TAG-rich lipoproteins (TRL) represent a very heterogeneous group of particles consisting of chylomicrons (CM), VLDL and their remnants. CM are assembled in the intestine and mainly contain lipids derived from the diet together with a single molecule of apoB48, while VLDL are generated in the liver and require the presence of apoB100. Once released in the bloodstream, these two types of particles compete for the process of TAG depletion by lipoprotein lipase( Reference Karpe and Hultin 1 ) and for the hepatic pathway of particle clearance mediated by receptors that recognise apoE, mainly LDL receptor (LDLR) and LDLR-related protein (LRP)( Reference Karpe, Humphreys and Samra 2 ). Therefore, plasma concentrations of TRL are determined by the rates of secretion from the intestine and liver as well as by the rates of catabolism of CM and VLDL( Reference Ginsberg 3 ).
The transport and metabolism of TRL, especially during the postprandial period, have been reported to be associated with atherosclerosis( Reference Patsch, Miesenböck and Hopferwieser 4 , Reference Ginsberg and Illingworth 5 ). TRL and their remnants can penetrate the arterial wall, where they remain trapped and are taken up by macrophages, causing lipid accumulation, which leads to the formation of foam cells and eventually to the formation of the fatty streak, the first visible lesion in atherogenesis. In previous studies, CM remnants have been shown to induce the formation of foam cells in a variety of macrophage populations, including those derived from the human monocyte cell line THP-1( Reference Batt, Avella and Moore 6 – Reference Moore, Bejta and Avella 8 ). The accumulation of cholesterol and TAG in these cells is influenced by the fatty acid composition of the remnants, which modulates their atherogenicity, regardless of their oxidative state( Reference Moore, Napolitano and Avella 7 ).
In addition to the macrophage-secreted lipoprotein lipase hydrolysis of TRL and the uptake of the released fatty acids, lipids are believed to enter macrophages by the uptake of whole particles via receptor-mediated pathways( Reference Napolitano and Bravo 9 ). Candidates involved in these pathways include LDLR and LRP, as well as VLDL receptor (VLDLR) and non-apoE-dependent receptors, such as the scavenger receptors A2 (SRA2), B1 (SRB1) and CD36, the major members of the scavenger receptor family expressed in macrophages( Reference Yu and Cooper 10 – Reference Goulter, Avella and Elliott 12 ). Direct phagocytosis has also been proposed as a mechanism for the uptake of TRL by macrophages, although it is thought to play only a minor role( Reference Yu and Mamo 13 ). Evidence from previous experiments with THP-1 macrophages suggests that CM remnants are mainly taken up by LRP and LDLR( Reference Bejta, Moore and Avella 14 ). However, the exact mechanisms by which TRL cause lipid accumulation in macrophages are not yet completely defined.
The processes by which TRL are taken up by macrophages are likely to influence the amount of lipids accumulated intracellularly. It is well known that the lipid composition of postprandial TRL affects their particle size and also influences the mechanisms related to their internalisation and intracellular accumulation( Reference Botham and Wheeler-Jones 15 – Reference De Pascale, Avella and Perona 17 ). Previously, it was thought that larger lipoproteins cannot penetrate the arterial intima and are, therefore, not available for uptake by macrophages in the subendothelial space( Reference Karpe 18 ), but now there is evidence indicating that CM-sized particles can be taken up by macrophages( Reference Palmer, Nova and Anil 19 ). However, these studies have also shown that the mechanisms responsible for internalisation are affected by the TAG composition of the particles, which, in turn, affects their size.
Earlier studies have established that TRL particles isolated at different time points during the postprandial period differ in their size and lipid composition( Reference Cabello-Moruno, Martinez-Force and Montero 20 ) and thus may be taken up differentially by macrophages. In the present study, we investigated the effects of TRL obtained from healthy subjects at 2, 4 and 6 h postprandially after a fat challenge on lipid accumulation and foam cell formation in macrophages derived from the human monocyte cell line THP-1. We also investigated the effect of postprandial TRL isolated at different time points on the gene expression of the apoE-dependent and scavenger receptors believed to be involved in the uptake of particles by these cells.
