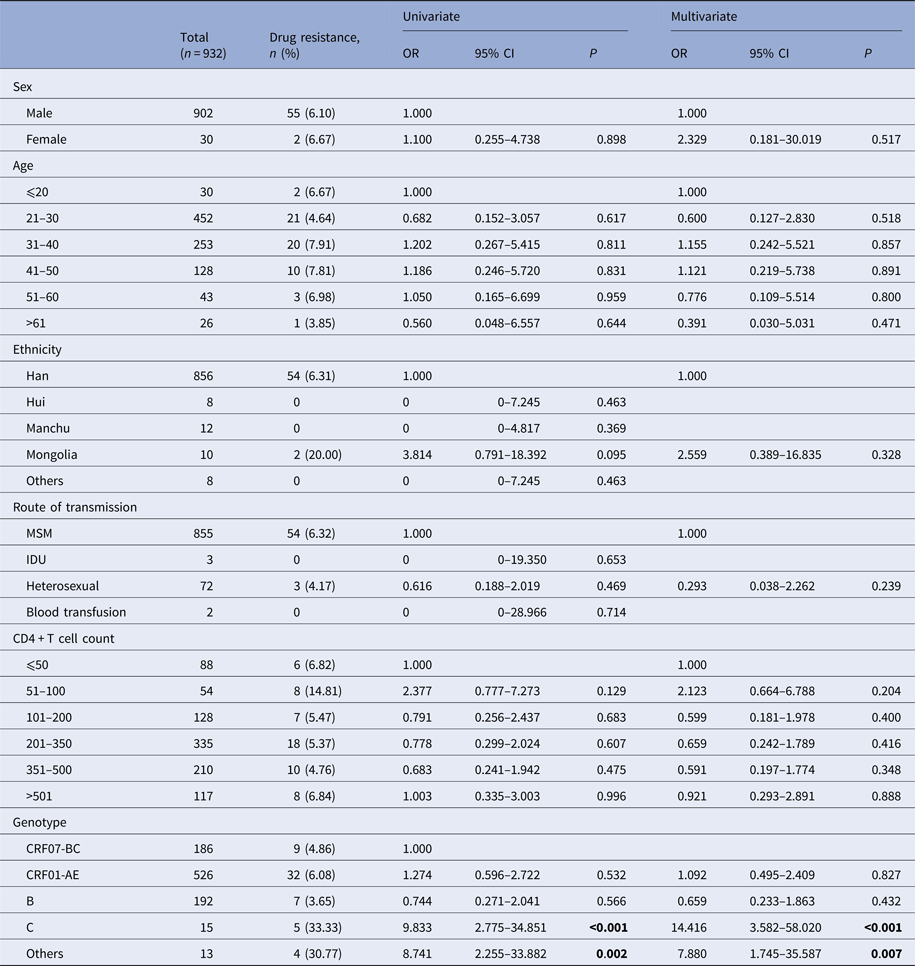
OR, odds ratio. CI, confidence interval. The values with OR of 0 by univariate analysis are Cornfield estimates.
Discussion
HIV drug resistance emerges when HIV replicates in the presence of ARV drugs. Transmission of drug-resistant HIV strains is associated with suboptimal virologic response to initial ART. Resistance testing at baseline can guide regimen selection to optimise virologic response.
The present study showed that the TDR rate in naïve patients in Beijing from April 2014 to February 2015 was 6.76%, in which the TDR rate for PIs, NRTIs and NNRTIs was 2.25%, 1.32% and 3.11%, respectively. According to the WHO SDRM list, the TDR rates in Beijing were at a moderate level, and this should be taken into account by clinicians and others concerned with disease control and prevention systems.
