The cholesterol-lowering properties of dietary phytosterols (PS) have been described in various animal models and in human trials. Currently, a daily intake of 2·5 g of plant PS or phytostanols is recommended to reduce the concentrations of LDL-cholesterol by up to 10 %, potentially decreasing the risk of CVD( Reference Lauer and Fontanarosa 1 ). Despite the unsettled debate about the exact mechanisms underlying these effects, it is well accepted that sterols and stanols can act at different levels during cholesterol absorption, mainly by interfering with the absorption of cholesterol and displacing it from the mixed micelle( Reference De Smet, Mensink and Plat 2 ) or enterocyte metabolism, thus affecting the assembly of chylomicrons( Reference Field, Born and Mathur 3 , Reference Liang, Wong and Guan 4 ) and the influx and efflux of cholesterol from enterocytes( Reference Plat and Mensink 5 – Reference Jia, Ebine and Demonty 7 ). However, another known mechanism for the elimination of cholesterol from the body is that via the bile acid (BA) synthesis pathway. In the liver, cholesterol can be oxidised by the cytochrome P450 enzyme cholesterol-7α-hydroxylase (encoded by CYP7A1), the main controller of BA synthesis( Reference Davis, Miyake and Hui 8 ). The hydroxylated cholesterol moiety is subsequently subjected to successive modifications resulting in different BA species, which get secreted into the gall bladder and eventually reach the duodenum. After recirculation into the liver through the so-called enterohepatic circulation, BA are eventually excreted from the body through the faeces( Reference Alrefai and Gill 9 ). Little is known about the effects of PS on the BA synthesis pathway; it has been shown in hamsters that the administration of soyabean sterol esters increases the faecal excretion of neutral lipids and the output of BA as well as changes the BA composition of the gall bladder( Reference Carr, Cornelison and Illston 10 ). In another study carried out in rats, stigmasterol has been found to increase the output of cholesterol and BA in a dose-dependent fashion( Reference Andriamiarina, Laraki and Pelletier 11 ). Interestingly, it has been reported that in humans the cholesterol-lowering response to plant sterol consumption is conditioned by the promoter variant − 204A>C of the CYP7A1 gene( Reference De Castro-orós, Pampín and Mozas 12 ), indicating that in humans PS are more effective if a more active promoter controls CYP7A1. Nevertheless, more studies are required to elucidate whether BA synthesis represents a mechanism for quantitative cholesterol elimination from the body in response to dietary PS.
Different investigations have ascribed other beneficial effects to dietary plant sterols and stanols apart from their cholesterol-lowering effects. A recent meta-analysis based on twelve randomised controlled trials has concluded that PS therapy can reduce blood TAG concentrations in humans( Reference Demonty, Ras and van der Knaap 13 ). Other supporting studies carried out in pre-clinical models have shown that the administration of β-sitosterol and stigmasterol over a period of 6 weeks decreases plasma TAG concentrations in hamsters( Reference Liang, Wong and Guan 4 ). In C57/BL6 mice, the administration of a plant sterol blend has been found to reduce hepatic and plasma TAG concentrations, but, surprisingly, not plasma cholesterol concentrations( Reference Rideout, Harding and Jones 14 ). Different mechanisms have been proposed to explain these effects; some authors have proposed that PS might block the absorption of fats( Reference Rideout, Harding and Jones 14 ), while others have concluded that plant sterols can reduce the release of VLDL from the liver( Reference Plat and Mensink 15 ) or affect the assembly of TAG in enterocytic chylomicrons( Reference Liang, Wong and Guan 4 ). Additionally, some studies have highlighted the protective effects of PS against insulin resistance( Reference Tanaka, Misawa and Ito 16 ), hyperglycaemia( Reference Alexander-Lindo, Morrison and Nair 17 ) or obesity( Reference Ikeda, Konno and Shimizu 18 ), thus placing these compounds as potentially effective agents against insulin resistance or the metabolic syndrome( Reference Nagao and Yanagita 19 ).
Despite growing evidence supporting the beneficial effects of plant sterols and stanols on TAG metabolism along with their hypocholesterolaemic activity, little is known about the involvement of the liver in the hypotriacylglycerolaemic properties of these bioactive compounds. Due to the key role that the liver plays in the control of the synthesis and distribution of lipids, we hypothesised that PS might attenuate triacylglycerolaemia by affecting hepatic processes related to fatty acid metabolism. We chose the golden Syrian hamster as the experimental model due to the common features that this mammal shares with humans but not with rats or mice regarding hepatic cholesterol and lipoprotein metabolism( Reference Gao, Zhang and Liu 20 , Reference Zhang, Wang and Jiao 21 ). Therefore, in the present study, we treated hamsters with combined dyslipidaemia with PS due to the hypotriacylglycerolaemic effects of PS in hypertriacylglycerolaemic subjects( Reference Plat, Brufau and Dallinga-Thie 22 ). We found that PS normalised fasting blood TAG concentrations several days after attenuating the cholesterolaemia. The hypotriacylglycerolaemic effects of PS were accompanied by the apparent protection against fatty liver development and the impairment of hepatic lipogenesis and fatty acid oxidation programme as well. In addition, PS-treated hamsters had a highly increased total BA:cholesterol ratio in the gall bladder. Altogether, the present study demonstrated that PS might have other beneficial properties apart from their hypocholesterolaemic properties, such as prevention of alterations of the hepatic lipid metabolism and, consequently, protection against steatosis and the combined dyslipidaemia associated with the consumption of a high-cholesterol diet.