Experimental methods
Subjects and study design
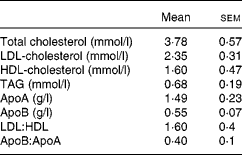
A total of nine healthy men aged 26·2 (sem 4·3) years with a BMI of 23·7 (sem 2·0) kg/m2 participated in the study. Subjects were excluded if they suffered from any digestive or metabolic disorder, were taking dietary supplements or were under medication of any kind. A fasting blood sample was collected to ensure that the recruited subjects had plasma TAG and glucose concentrations within normal limits (Table 1). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki of 1975 (revised in 2000), and all procedures involving human subjects were approved by the Institutional Committee on Human Research (Hospital Universitario Virgen del Rocio, Seville, Spain). Written informed consent was obtained from all subjects.
Table 1 Baseline data of normolipidaemic men who participated in the present study (Mean values with their standard errors, n 9)
The experiment was carried out for 3 weeks, with the 2nd week being the washout period during which the subjects resumed consuming their normal diet. The subjects were given 1 litre of refined olive oil 1 week before the start of the study to minimise the effect of their habitual dietary oil. On the last day of this week, the subjects consumed a meal that consisted of one slice of brown bread (28 g), one cup of skimmed yogurt (125 g) and plain pasta (100 g, cooked with 200 ml of water) with tomato sauce (130 g) previously mixed with refined olive oil (70 g). The oil provided 2587 kJ of energy, while the whole meal provided 4523 kJ, distributed as follows: 32·5 % carbohydrate; 7·6 % protein; 59·9 % fat. The refined olive oil, with oleic acid (18 : 1n-9), palmitic acid (16 : 0), linoleic acid (18 : 2n-6) and stearic acid (18 : 0) proportions of 78·7, 10·6, 5·2 and 3·3 %, respectively, was kindly supplied by Oleicola El Tejar, S.A.
The subjects were asked to consume a low-fat dinner the evening before the postprandial study and to abstain from drinking alcohol and smoking for 24 h. On arrival to the laboratory, after an overnight fast (12 h), a cubital vein was catheterised and a baseline blood sample was collected immediately before the consumption of the test meal. After the consumption of the test meal, blood samples were collected hourly for a period of 7 h. During the course of the experiment, the subjects were allowed to drink water and to undertake only light activities.
Serum was recovered by centrifugation (1620 g , 30 min, 4°C) and sodium azide, phenylmethylsulfonyl fluoride and aprotinin (Sigma-Aldrich) were added to it at a final concentration of 1 mmol/l, 10 μmol/l and 0·5 mg/l, respectively.
Isolation of postprandial TAG-rich lipoproteins
Postprandial TRL were isolated from 4·5 ml of serum collected at 2, 4 and 6 h after the consumption of the test meal. Serum was layered under 6 ml of NaCl solution (d= 1·006 kg/l), and TRL were obtained by a single ultracentrifugation spin (39 000 rpm, 18 h, 12°C). Ultracentrifugation was performed using a SW 41Ti swinging-bucket rotor in a Beckman L8-70M preparative ultracentrifuge (Beckman Instruments). Different postprandial time points were chosen for the isolation of TRL according to the hours at which maximum and minimum serum TAG concentrations are found( Reference Cabello-Moruno, Martinez-Force and Montero 20 ). TRL were rapidly frozen at − 80°C and thawed just before use. As extreme changes in temperature might cause alterations in TRL structure, particles were checked for aggregations before use in culture.
Determination of TAG-rich lipoprotein lipid class and apoB compositions
TRL obtained from 4·5 ml of serum (approximately 1 ml) were adjusted to a final volume of 1·5 ml with KCl solution (0·1 m). Total lipids in TRL were extracted according to the method of Folch et al. ( Reference Folch, Lees and Sloane-Stanley 21 ) with slight modifications. The extracted lipids were dissolved in 1 ml of chloroform–methanol (2:1, v/v). Aliquots were prepared and kept at − 20°C until use.
