Iodine is a component of the thyroid hormones which are crucial for brain and neurological development, particularly during gestation and early life(Reference Zimmermann1). Iodine requirements are therefore two-thirds higher for women when pregnant than when non-pregnant(Reference Andersson, de Benoist and Delange2). Iodine deficiency was common in the UK up to the 1960s(Reference Phillips3) and was eradicated, not by the usual practice of an iodised-salt programme, but through the adventitious increase in milk-iodine concentration and increased milk intake(Reference Phillips3). Though the UK has therefore been assumed to be iodine sufficient, a recent national study has revealed mild iodine deficiency in adolescent girls, giving cause for concern(Reference Vanderpump, Lazarus and Smyth4). While randomised, controlled trials in regions of severe iodine deficiency have shown that prenatal iodine deficiency causes developmental defects in cognition, there is less information on the effects in regions of mild iodine deficiency.
Our study investigated maternal iodine status in 1,000 women of the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort who were recruited in the 1990s(Reference Golding, Pembrey and Jones5). Iodine concentration (and creatinine to adjust for urine volume) was measured in urine samples from pregnant women of median gestational age 13 weeks (IQR 10-16). Women were grouped as iodine-deficient or sufficient according to WHO criteria(Reference Andersson, de Benoist and Delange2). The relationships between maternal iodine status and child's IQ at age 8 (Wechsler Intelligence Scale for Children, WISC), reading ability at age 9 (Neale Analysis of Reading Ability), and Key Stage 2 scores at age 11 were analysed using logistic regression.
The group was mildly-to-moderately iodine deficient based on a median iodine concentration of 91.8 μg/L (123 μg/g creatinine)(Reference Andersson, de Benoist and Delange2) and 61% of women were classed as iodine deficient when using the creatinine-adjusted data. The children of women deficient in iodine were more likely to have a total IQ score below the 25th centile (unadjusted OR=1.42, 95% CI 1.05–1.94). The Table shows the risk of being in the bottom quartile of scores, after controlling for relevant confounders (mother's parenting score, home score, family adversity during pregnancy, life-event score, dietary intake of n-3 fatty acids and iron, gender, ethnicity, maternal age, smoking, alcohol intake, parity, breastfeeding, partner at birth, parental education, housing status, crowding and use of iron, fish oil and vitamin/mineral supplements).
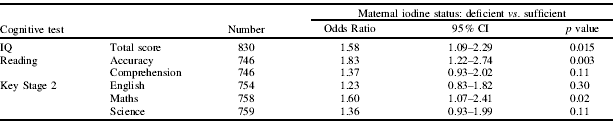
Although these results cannot prove causality, they are suggestive of the importance of achieving adequate iodine status during pregnancy and highlight the possibility that iodine deficiency can pose a risk to the developing infant, even in a country considered to be iodine replete. A limitation of this study is the estimation of iodine in a single spot-urine sample which may not reflect maternal diet for the entire pregnancy. We plan further study in this cohort with both a larger sample size and repeated iodine measures throughout gestation.
S. Bath gratefully acknowledges PhD studentship funding by the Waterloo Foundation and Wassen International.