Materials and methods
Animals
The Animal Ethics Committee of the Universitat Rovira i Virgili (Tarragona, Spain) approved all the procedures. The animals used were 3-month-old male golden Syrian hamsters (Charles River Laboratories) weighing about 100 g. The hamsters were housed individually in cages at 22°C under a 12 h light–12 h dark cycle (lights on at 09.00 hours) and with free access to food and water. After an adaptation period of 4 d, twenty hamsters were fed a high-fat diet (HFD) providing 21 % of energy as fat and 0·1 % of cholesterol (D10051907; Research Diets, Inc.) for 15 d to induce combined dyslipidaemia and ten hamsters (standard diet (STD) group) were fed a normal fat diet (D10051906; Research Diets, Inc.) throughout the study. On day 15, blood samples were obtained by saphenous vein puncture and hamsters fed the HFD were further divided into two groups. Of these two groups, one (n 10, HFD) was fed the described HFD and the other (n 10, HFD+PS) was fed the same HFD supplemented with 1 % of PS (D10051908; Research Diets, Inc.). The composition of diets is given in Table 1. According to the manufacturer (Lipofoods), the PS provided were in the dispersible form (Lipophytol®) and contained 23·3 % campesterol, 0·4 % campestanol, 28·1 % estigmasterol, 43 % β-sitosterol and 1 % estigmastanol. According to the Reagan-Shaw formula( Reference Reagan-Shaw, Nihal and Ahmad 23 ), the PS dose used in the present study is equivalent to a daily human intake value of 3·8 g and far below the doses tested for adverse effects( 24 ). Nevertheless, it has been shown that PS at high doses can decrease the bioavailability of β-carotene and α-tocopherol, despite the relevance of the consequences being still not known( Reference Richelle, Enslen and Hager 25 ). Body weight and food intake were recorded every 4 d, and additional blood sampling was done on day 36 of the experiment. Faeces were collected 48 h before killing the animals. On day 57, hamsters were fasted for 6 h and anaesthetised with sodium pentobarbital and blood was collected by cardiac puncture. Plasma was obtained by centrifugation, and tissues were dissected, weighed, snap-frozen in liquid N2 and stored at − 80°C until further analyses. Before freezing, a portion of the liver was sectioned and fixed in 4 % paraformaldehyde for 24 h and stored at 4°C in phosphate buffer for histological examination. Bile was obtained by gall bladder puncture, snap-frozen in liquid N2 and stored at − 80°C.
Table 1 Composition of the diets used in the study
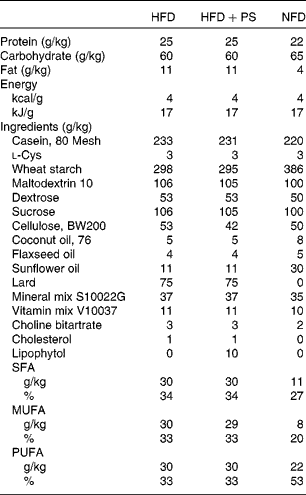
HFD, high-fat diet; HFD+PS, high-fat diet with phytosterols; NFD, normal fat diet.
Gene expression analysis
Total RNA from the liver and retroperitoneal white adipose tissue was extracted as described previously( Reference Caimari, del Bas and Crescenti 26 ). RNA quality was checked spectrophotometrically and by agarose gel electrophoresis. Primers for the different genes are listed in Table S1 (available online), and these were obtained from biomers.net. Real-time quantitative PCR using β-actin as the reference gene were carried out as described previously( Reference Caimari, del Bas and Crescenti 26 ).
Plasma analysis
Commercial enzymatic colorimetric kits were used for the determination of the concentrations of plasma glucose (intra-assay reliability, CV%, 0·83–0·59; inter-assay reliability, CV%, 1·58–1·50), TAG (intra-assay reliability, CV%, 0·39–0·43; inter-assay reliability, CV%, 3·62–3·60) (QCA) and NEFA (intra-assay reliability, CV%, 0·75–0·61; inter-assay reliability, CV%, 0·75–4·91) (Wako). The concentrations of plasma total cholesterol, HDL-cholesterol and VLDL/LDL-cholesterol were determined using a colorimetric kit according to the manufacturer's instructions (BioAssay Systems) (intra-assay reliability, CV%, 0·83–0·52; inter-assay reliability, CV%, 0·75–4·2). The concentrations of plasma insulin were measured using a mouse/rat insulin ELISA kit (intra-assay reliability, CV%, 0·9–8·4; inter-assay reliability, CV%, 6·0–17·9) (Millipore Iberica S.A.). The Revised Quantitative Insulin Sensitivity Check Index (R-QUICKI) was computed as described previously( Reference Caimari, del Bas and Crescenti 26 ).
Extraction and quantification of lipids
Lipids were extracted from the liver (80 mg) and dried faeces (100 mg) of hamsters using the methods described previously( Reference Caimari, del Bas and Crescenti 26 ). TAG and total phospholipids were quantified using colorimetric kits (QCA), and cholesterol was quantified by GC–MS/MS. Briefly, samples were lyophilised, ground and subjected to solid–liquid extraction using methanol–chloroform (2:1) as an extractant. Samples and cholesterol standards (Sigma Aldrich Química SA) were dried under N2 steam, derivatised with N-methyl-N-(trimethylsilyl)trifluoroacetamide and further diluted with hexane. Cholesterol analysis was carried out using a 7890A GC coupled to a 7890A QqQ/MS (Agilent Technologies). Chromatographic column used was a HP-5MS 5 % phenyl 95 % polydimethylsiloxane (Agilent Technologies). He gas of 9·99995 % purity was used as a carrier gas, at a constant flow of 1 ml/min. Sample volume was 1 μl and the total run time was 20 min. Ionisation was done by electronic impact. Acquisition was done in selected-ion monitoring (SIM) mode, using 329 m/z as a quantifier ion and 368 and 458 m/z as the qualifier ions. The reproducibility of the method was estimated with a relative standard deviation (%) of 3·64. The retention of cholesterol was computed as the difference between ingested and excreted cholesterol amounts during 48 h.