The lipid class composition of TRL was determined by HPLC as described by Perona & Ruiz-Gutierrez( Reference Perona and Ruiz-Gutierrez 22 ). In brief, 10 ml of lipids were dissolved in chloroform–methanol (2:1, v/v) and injected by an automatic sampler into a 2690 Alliance liquid chromatograph (Waters) controlled by the Empower System (Waters). Detection was accomplished with a model 2420 light-scattering detector (Waters). Lipid classes were separated on a LiChrospher diol column (250, 4·6, 5 mm particle sizes; Merck) using a gradient-elution system containing hexane, 2-propanol and methanol. Stock solutions of cholesteryl oleate, triolein, cholesterol and phosphatidylcholine were prepared in chloroform–methanol (2:1, v/v) and used as standards for the identification and quantification of cholesteryl esters (CE), TAG, free cholesterol (FC) and phospholipids, respectively. The precision of the method ranged from 2·4 to 4·0 % for lipid standards and from 6·8 to 11·7 % for CM obtained 4 h after the fat challenge .

Fig. 1 Microphotographs of THP-1 macrophages incubated for 24 h in the presence or absence (control) of TAG-rich lipoproteins (TRL, 15 μg total cholesterol/ml) isolated at 2, 4 and 6 h after the consumption of the test meal and stained with Oil Red O for visualising lipid accumulation. Typical images from nine experiments are shown. (a) 2 h control, (b) 2 h TRL, (c) 4 h control, (d) 4 h TRL, (e) 6 h control, and (f) 6 h TRL.
ApoB48 and apoB100 were quantified after separation by SDS–PAGE (Fig. 2). Electrophoresis was carried out using the buffer system described by Laemmli( Reference Laemmli 23 ). Gels contained a 4–15 % acrylamide gradient, 0·1 % SDS and 0·375 m-Tris. The acrylamide gradient was poured using a Hoefer SG-50 two-chamber, gravity-flow gradient maker (Hoefer). A stacking gel (5 % acrylamide, 0·1 % SDS, 2 mm-EDTA, and 0·11 m-Tris–HCl, pH 6·8) was added with a ten-slot well-forming comb. Non-delipidated lipoprotein samples (3 mg of protein) were reduced in SDS sample buffer (3 % SDS, 0·8 mm-EDTA, 5 % mercaptoethanol, 0·004 % bromophenol blue, 0·05 m-Tris–HCl, and 10 % glycerol, pH 6·8) for 3 min at 100°C. The gels were run at 60 V for 3 h. The gels were fixed with a 10 % methanol–7 % acetic acid solution for 30 min and stained overnight with SYPRO Ruby Protein Gel Stain (Molecular Probes). Destaining was achieved by 1 h wash with the 10 % methanol–7 % acetic acid solution. The two molecular weight forms of apoB were clearly separated in this gel system. Apo were identified by comparing the distance that they migrated in the gels with the distance travelled by known molecular weight standards (SigmaMarker, Wide Range (molecular weight 6·5–250·0 kDa)). The gels were scanned using the Gel Doc 1000 system (Bio-Rad) and analysed using the software Molecular Analyst version 1.6 (Bio-Rad). All samples from the same subject were run on the same gel to enable comparison after separation and staining under the same conditions. Considering that apoB100 and apoB48 exhibit the same chromogenicity( Reference Karpe and Hamsten 24 ), the relative amounts of the two isoforms were calculated from the staining intensity, and their absolute masses were calculated from the total apoB mass determined by immunoturbidimetry (Sigma Diagnostics).

Fig. 2 SDS–PAGE analysis of apoB48 and apoB100 separated from human TAG-rich lipoproteins isolated at 2, 4 and 6 h after the consumption of the test meal. MW, molecular weight markers.