We found, drug resistance was more prevalent in genotype C and other genotypes than CRF01-AE, CRF07-BC and B subtypes. In the resistance to NNRTIs, TDR rates were significantly different between HIV subtypes, among which the rate in the C subtype was the highest (33.33%). Similar to the C subtype, we only found TDR to NNRTIs in the subtypes classed as ‘others’. In contrast to the results in NNRTIs, the major gene subtypes with drug resistance to NRTIs and PIs were CRF07-BC and CRF01-AE. There was no NRTIs or PIs resistance in the B genotype. These findings suggested that genotype C was the dominant strain which was resistant to ART drugs. These results, showing more TDR in subtype C and no PI or NRTI TDR in subtype B, differ from previous studies in China [Reference Zhao6, Reference Sui7] and worldwide [Reference Hattori8–Reference Chehadeh11]. However, in particular, there are reasons why our results for subtype C may be different from other studies. We found that only 15 patients (1.61%) were infected by subtype C in our study. Subtype C is apparently at a low rate in Beijing, and these cases may be due to people coming to Beijing from other provinces. Among them, five out of 15 cases possessed NNRTI mutations, a rate of nearly 33%. We think there are some occasional factors with a comparable lower number of cases, and the same phenomena were observed in other subtypes (30.77%, four in 33). The rate of PI use in our current ART regimen was low, and virological failure was occasionally observed. It is important to consider that some mutations are in fact natural polymorphisms in subtype C, as interpreted by the Stanford University HIV-1 DR database; here, the PI ‘mutations’ are most likely to be characteristic of some of the prevailing strains, rather than an indication of the existence of lower sensitivity to certain PIs. Although genotype C is not the main strain in Beijing area, in other areas in China, such as Yunnan province, it accounts for about 41.7% [Reference Ma2]. Thus, physicians should pay attention to TDR of NNRTIs in regions with genotype C is the dominant strain in that region.
In the present study, the TDR among 932 patients was 6.8% which was in agreement with the findings from Beijing area in 2011 [Reference Ye12, Reference Ye13]. Further analysis showed that these patients were mainly resistant to NNRTIs, among which 23 patients each (2.47%) were resistant to EFV, NVP and RPV. No drug resistance to LPV/r was found in these patients. Currently, EFV and NVP are the first-line anti-HIV drugs in China; therefore, in regions where baseline drug resistance test is available, genotypic testing is recommended as the preferred resistance testing to guide therapy in ARV-naive patients. In addition, the responses to the ART should be closely monitored. In the case of the regions where the baseline drug resistance surveillance could not be performed, the treatment of the patients should be closely monitored. In patients with good treatment adherence, while not achieving HIV suppression after 6 months’ treatment, TDR should be considered as a possibility and the treatment should be modified.
On a technical note, we must highlight that among the 1241 patients, 932 patients’ pol regions sequences were amplified and analysed in this study. However, in nearly one-fourth of the patients, we failed to get pol gene amplification. There may be some factors influencing the poor rate of PCR amplification, including: (1) some patients may have received prophylaxis treatment after exposure to high-risk behaviour, and they may conceal that they received official treatment; (2) some cases may have a relatively low viral load, even without drug treatment; (3) some failure may be caused by the low connection of primer pairs for pol gene to the genome of HIV-1 strains. Empirically, we would expect nearly 10–15% of the failures may be caused by this factor.
In summary, the major causes of treatment failure in patients on ART are mainly due to infection with virus strains that have drug resistance mutations. Therefore, for treatment-naive HIV/AIDS patients with TDR mutations in Beijing, the medication strategy should be decided according to pre-treatment drug resistance testing results. For patients not subjected to drug resistance testing before treatment, the HIV viral load should be closely monitored after treatment. In cases of virological failure, drug resistance testing should be performed for early identification of drug resistance and treatment should be appropriately modified.
Acknowledgements
This study was supported by The National Natural Science Foundation of China (8127316 & 81601795), The Preclinical Medicine-Clinical Medicine Scientific Research Cooperation Fund of Capital Medical University (16JL18) and The Capital Health Research and Development of Special Found (2016-1-2182).
Declaration of interest
The authors declare that they have no conflict of interest.