Bile composition analysis
Bile was used for the quantification of total BA by a method based on the 3α-hydroxysteroid dehydrogenase activity (Spinreact) (intra-assay reliability, CV%, 2·69–1·2; inter-assay reliability CV%, 5·17–1·33) and cholesterol (intra-assay reliability, CV%, 0·71–1·08; inter-assay reliability, CV%, 1·24–0·76) using colorimetric kits (Spinreact and QCA, respectively) according to the manufacturer's instructions.
Liver histology
Morphometric analysis of the liver was carried out as described previously( Reference García, Palou and Sánchez 27 ). Macrovesicular steatosis was evaluated according to the method of Brunt et al. ( Reference Brunt, Janney and Di Bisceglie 28 ) by estimating the percentage of area covered by fat droplets using a score graded from 0 to 3 (0, null; 1, steatosis detected in up to 30 % of the microscopic fields; 2, steatosis detected in between 30 and 60 % of the fields; and 3, steatosis detected in more than 60 % of the fields).
Statistical analysis
Data are expressed as means with their standard errors. For endpoint measurements, differences among the three experimental groups were assessed using one-way ANOVA. The homogeneity of variance assumption was confirmed by Levene's test. When the homogeneity of variances was not confirmed, a Greenhouse–Geisser correction was applied for one-way ANOVA (where indicated). When the one-way ANOVA indicated significant differences among the groups, post hoc contrasts were applied using the Bonferroni method. Results of the post hoc contrasts are shown in the figures and tables using lower-case letters. Student's t test was used where indicated exclusively to compare the means of two experimental groups. In the case of parameters measured at different time points, a mixed-design ANOVA based on a univariate type III repeated-measures ANOVA was used, setting time as the within-subjects variable to assess the effects of the treatments over time and the three experimental groups (STD, HFD and HFD+PS) as the between-subjects variable. The homogeneity of variances was confirmed by Mauchly's test. Results of the repeated-measures ANOVA are reported in the figures with capital letters indicating significant differences between the measures (T), significant differences among the experimental groups (G) or a significant interaction between the two (T × G) where indicated. The level of statistical significance was set at P< 0·05 (two-tailed) for all the tests. All the statistical tests were conducted using the software R version 3.1.0 for Windows (R Project for Statistical Computing).
Multivariate clustering analysis
Principal component analysis and partial least-squares discriminant analysis (PLS-DA) were carried out using the statistical software Multibase 2013 (Numerical Dynamics). The resulting scores for the three first components were plotted on a 3D representation using the software R version 3.1.0 for Windows (R Project for Statistical Computing).
Results
Body and tissue weights
No differences were observed in food intake among the experimental groups (data not shown). Body weight curves (Fig. 1(A)), compared using repeated-measures ANOVA, revealed significant changes with time (F 14,378= 27·8, P <0·001), but not among the experimental groups (F 2,27= 2·34, P =0·115). Nevertheless, no significant T × G interaction was observed (F 28,378= 4·05, P <0·001), as body weight changes were dependent on the diet throughout the study duration. At the end of the experiment, liver and different adipose tissues were dissected and weighed (Fig. 1(B)). The weight of the different adipose tissue depots in the HFD groups, either with or without PS, was slightly increased, but changes were not statistically significant as revealed by one-way ANOVA. In contrast, liver weights differed significantly among the experimental groups (F 2,27= 14·2, P <0·001). The post hoc contrasts indicated that the liver weights of the HFD group were significantly increased by 35 % compared with those of the STD group (P <0·001) and the HFD+PS group (P =0·009). No differences were observed between the HFD+PS and the STD groups (P =0·150). Liver enlargement observed in the HFD group was accompanied by a clear steatotic profile (Fig. 1(C)), but not in the other two groups. Accordingly, histological analysis (Fig. 1(D)) revealed grade 3 microvesicular steatosis with no apparent fibrosis in the livers of the HFD group, while the degree of steatosis in the HFD+PS group was similar to that in the STD group. As expected, the hepatic lipid profile was different among the experimental groups (Fig. 1(E)). Significant differences (F 2,27= 25·64, P <0·001) were observed in hepatic total cholesterol concentrations between the HFD and the other two groups (P <0·001 for both), though no significant differences could be observed between the STD and the HFD+PS groups (P =1). Hepatic TAG concentrations were significantly different among the experimental groups (F 2,27= 7·317, P =0·003), with the HFD group having higher concentrations than the STD and the HFD+PS groups (P =0·006 and P =0·015, respectively), but not between the HFD+PS and the STD groups (P =1). The concentrations of phospholipids were significantly different among the experimental groups (F 2,27= 3·841, P =0·034), with the highest concentrations being detected in the HFD group than in the STD group (P =0·033) and the HFD+PS group (P =0·047), but not between the STD and the HFD+PS groups (P =1).