Culture of THP-1 macrophages
THP-1 monocytes (3–9 × 105 cells/ml) grown in Roswell Park Memorial Institute 1640 medium supplemented with fetal bovine serum (10 % v/v), penicillin (60 μg/ml; 100 U/ml), streptomycin (100 mg/ml) and 2-mercaptoethanol (20 μm) were induced to differentiate into macrophages by incubation with phorbol 12-myristate 13-acetate (200 ng/ml) for 72 h at 37°C in 5 % CO2–95 % air. The confluency of adherent cells was 70–80 % in all cell-culture experiments, and cell viability was >95 % and was unaffected on incubation with TRL.
Oil Red O staining
Cells (3 × 105 cells/ml) were incubated with TRL (15 μg cholesterol/ml) for 24 h, washed with PBS and 60 % propan-2-ol, and then stained with Oil Red O (0·5 % (w/v) in 40 % propan-2-ol/H2O (v/v)). After 15 min, the stain was removed and cells were washed twice with PBS.
Determination of cell lipid composition
Cells (8 × 105 cells/ml) were incubated with TRL (15 μg cholesterol/ml) for 48 h and then washed with PBS and scraped off the plates with 700 ml of PBS. Cells were lysed by sonication for 5 s (two cycles) at 50 W using a Bandelin SONOPULS HD2070 apparatus (Bandelin Electronics). An aliquot of 50 ml was used to determine the protein content using the method of Bradford. Lipids were extracted using the method of Folch et al. ( Reference Folch, Lees and Sloane-Stanley 21 ). TAG, FC and CE (total cholesterol − CE) concentrations were determined by enzymatic analysis using commercial enzymatic reagent kits (Alpha Laboratories).
Analysis of mRNA
THP-1 macrophages (6 × 106 cells/ml) were incubated with TRL (15 μg cholesterol/ml) for 16 h and then total RNA was extracted (GenElute Mammalian Total RNA Kit; Sigma-Aldrich) with DNAase I treatment according to the manufacturer's instructions. RT and oligo(deoxythymidine) primers were obtained from Promega and were used for reverse transcription. The mRNA abundances of LDLR, LRP, VLDLR, CD36, SRA2, SRB1 and the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were determined by real-time PCR (quantitative PCR) using SYBR Green quantitative fluorescence (SYBR Green JumpStart Taq ReadyMix, Sigma-Aldrich) and an Opticon 2 LightCycler system (MJ Research). The forward and reverse primers and the PCR conditions employed are summarised in Table S1 (available online). C t values were determined using the automated threshold analysis (Opticon Monitor 2 software; Bio-Rad). Data were normalised using values obtained for GAPDH, and the fold change in mRNA expression in TRL-treated macrophages v. control macrophages was determined using the method of Pfaffl( Reference Pfaffl 25 ).
Statistical analyses
Results are expressed as means with their standard errors (n 9), unless otherwise stated. Statistical analyses were conducted using the GraphPad Prism® 5 statistical package (GraphPad Software, Inc.). Statistical significance of postprandial changes was analysed using one-way ANOVA followed by Tukey's multiple comparison test. Differences were considered statistically significant at P< 0·05.
Results
Lipid class and apoB compositions of postprandial TAG-rich lipoproteins
The lipid class composition of TRL isolated at 2, 4 and 6 h after the consumption of the test meal is summarised in Table 2. The proportion of TAG in TRL was highest at 2 h and declined subsequently, becoming significantly lower at 6 h when compared with that at 2 and 4 h. The reduction of TAG content resulted in a proportional significant increase in CE and phospholipid concentrations at 4 and 6 h. The relative proportions of FC and total cholesterol in TRL remained unaltered throughout the postprandial period. Both apoB48 and apoB100 were quantified in postprandial TRL, and the highest concentrations were observed at 2 h and the lowest at 4 h. The TAG:apoB100 and TAG:apoB48 ratios were used as estimates of the particle size at different postprandial time points. The highest ratio (largest particles) was found at 2 h and the lowest ratio (smallest particles) at 6 h, with the latter being significantly different from the ratios at 2 and 4 h.
Table 2 Lipid class composition, apoB content and TAG:apoB ratios of TAG-rich lipoproteins (TRL) obtained from healthy subjects after the consumption of the test meal (Mean‡ values with their standard errors, n 9)
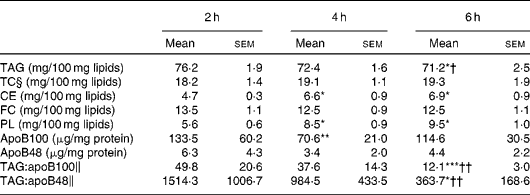
TC, total cholesterol; CE, cholesteryl esters; FC, free cholesterol; PL, phospholipids.
Mean value was significantly different from that at 2 h: * P< 0·05; ** P< 0·01; *** P< 0·001.
Mean value was significantly different from that at 4 h: † P< 0·05; †† P< 0·01.
‡ Mean from nine separate experiments each using TRL obtained from different subjects.
§ TC is calculated as the sum of FC and CE.
∥ TAG:apoB ratios were calculated after normalising by mg of TRL protein.
Intracellular lipid composition in THP-1 macrophages
Microphotographs of THP-1 macrophages incubated for 24 h in the absence (control) or presence of TRL isolated at 2, 4 or 6 h after the consumption of the test meal are shown in Fig. 1. Cells were stained with Oil Red O to visualise the incorporation of lipids into the cytoplasm. Images revealed a light staining in control cells and a strong red staining in cells incubated with TRL, confirming the intracellular incorporation of lipids from lipoproteins. There were also some differences related to the postprandial time point, with particles isolated at 6 h inducing a visually noticeable lower staining intensity compared with those isolated at 2 or 4 h after the consumption of the test meal.
THP-1 macrophages were also incubated with postprandial TRL to evaluate their influence on intracellular lipid mass (Fig. 3). The incubation of macrophages with TRL induced a statistically significant increase in the intracellular concentrations of TAG compared with control cells (absence of TRL). The strongest effect was observed when macrophages were incubated with TRL isolated at 4 h after the consumption of the test meal than when incubated with TRL isolated at 2 and 6 h. The contribution of FC and CE to the accumulation of lipids in cells was statistically indistinguishable from that in control cells at all time points.

Fig. 3 Intracellular lipid accumulation in macrophages incubated for 24 h in the absence (control, □) or presence of TAG-rich lipoproteins (TRL, 15 μg total cholesterol/ml) isolated at 2, 4 and 6 h after the consumption of the test meal (■). (a) TAG, (b) cholesteryl esters (CE), (c) free cholesterol (FC) and (d) total cholesterol (TC). Values are means from nine separate experiments each using TRL obtained from different subjects, with their standard errors represented by vertical bars. Mean value was significantly different from that of control cells: ** P< 0·01; *** P< 0·001.
Macrophage receptor gene expression
The effect of TRL isolated at 2, 4 and 6 h after the consumption of the test meal on the mRNA expression of membrane receptors in macrophages is shown in Fig. 4. The relative abundance of LDLR mRNA transcripts exhibited a reduction of nearly 50 % after the incubation of cells with TRL. The strength of the inhibition changed according to the postprandial time point, being significantly stronger with particles isolated at 2 h than with those isolated at 4 h. The mRNA levels of LRP were not affected after the incubation of cells with TRL, although a tendency towards inhibition of the expression was observed after the incubation of cells with TRL isolated at 2 h. In contrast to those of LDLR and LRP, the mRNA levels of VLDLR were elevated by TRL treatment, but there were no significant differences between the effects exerted by TRL isolated at different postprandial time points.

Fig. 4 Fold change in the mRNA expression of membrane receptors in macrophages incubated with (when compared with without) TAG-rich lipoproteins (TRL, 15 μg total cholesterol/ml) isolated at 2, 4 and 6 h after the consumption of the test meal. (a) LDL receptor (LDLR), (b) LDLR-related protein (LRP), (c) VLDL receptor (VLDLR), (d) scavenger receptor CD36 (CD36), (e) scavenger receptor class A type 2 (SRA2) and (f) scavenger receptor class B type 1 (SRB1). Values are means from nine separate experiments each using TRL obtained from different subjects, with their standard errors represented by vertical bars. * Mean value was significantly different from that of cells incubated with TRL isolated at 2 h (P< 0·05).