Fig. 1 (A) Evolution of body weight in hamsters fed a standard diet (STD, ![]() ) or a high-fat diet without phytosterols (HFD,
) or a high-fat diet without phytosterols (HFD, ![]() ) or a HFD with phytosterols (HFD+PS,
) or a HFD with phytosterols (HFD+PS, ![]() ). The arrow denotes the PS treatment starting point. Values are means (n 10), with their standard errors represented by vertical bars. T denotes a significant effect of time (P< 0·001) and T × G denotes the significant interaction of time and group (P< 0·001) after a repeated-measures ANOVA comparison. Homogeneity of variances was confirmed by Mauchly's test. (B) Weights of the white adipose tissues (retroperitoneal white adipose tissue (RWAT), inguinal white adipose tissue (IWAT), mesenteric white adipose tissue (MWAT) and epididymal white adipose tissue (EWAT)) and livers of the STD (
). The arrow denotes the PS treatment starting point. Values are means (n 10), with their standard errors represented by vertical bars. T denotes a significant effect of time (P< 0·001) and T × G denotes the significant interaction of time and group (P< 0·001) after a repeated-measures ANOVA comparison. Homogeneity of variances was confirmed by Mauchly's test. (B) Weights of the white adipose tissues (retroperitoneal white adipose tissue (RWAT), inguinal white adipose tissue (IWAT), mesenteric white adipose tissue (MWAT) and epididymal white adipose tissue (EWAT)) and livers of the STD (![]() ), HFD (■) and HFD+PS (□) groups. Values are means (n 10), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; Bonferroni post hoc test when one-way ANOVA revealed significant differences among the groups). Homogeneity of variances was confirmed by Levene's test. (C) Livers of the STD, HFD and HFD+PS groups (one animal representative of each group is shown). (D) Histology (haematoxylin and eosin staining, 20 × , scale bar 100 μm) of representative liver sections of the STD, HFD and HFD+PS groups. (E) Hepatic concentrations of cholesterol (CHOL), TAG and phospholipids (PL). Values are means (n 10), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; Bonferroni post hoc test when one-way ANOVA revealed significant differences among the groups). Homogeneity of variances was confirmed by Levene's test. The level of statistical significance was set at P< 0·05 (two-tailed) for all the statistical tests. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
), HFD (■) and HFD+PS (□) groups. Values are means (n 10), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; Bonferroni post hoc test when one-way ANOVA revealed significant differences among the groups). Homogeneity of variances was confirmed by Levene's test. (C) Livers of the STD, HFD and HFD+PS groups (one animal representative of each group is shown). (D) Histology (haematoxylin and eosin staining, 20 × , scale bar 100 μm) of representative liver sections of the STD, HFD and HFD+PS groups. (E) Hepatic concentrations of cholesterol (CHOL), TAG and phospholipids (PL). Values are means (n 10), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; Bonferroni post hoc test when one-way ANOVA revealed significant differences among the groups). Homogeneity of variances was confirmed by Levene's test. The level of statistical significance was set at P< 0·05 (two-tailed) for all the statistical tests. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Plasma metabolites and cholesterol balance
Plasma cholesterol and TAG concentrations were determined at three different time points (Fig. 2). On day 15, the HFD group exhibited a significant (20 %) increase in serum cholesterol concentrations (P <0·001; Student's t test) and a 35 % increase in TAG concentrations (P =0·003; Student's t test) compared with the STD group, confirming that the HFD animals had developed combined hyperlipidaemia. At that point, PS were introduced into the diets of the HFD+PS group for the remainder of the study duration. Repeated-measures ANOVA revealed serum total cholesterol (Fig. 2(A)) concentrations to be significantly different among the experimental groups (F 2,27= 20·7, P <0·001) and throughout the study duration (F 2,54= 4·9, P =0·01). A significant T × G interaction was observed (F 4,54= 10·8, P< 0·001), indicating that the change in cholesterol concentrations was dependent on the diet. Serum TAG concentrations (Fig. 2(B)) differed significantly among the experimental groups (F 2,27= 5·0, P =0·01), but not throughout the study duration (F 2,54= 0·2, P =0·79), and no significant T × G interaction was observed.

Fig. 2 Relative quantification of (A) serum cholesterol and (B) TAG concentrations in normolipidaemic hamsters (standard diet (STD), ![]() ) and dyslipidaemic hamsters fed a high-fat diet without phytosterols (HFD, ■) or a HFD with phytosterols (HFD+PS, □) on days 15, 36 and 57 of the experiment. Values are means (n 10), with their standard errors represented by vertical bars. T denotes a significant effect of time (P< 0·001), G denotes a significant effect among the experimental groups (P< 0·001), and T × G denotes the significant interaction of time and group (P< 0·001) after a repeated-measures ANOVA comparison. Homogeneity of variances was confirmed by Mauchly's test. The level of statistical significance was set at P< 0·05 (two-tailed) for all the tests.
) and dyslipidaemic hamsters fed a high-fat diet without phytosterols (HFD, ■) or a HFD with phytosterols (HFD+PS, □) on days 15, 36 and 57 of the experiment. Values are means (n 10), with their standard errors represented by vertical bars. T denotes a significant effect of time (P< 0·001), G denotes a significant effect among the experimental groups (P< 0·001), and T × G denotes the significant interaction of time and group (P< 0·001) after a repeated-measures ANOVA comparison. Homogeneity of variances was confirmed by Mauchly's test. The level of statistical significance was set at P< 0·05 (two-tailed) for all the tests.
Analysis of serum metabolites at the end of the experiment (Table 2) indicated significant changes in total cholesterol concentrations among the experimental groups (F 2,27= 36·9, P <0·001) due to increased concentrations being detected in the HFD group compared with those in the STD and the HFD+PS groups (P <0·001 for both), but not between the STD and the HFD+PS groups (P =0·31). The same pattern was observed for the concentrations of HDL-cholesterol (F 2,27= 4·9, P =0·02) and VLDL+LDL-cholesterol (F 2,27= 19·5, P <0·001). The post hoc contrasts confirmed that the differences were due to the HFD group, which had higher concentrations of all the fractions compared the STD and the HFD+PS groups (P <0·05 for all the cases), while no differences were observed between the STD and the HFD+PS groups. Similarly, plasma TAG concentrations were found to be significantly altered (F 2,27= 7·6, P =0·003). The post hoc contrasts indicated that the 56 % increase in the HFD group was significantly higher than that in the STD and the HFD+PS groups (P =0·005 and P =0·013, respectively), but no significant differences were observed between the STD and the HFD+PS groups (P =0·98). No significant differences were observed in plasma glucose or insulin concentrations among these groups either. Nevertheless, plasma NEFA concentrations were significantly different among the experimental groups (F 2,27= 4·9, P =0·015), presumably due to the significantly lower concentrations in the HFD+PS group compared with those in the HFD group (P =0·012), while differences between the STD and the HFD groups can only be described as a trend (P =0·102). As a consequence, the insulin resistance index calculated as R-QUICKI indicated reduced insulin sensitivity in the HFD group with respect to the STD and the HFD+PS groups, despite the fact that no statistical significance was revealed by one-way ANOVA.