The incubation of macrophages with postprandial TRL had similar effects on scavenger, non-apo E-dependent receptors as well as on the mRNA expression of each of the genes studied, as the abundance of transcripts was increased at all time points, with the exception of a slight inhibition of SRA2 expression in cells incubated with TRL isolated at 2 h postprandially. The lowest mRNA expression of these receptors was observed when cells were incubated with TRL isolated at 2 h after the consumption of the test meal, and it was significantly increased above this level when cells were incubated with TRL isolated at 4 h, except in the case of VLDLR. A similar pattern was observed in the mRNA expression of CD36, with a significantly higher expression being observed with particles isolated at 4 h than with particles isolated at 2 h. No significant differences were observed in the abundance of transcripts when cells were incubated with particles isolated at 4 and 6 h when compared with control cells. The increase in mRNA expression in cells after treatment with TRL isolated at 6 h when compared with that in cells after treatment with TRL isolated at 2 h postprandially was significant for only SRB1.
Discussion
TRL are considered to be atherogenic particles, as they can be taken up by macrophages, leading to the formation of foam cells( Reference Yu and Mamo 13 ). However, there is evidence indicating that not all TRL are equally taken up by macrophages and that the particle size and composition of TRL may have a significant influence on the process of uptake and lipid accumulation( Reference Botham and Wheeler-Jones 15 ). In the present study, we investigated the influence of postprandial phase time on the composition of TRL and their receptor-related uptake by macrophages.
The highest concentration of TAG in TRL was observed at 2 h after the consumption of the test meal, which declined at later time points (Table 2). TRL exhibited a progressive decrease in TAG concentrations throughout the postprandial period, but were proportionally enriched with phospholipids and CE. There was a change in the concentrations of apoB100 and apoB48 in TRL, although in this case the lowest concentrations were observed at 4 h postprandially. Estimation of the particle size of TRL from the TAG:apoB100 and TAG:apoB48 ratios indicated that there was a progressive decrease in particle size with time after the consumption of the test meal, as ratios were highest at 2 h and lowest at 6 h. We have previously reported that TRL particles differing in size and composition can modulate the clearance of TRL postprandially, and this may affect the incorporation of TAG into the tissues( Reference Cabello-Moruno, Perona and Osada 16 – Reference Cabello-Moruno, Perona and Ruiz-Gutierrez 26 ). Palmer et al. ( Reference Palmer, Nova and Anil 19 ) carried out a study to investigate the effect of particle size on the accumulation of TAG in THP-1 macrophages. They found a preferential accumulation of TAG in macrophages in response to incubation with large VLDL (Svedberg flotation rate (Sf) 60–400), i.e. intermediate-sized TRL particles, compared with small VLDL (Sf 20–60) or large-sized CM (Sf >400). In agreement with this, the present results show that the highest uptake of TAG occurred when cells were incubated with TRL isolated at 4 h after the fat challenge, which was also the time point at which the estimated particle size was intermediate between that of particles isolated at 2 and 6 h (Fig. 3(a)). The use of the TAG:apoB ratio as an estimate of particle size is a limitation of the present study, but this ratio, as well as the cholesterol:apoB ratio, has been used for this purpose previously and proved to be reliable and convenient( Reference Cabello-Moruno, Perona and Osada 16 , Reference Cabello-Moruno, Martinez-Force and Montero 20 , Reference Tani, Saito and Anazawa 27 ). The fatty acid composition of dietary fat has been shown to modify the particle size of postprandial TRL in earlier studies. In this regard, Mekki et al. ( Reference Mekki, Charbonnier and Borel 28 ) showed that the intake of butter led to smaller circulating CM compared with the intake of olive or sunflower oils. However, it is unlikely that the changes observed in total TAG content and estimated particle size in the present study were related to modifications in the fatty acid TAG molecular species composition of postprandial TRL. In a recent study, we observed no significant changes in fatty acid or TAG molecular species composition related to the postprandial time point after the consumption of the same meal containing refined olive oil( Reference Cabello-Moruno, Martinez-Force and Montero 20 ). Vors et al. ( Reference Vors, Pineau and Gabert 29 ) reported that in obese individuals, but not in normal-weight subjects, CM were larger when fat was administered as an emulsion than when consumed as a spread on bread. Although the metabolic consequences need to be investigated further, these results are of importance, as it has been proposed that milk derivatives should be used to standardise protocols for postprandial tests( Reference Lairon, Lopez-Miranda and Williams 30 ).
The incubation of macrophages with postprandial TRL caused a highly significant increase in intracellular TAG concentrations, but did not have any effect on the cellular accumulation of FC and CE. This result, together with the visualisation of the lipid content with Oil Red O staining, suggests that postprandial TRL induce the formation of foam cells in THP-1 cell populations mainly by TAG, rather than cholesterol, accumulation. Although there is a great deal of evidence from studies carried out both in vivo and with THP-1 macrophages to indicate that foam cell formation induced by native or modified LDL is caused by the accumulation of CE( Reference Ghosh, Zhao and Bie 31 ), our findings are consistent with those of extensive previous work showing that CM remnants cause excessive TAG accumulation in human monocyte-derived macrophages as well as in the mouse macrophage cell line J774 and THP-1 macrophages( Reference Batt, Avella and Moore 6 , Reference Bejta, Moore and Avella 14 , Reference De Pascale, Avella and Perona 17 , Reference Napolitano, Avella and Botham 32 , Reference Moore, Bejta and Avella 33 ).
In macrophages, TAG are internalised not only via the direct uptake of fatty acids after lipoprotein lipase hydrolysis and subsequent re-esterification, but also by the uptake of whole particles by receptor-mediated pathways( Reference Palmer, Nova and Anil 19 ); however, the exact mechanisms involved have not been defined completely. Current evidence suggests that CM remnants are mainly taken up via apoE-dependent pathways, mediated by LDLR and LRP1. In the present study, we observed that the incubation of macrophages with human TRL, isolated at different postprandial time points, induced a decrease in the mRNA levels of LDLR in all cases (Fig. 4(a)). These findings are in agreement with those of previous work demonstrating a decreased mRNA expression of LDLR after the incubation of THP-1 macrophages with CM remnants( Reference Bejta, Moore and Avella 14 , Reference Batt, Patel and Botham 34 ). It is known that the expression of this receptor is inhibited by the intracellular accumulation of cholesterol due to lipoprotein uptake( Reference Batt, Patel and Botham 34 , Reference Albertini, Moratti and De Luca 35 ). However, the results of the present study indicate that the reduction of LDLR mRNA expression might also be caused by excessive TAG accumulation, as an increase in intracellular cholesterol concentrations was not observed. It is clear from the extensive studies carried out over the past few years that receptors other than LDLR are involved in the uptake of TRL by macrophages( Reference Bejta, Moore and Avella 14 , Reference Kowala, Recce and Beyer 36 ). Bejta et al. ( Reference Bejta, Moore and Avella 14 ) showed that LRP has a major role in the uptake of CM remnants by THP-1 macrophages. Others have also demonstrated that a decrease in the uptake of CM remnants by macrophages occurs when LRP is blocked by inhibitors( Reference Fujioka, Cooper and Fong 37 ), although Mamo et al. ( Reference Mamo, Elsegood and Gennat 11 ) did not find any evidence implicating LRP in this process. Palmer et al. ( Reference Palmer, Nova and Anil 19 ) reported that VLDL and CM exert different effects on the expression of this receptor, which could be related to their particle size, but we were unable to find any significant effects of TRL isolated at different postprandial time points (Fig. 4(b)). VLDLR is another member of the LDLR superfamily that can bind to TRL remnants( Reference Takahashi, Suzuki and Kohno 38 , Reference Niemeier, Gàfvels and Heeren 39 ) and may be involved in the formation of foam cells( Reference Kosaka, Takahashi and Masamura 40 ). A modest up-regulation in the expression of this receptor was observed in the present study (Fig. 4(c)). However, no influence of the postprandial phase time was observed.