Table 2 Plasma concentrations of metabolites in normolipidaemic hamsters fed a standard diet (STD) and dyslipidaemic hamsters fed a high-fat diet (HFD) and a HFD supplemented with phytosterols (HFD+PS)* (Mean values with their standard errors, n 10)
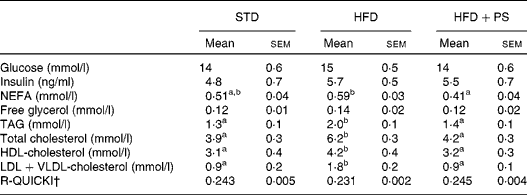
R-QUICKI, Revised Quantitative Insulin Sensitivity Check Index.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05; one-way ANOVA using a Bonferroni post hoc contrast with a CI of 0·95).
* Serum metabolite concentrations were determined at the end of the study after 6 h of fasting in blood samples collected by cardiac puncture.
† R-QUICKI was computed as indicated in the Materials and methods section.
Quantification of cholesterol in the diets and faeces (Table 3) revealed that cholesterol consumption patterns differed significantly among the experimental groups (F 2,27= 177, P <0·001; Greenhouse–Geisser corrected). This difference was due to increased cholesterol consumption in the HFD and the HFD+PS groups compared with that in the STD group (P <0·001 for both pairwise comparisons), while no differences were observed between the HFD and the HFD+PS groups (P =0·92). Nevertheless, the significant change in cholesterol excretion (F 2,27= 63, P <0·001; Greenhouse–Geisser correction) was due to differences between the HFD+PS group and both the STD and the HFD groups (P <0·001 for both pairwise comparisons). These changes were reflected in differences in cholesterol retention (F 2,27= 64·7, P <0·001; Greenhouse–Geisser correction), with the HFD+PS group displaying higher cholesterol retention than the STD group (P =0·009), though lower than that in the HFD group (P <0·001). In turn, the HFD group also differed significantly from the STD group with respect to cholesterol retention (P <0·001).
Table 3 Cholesterol intake and excretion in normolipidaemic hamsters fed a standard diet (STD) and dyslipidaemic hamsters fed a high-fat diet (HFD) or a HFD supplemented with phytosterols (HFD+PS)* † (Mean values with their standard errors, n 10)
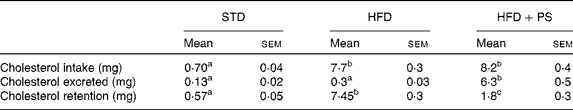
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05; one-way ANOVA using a Bonferroni post hoc contrast with a CI of 0·95).
* Chow intake was monitored and faeces were collected for 48 h. Cholesterol in the diets and faeces was quantified by GC.
† Data were analysed by a one-way ANOVA using a Greenhouse–Geisser correction when the homogeneity of variance assumption was discarded by Levene's test.
Expression of lipogenic genes in the liver and retroperitoneal white adipose tissue
As has been reported, PS protected against hepatic lipid accumulation and liver enlargement induced by the HFD. Therefore, we analysed the expression of genes involved in fatty acid synthesis, esterification and oxidation. There were no changes in the expression of lipogenic genes acetyl-CoA carboxylase (ACC1) and diacylglycerol O-acyltransferase 2 (DGAT2) in the liver of all the experimental groups (Fig. 3(A)). In contrast, there were significant changes in the expression of fatty acid synthase (FASN) and stearoyl-CoA desaturase-1 (SCD1) (F 2,27= 5·2, P =0·01 and F 2,27= 3·9, P =0·03; respectively). Despite applying the post hoc contrasts, no differences were observed in the expression of FASN between the HFD and the HFD+PS groups (P =1); in the case of SCD1, a clear tendency (P =0·098) to recover the normal expression levels was observed in these groups. Clear differential effects were observed in the expression of β-oxidation-regulatory genes carnitine palmitoyltransferase-Iβ (CPT1B) (F 2,27= 6·3, P =0·006), PPARA (F 2,27= 12·2, P <0·001) and phosphoenolpyruvate carboxykinase 1 (PCK1) (F 2,27= 10·8, P <0·001). The post hoc contrasts indicated the HFD+PS group to differ significantly from the HFD group (P <0·05) but not from the STD group with respect to the expression of these three genes. In contrast to our findings in the liver, no differences were observed in the expression of these genes in the retroperitoneal white adipose tissue between the HFD and the HFD+PS groups, despite a clear effect of the HFD either with or without PS being observed (Fig. 3(B)).

Fig. 3 Expression levels of genes related to fatty acid and glycerolipid metabolism in the (A) liver and in the (B) retroperitoneal adipose tissue of normolipidaemic hamsters (standard diet (STD), ![]() ) and dyslipidaemic hamsters fed a high-fat diet without phytosterols (HFD, ■) or a HFD with phytosterols (HFD+PS, □). Gene expression was quantified by real-time quantitative PCR using β-actin (ACTB) as the endogenous control. Values are means (n 10), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; Bonferroni post hoc contrast when one-way ANOVA revealed significant differences among the groups). Homogeneity of variances was confirmed by Levene's test. The level of statistical significance was set at P< 0·05 (two-tailed) for all the tests. The genes analysed were acetyl-CoA carboxylase (ACC1), fatty acid synthase (FASN), diacylglycerol O-acyltransferase 2 (DGAT2), stearoyl-CoA desaturase-1 (SCD1), carnitine palmitoyltransferase-Iα (CPT1A), carnitine palmitoyltransferase-Iβ (CPT1B), PPARA, and phosphoenolpyruvate carboxykinase 1 (PCK1).