The expression of scavenger receptors (CD36, SRB1 and SRA2) is a characteristic feature of macrophages, and there is evidence suggesting that some of them have relevant roles in the uptake of TRL by different cells, including primary macrophages and THP-1 monocyte/macrophages( Reference Yu and Cooper 10 , Reference Bejta, Moore and Avella 14 , Reference Botham and Wheeler-Jones 15 ). Adenovirus-mediated overexpression of hepatic SRB1 can reduce serum VLDL- and CM-associated TAG concentrations( Reference Out, Hoekstra and de Jager 41 ). Immunoblocking of CD36, and probably SRB1( Reference Napolitano and Bravo 9 ), has been shown to lower the incorporation of CM remnants into macrophages by 35–40 %( Reference Bejta, Moore and Avella 14 ), leading to reduced CE, but not TAG, accumulation in cells, probably because the uptake of this lipid class is mainly mediated by lipoprotein lipase( Reference Napolitano and Bravo 9 ). SRA2 plays a role in the formation of foam cells induced by LDL( Reference Dhaliwal and Steinbrecher 42 ), but little evidence is available on the part it may play in the uptake of TRL by macrophages. In the present study, the gene expression of these receptors was increased after the incubation of cells with all TRL particles, but to different extents, depending on the postprandial time point (Fig. 4(d)–(f)). The expression was low (SRA2 and CD36) or even inhibited (SRB1) when cells were incubated with particles isolated at 2 h, while it was high when cells were incubated with particles isolated at 4 h in all cases. It is worth noting that the time-related effects of TRL on the expression of scavenger receptors were associated with the intracellular accumulation of TAG in macrophages. Indeed, the highest expression of these receptors occurred at 4 h postprandially, the time point at which TAG accumulation in cells was highest, suggesting that this receptor pathway may be actively contributing to the accumulation of TAG and the formation of foam cells.
In summary, the size and lipid composition of TRL were affected by the postprandial time point at which they were collected. TRL isolated at all time points studied caused considerable intracellular TAG accumulation in THP-1 macrophages, but the greatest increase was observed with particles of intermediate size, isolated at 4 h after the consumption of the test meal. For addition into the cell culture, TRL were normalised by cholesterol concentrations (15 μg cholesterol/ml); thus, for each postprandial time point, a similar number of particles were added to the cell culture. It is known that receptor-mediated pathways play a role in the uptake of TRL by macrophages. Among those examined in the present study, the apoE-dependent receptors were expressed differentially in cells after treatment with postprandial TRL, with the expression of LDLR being down-regulated and no effect being observed on that of LRP. In contrast, the expression of the apoB100-dependent VLDLR was slightly up-regulated. A common feature of these receptors, however, was the absence of any sensitivity to the time point at which TRL were isolated. Conversely, scavenger receptors were more sensitive to the postprandial time point, with a higher mRNA expression being observed after the incubation of cells with the intermediate-sized TRL isolated at 4 h, which also had the strongest effect on intracellular TAG concentrations. The results of the present study thus suggest that the atherogenicity of postprandial TRL varies with the time they are in the circulation, with those isolated at 4 h after the consumption of the meal being potentially most atherogenic, as they promote the formation of foam cells by increasing the accumulation of TAG in cells, possibly because of the up-regulation of the expression of scavenger receptors and, to a lesser extent, that of VLDLR.
Acknowledgements
The present study was supported by funds from the Spanish Ministry of Economy and Competitiveness (AGL2011-23810).
The authors’ contributions are as follows: R. C.-M. and M. A. performed the experiments; E. M. recruited the subjects and performed the biochemical analyses; K. M. B., J. S. P. designed the study; L. S. and J. S. P. analysed the results; K. M. B., L. S. and J. S. P. wrote the article.
None of the authors has any conflicts of interest to declare.