) and dyslipidaemic hamsters fed a high-fat diet without phytosterols (HFD, ■) or a HFD with phytosterols (HFD+PS, □). Gene expression was quantified by real-time quantitative PCR using β-actin (ACTB) as the endogenous control. Values are means (n 10), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; Bonferroni post hoc contrast when one-way ANOVA revealed significant differences among the groups). Homogeneity of variances was confirmed by Levene's test. The level of statistical significance was set at P< 0·05 (two-tailed) for all the tests. The genes analysed were acetyl-CoA carboxylase (ACC1), fatty acid synthase (FASN), diacylglycerol O-acyltransferase 2 (DGAT2), stearoyl-CoA desaturase-1 (SCD1), carnitine palmitoyltransferase-Iα (CPT1A), carnitine palmitoyltransferase-Iβ (CPT1B), PPARA, and phosphoenolpyruvate carboxykinase 1 (PCK1).
We also used a multivariate approach including the expression results of genes ACC1, FASN, DGAT2, SCD1, CPT1A, PPAR and PCK1 to assess the clustering trends of the animals. W chose a principal component analysis as an unsupervised (Fig. 4(A)) and a PLS-DA as a supervised (Fig. 4(B)) clustering analysis. The explained variance was 81·9 % for the principal component analysis and 62·8 % for the PLS-DA.

Fig. 4 Results of multivariate analysis of hepatic gene expression. Expression values of the genes acetyl-CoA carboxylase (ACC1), fatty acid synthase (FASN), diacylglycerol O-acyltransferase 2 (DGAT2), stearoyl-CoA desaturase-1 (SCD1), carnitine palmitoyltransferase-Iα (CPT1A), PPAR and phosphoenolpyruvate carboxykinase 1 (PCK1) in the livers of normolipidaemic hamsters (STD, ![]() ) and dyslipidaemic hamsters fed a high-fat diet without phytosterols (HFD,
) and dyslipidaemic hamsters fed a high-fat diet without phytosterols (HFD, ![]() ) or a HFD with phytosterols (HFD+PS,
) or a HFD with phytosterols (HFD+PS, ![]() ) were used for an unsupervised clustering analysis by a principal components analysis (PCA) and for a supervised clustering analysis by a partial least-squares discriminant analysis (PLS-DA). The resulting scores for components (Comp) 1, 2 and 3 of each animal are given for the (A) PCA and (B) PLS-DA. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
) were used for an unsupervised clustering analysis by a principal components analysis (PCA) and for a supervised clustering analysis by a partial least-squares discriminant analysis (PLS-DA). The resulting scores for components (Comp) 1, 2 and 3 of each animal are given for the (A) PCA and (B) PLS-DA. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Effects of phytosterols on the metabolism of bile acids
The observed liver-specific changes prompted us to study an exclusive hepatic process tightly linked to lipid metabolism and energy homeostasis such as BA synthesis. Analysis of the bile revealed that biliary cholesterol content (Fig. 5(A)) was significantly different among the experimental groups (F 2,27= 4·8, P =0·016), mainly due to differences between the HFD and the STD groups (P =0·045), despite the fact that the HFD+PS group had lower, though non-significant, levels compared with the HFD group. Biliary total BA content (Fig. 5(B)) exhibited significant changes (F 2,27= 3·4, P= 0·048) due to increased levels being detected in the HFD and the HFD+PS groups with respect to the STD group (P =0·043 and P =0·042, respectively). The BA:cholesterol ratio (Fig. 5(C)) was significantly affected (F 2,27= 6·4, P =0·005) mainly due to the 2-fold increase in the ratio of the HFD+PS group compared with that of the HFD group (P =0·006). We quantified the mRNA levels of hepatic CYP7A1 and found the expression of CYP7A1 to be 2-fold higher in the HFD+PS group than in the HFD group (Fig. 5(D)), although these changes were not significant when the three experimental groups were compared using one-way ANOVA (F 2,27= 2·45, P =0·105). We also analysed the gene expression of farnesoid X receptor (FXR), small heterodimer partner (SHP) and hepatocyte nuclear factor-4α (HNF4A). The expression of HNF4A was found to be significantly altered (F 2,27= 3·9, P =0·03), mainly in the STD and the HFD groups (P =0·036), but no differences were observed between the HFD+PS and the HFD groups (P =0·149) or the STD group (P =0·987). In turn, the expression of FXR and SHP was not altered by PS.

Fig. 5 Quantification of (A) cholesterol and (B) total bile acids (TBA) in the bile obtained from the gall bladder of normolipidaemic hamsters (STD) and dyslipidaemic hamsters fed a high-fat diet without phytosterols (HFD) or a HFD with phytosterols (HFD+PS). (C) TBA:total cholesterol ratio computed as described in the Materials and methods section. (D) Expression levels of genes related to bile acid metabolism in the liver of hamsters quantified by real-time quantitative PCR using β-actin (ACTB)) as the endogenous control. ![]() , STD; ■, HFD; □, HFD+PS. Values are means (n 10), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; Bonferroni post hoc test when one-way ANOVA revealed significant differences among the groups). Homogeneity of variances was confirmed by Levene's test. The level of statistical significance was set at P< 0·05 (two-tailed) for all the tests. The genes analysed were cholesterol-7α-hydroxylase (CYP7A1), farnesoid X receptor (FXR), small heterodimer partner (SHP) and hepatocyte nuclear factor-4α (HNF4A).
, STD; ■, HFD; □, HFD+PS. Values are means (n 10), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; Bonferroni post hoc test when one-way ANOVA revealed significant differences among the groups). Homogeneity of variances was confirmed by Levene's test. The level of statistical significance was set at P< 0·05 (two-tailed) for all the tests. The genes analysed were cholesterol-7α-hydroxylase (CYP7A1), farnesoid X receptor (FXR), small heterodimer partner (SHP) and hepatocyte nuclear factor-4α (HNF4A).
Discussion
Different studies have shown that PS might have additional beneficial properties apart from their hypocholesterolaemic activity, mainly plasma TAG-lowering or insulin resistance-ameliorating properties. In the present study, we investigated the potential beneficial effects of soyabean PS on hepatic and blood lipids of golden hamsters with diet-induced combined hyperlipidaemia.
Currently, it is well accepted that PS inhibit the intestinal absorption of dietary cholesterol( Reference De Smet, Mensink and Plat 2 ). The results of the present study are in good agreement with the different mechanisms proposed, as the inclusion of PS into the HFD resulted in a 20-fold increase in faecal cholesterol content. However, additionally, we found evidence pointing to a supplementary mechanism of action in the hamster. HFD feeding followed by PS supplementation in hamsters led to a remarkable increase in the biliary BA:cholesterol ratio, induced the expression of CYP7A1, which governs the synthesis of BA and controls its activity at the transcriptional level( Reference Davis, Miyake and Hui 8 ), and normalised the expression of HNF4A, a key controller of BA metabolism( Reference Inoue, Yu and Yim 29 – Reference Hayhurst, Lee and Lambert 31 ), indicating that PS enhanced BA synthesis. Additionally, it is worth considering that cholesterol retention in HFD+PS animals was still higher than that in STD animals, while the hepatic cholesterol concentrations were similar in both groups. Altogether, these results indicate that the increased excretion of cholesterol via BA represents an additional mechanism of cholesterol reduction in the liver, thus explaining the striking protection against fatty liver development. In contrast to our findings, treatment of mice with β-sitosterol, which represents 38 % of the PS used in the present experiment, has been found to decrease the faecal excretion of BA( Reference Uchida, Takase and Nomura 32 ). In Wistar–Kyoto and Wistar rats, stigmasterol, another PS present in the diets used in the present experiment, has been found to decrease hepatic cholesterol synthesis and, in parallel, reduce BA synthesis( Reference Batta, Xu and Honda 33 ). However, the results of the present study are in agreement with those of previous studies showing increased BA synthesis and altered BA composition in hamsters treated with soyabean sterol esters( Reference Carr, Cornelison and Illston 10 ). The possible explanation for the contrasting data can be the inherent differences in the animal models used. In hamsters and humans, the expression of CYP7A1 is not controlled by the concentrations of hepatic cholesterol as tightly as in rats and mice( Reference Horton, Cuthbert and Spady 34 , Reference Goodwin, Watson and Kim 35 ). As expected, BA synthesis was not coupled to hepatic cholesterol concentrations in hamsters in the present study. As hamsters and humans share many common features related to cholesterol and BA metabolism( Reference Zhang, Wang and Jiao 21 ), additional studies carried out in human subjects could shed some additional light on the effects of PS therapy on BA metabolism. Interestingly, it has been reported that the cholesterol-lowering response to PS intake in humans is conditioned by a more active variant ( − 204A>C) of the CYP7A1 gene promoter( Reference De Castro-orós, Pampín and Mozas 12 ). Therefore, the effectiveness of PS therapy in humans depends, at least in part, on the modulation of CYP7A1 gene expression. Nevertheless, it should be considered that the effects of PS on the excretion of cholesterol could take place through different pathways, such as dietary or hepatobiliary absorption to plasma as well as transintestinal cholesterol excretion( Reference De Smet, Mensink and Plat 2 – Reference Jia, Ebine and Demonty 7 ). We currently do not know the contribution of the BA synthesis pathway to the observed effects of PS and whether these effects might be due to the direct or indirect actions of PS in the liver. Therefore, further research is required to determine the exact mechanisms, the quantitative contribution of each pathway and the relevance of the BA synthesis pathway to the effects of PS.
The HFD-fed animals had fatty liver as confirmed by the clear accumulation of lipids. This phenotype was accompanied by changes in the expression of genes that have previously been reported to be related to fatty liver in different models. It has previously been reported that mice lacking or with a low expression of hepatic PCK1 can develop severe fatty liver due to impaired control of energy homeostasis, involving the imbalance of cataplerotic and anaplerotic processes( Reference She, Shiota and Shelton 36 ) and the subsequent impairment of β-oxidation. In the model used in the present study, HFD feeding resulted in a reduced expression of hepatic β-oxidation-regulatory genes, such as PPARA and CPT1A. Despite the debatable relevance of β-oxidation impairment in the development of fatty liver, the results of the present study indicate that this process might play an important role under the present experimental conditions. Together with these changes, a slight induction of SCD1 expression was observed, while the expression of other lipogenic genes remained unaltered. These results are in agreement with the results of previous studies showing that SCD1 is the lipogenic enzyme responding at the gene expression level in the fatty livers of golden hamsters( Reference Basciano, Miller and Naples 37 ). A similar pattern was observed in the expression of HNF4A. It has been shown that this nuclear receptor is essential for several hepatic processes that were found to be altered in the model used in the present study, such as lipid synthesis, oxidation and secretion or BA synthesis( Reference Inoue, Yu and Yim 29 , Reference Hayhurst, Lee and Lambert 31 ). These results can explain, at least partially, the excessive accumulation of TAG and phospholipids due to impaired fatty acid oxidation and increased fatty acid synthesis, together with excess dietary fat reaching the liver during the postprandial state. The addition of PS to the HFD normalised the hepatic lipid profile and the expression of the above-mentioned genes.
Differences in the expression of fatty acid metabolism-related genes were statistically significant, but quantitatively mild when analysed individually. Therefore, we used two widely accepted multivariate clustering techniques, principal component analysis and PLS-DA, to assess whether the sum of these mild differences could be translated into clear contrasts. The outcome revealed that the HFD+PS-fed animals were partially similar to the STD-fed animals and significantly differed from the HFD-fed animals if all the analysed genes of fatty acid metabolism were used as clustering criteria. The clustering assay, therefore, reinforces and is consistent with the protection provided by the PS treatment against hepatic fatty acid metabolism. Explanation for these results could be found in the striking decrease in the hepatic cholesterol pool observed in the HFD+PS group. Previous studies have shown that a HFD and a high-fructose diet do not induce hepatic fat accumulation in hamsters, but that the addition of cholesterol to this background diet results in reduced PPARα protein expression, increased lipogenesis and fatty liver development( Reference Basciano, Miller and Naples 37 ). According to these data, a significant decrease in hepatic cholesterol pool is sufficient to prevent the development of fatty liver in hamsters. In fact, it has been suggested that excessive dietary cholesterol can trigger a cascade of events resulting in the impairment of hepatic fatty acid metabolism and, eventually, in fat accumulation in the liver of a broad range of mammalian species( Reference Enjoji, Yasutake and Kohjima 38 ).
Another relevant finding is the decrease in fasting plasma TAG and NEFA concentrations in the HFD+PS group with respect to the HFD group. This is in agreement with the results of previous studies in human subjects( Reference Demonty, Ras and van der Knaap 13 ) and hamsters( Reference Liang, Wong and Guan 4 ). In the present experiment, the normalisation of fasting serum TAG concentrations by PS was a long-term effect, occurring between day 20 and day 40 of the treatment. In contrast, serum cholesterol concentrations decreased before day 20 of the treatment. These results indicate that the normalisation of plasma TAG concentrations by PS depends, at least in part, on the normalisation of hepatic lipogenesis and β-oxidation processes. In this scenario, the hepatic clearance of TAG in dyslipidaemic hamsters treated with PS might be slow, depending on fatty acid synthesis, increased fatty acid oxidation and the shuttling of TAG into VLDL for secretion. Consequently, this gradual removal of TAG might not be reflected in plasma VLDL concentrations until the hepatic concentrations of TAG are normalised. Nevertheless, other possibilities cannot be ruled out, as it has been shown that the intake of different stanols and sterols can modulate the mobilisation of hepatic fat. Thus, PS intake may enhance energy expenditure and hepatic β-oxidation in rats( Reference Ikeda, Konno and Shimizu 18 ). In humans, plant stanols are more efficient at reducing the release of TAG-rich VLDL from the liver in dyslipidaemic patients than in normolipidaemic subjects( Reference Plat and Mensink 15 ) and, according to the authors, this observation fits with an enhancement of the hepatic β-oxidation programme. The results of the present study are in agreement with those of these studies, as PS caused a remarkable decrease in serum NEFA concentrations and a parallel increase in the expression of PPARA and CPT1A in the HFD-fed animals. Therefore, it is plausible to hypothesise that PS can enhance the oxidation of fatty acids in the liver, and perhaps in other tissues, contributing to a reduction of the overall pool of NEFA and TAG. Nevertheless, more research is still required to elucidate the exact mechanism leading to these effects. Increased concentrations of circulating NEFA have been proposed as a key factor for the development of insulin resistance( Reference Boden 39 ). Despite the suggested beneficial effects of PS consumption on insulin resistance via decreased circulating NEFA concentrations, more research is required to assess these effects.
Currently, combined hyperlipidaemia represents a risk factor for the development of CVD and is a hallmark of the metabolic syndrome, an altered state with a growing prevalence worldwide( Reference Alberti, Zimmet and Shaw 40 ). The prevalence of fatty liver or hepatic steatosis is also increasing, accompanied by other altered states such as obesity and/or insulin resistance( Reference Farese, Zechner and Newgard 41 ). The results of the present study, together with those of the other studies carried out in human subjects, indicate that long-term consumption of PS might provide remarkable protection to hepatic lipid metabolism against the deleterious effects of cholesterol and fatty acid overload, placing PS as effective agents against fatty liver and combined hyperlipidaemia development, including cholesterol, TAG and NEFA. At least two mechanisms could be responsible for these effects: first, increased faecal cholesterol excretion, which is in agreement with the classically accepted mechanism of action ascribed to PS and, second, the identified supplementary mechanism based on an increased efflux of cholesterol into the BA synthesis pathway. Even though we currently do not know the quantitative contribution of the BA synthesis pathway to the hypocholesterolaemic effects of PS, the results of the present study indicate that analysis of BA-related parameters in human trials including PS should be considered in future studies. In conclusion, the present study indicates that PS may be considered as promising agents in the treatment of lipid metabolism alterations beyond the reduction of blood cholesterol concentrations.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514001342
Acknowledgements
The authors cordially thank Mr David Moriñas for statistical support, Mr Ignasi Papell for management support and also Vanessa Grifoll and Silvia Pijuan, the laboratory technicians.
A. Caimari, J. L., L. A., F. P. and J. M. d. B. designed the research. S. L., A. Caimari, A. Crescenti and J. M. d. B. carried out the animal treatment, sample collection and analyses. J. M. d. B., A. Caimari and S. L. carried out the discussion of the results. J. L and J. M. d. B. wrote the paper.
J. L. is an employee of Lipotec Company. This does not alter the authors' adherence to all the British Journal of Nutrition policies on sharing data and materials.










