Introduction
Species conservation and management decisions require information on population size, a parameter that is difficult to obtain for species that are rare, elusive or that inhabit places that are difficult for researchers to reach. To compare population numbers over time, and to detect trends effectively, systematic monitoring studies are required. Such monitoring is also useful for the development of conservation and management plans (Butchart et al., Reference Butchart, Stattersfield and Collar2006). Monitoring is particularly important for large scavenging raptors, several species of which are rare and declining (Ferguson-Lees & Christie, Reference Ferguson-Lees and Christie2001). These birds are at the top of the food chain, have low reproductive rates, and their populations typically have a high percentage of non-reproductive birds, making the species susceptible to extinction from human persecution (Meretsky et al., Reference Meretsky, Snyder, Beissinger, Clendenen and Wiley2000; Owens & Bennett, Reference Owens and Bennett2000; Ferguson-Lees & Christie, Reference Ferguson-Lees and Christie2001).
Scavengers such as vultures are exposed to several critical threats (Koenig, Reference Koenig2006). In Africa, for example, vultures are unintended victims of poisoned carcasses laid out for other carnivores (Brown, Reference Brown1991; Koenig, Reference Koenig2006). Residues of diclofenac, an anti-inflammatory drug used for livestock in Asia, have brought the populations of three vulture species close to extinction (Oaks et al., Reference Oaks, Gilbert, Virani, Watson, Meteyer and Rideout2004). California condors Gymnogyps californianus are threatened by lead poisoning in North America (Meretsky et al., Reference Meretsky, Snyder, Beissinger, Clendenen and Wiley2000), and lead and other poisons are threatening vultures in Europe (e.g. Donázar et al., Reference Donázar, Palacios, Gangoso, Ceballos, González and Hiraldo2002) and condors in South America (Díaz et al., Reference Díaz, Cuesta, Abreu and Mujica2000; Lambertucci, Reference Lambertucci2007). Knowledge of the population size and age class structure of these species is required for estimating future viability.
The Andean condor Vultur gryphus is distributed throughout the Andes in South America, from Venezuela to southern Argentina and Chile. It is categorized as Near Threatened on the IUCN Red List, listed in Appendix I of CITES and is extinct in some parts of its range (BirdLife International, 2008). It is a long-lived species with one of the lowest reproductive rates among birds (Wallace & Temple, Reference Wallace and Temple1988; Lambertucci, Reference Lambertucci2007). Continuing declines have been documented in some areas where the species is already disappearing or scarce (Calchi & Viloria, Reference Calchi and Viloria1991; Lieberman et al., Reference Lieberman, Rodriguez, Paez and Wiley1993; Koenen et al., Reference Koenen, Koenen and Yanez2000) but little is known about population sizes and trends in areas where the species is more abundant. Local population estimates are available for Peru (109 individuals; Wallace & Temple, Reference Wallace and Temple1988), Bolivia (78; Ríos-Uzeda & Wallace, Reference Ríos-Uzeda and Wallace2007), central Argentina (100; Donázar & Feijóo, Reference Donázar and Feijóo2002), southern Argentina (196; Lambertucci et al., Reference Lambertucci, Jácome and Trejo2008) and southern Chile (73; Kusch, Reference Kusch2004). The northern population has been estimated to be c. 200, from northern Ecuador to Venezuela (Díaz et al., Reference Díaz, Cuesta, Abreu and Mujica2000). Díaz et al. (Reference Díaz, Cuesta, Abreu and Mujica2000) proposed a figure of 6,200 individuals for the species but this estimate was based on data from only a few sites.
Condors breed solitarily but roost and eat communally (del Hoyo et al., Reference del Hoyo, Elliott and Sargatal1994; Lambertucci, Reference Lambertucci2007). Although the use of communal roosts is variable across days, seasons and years, they are good places to estimate abundance (Kusch, Reference Kusch2004; Lambertucci et al., Reference Lambertucci, Jácome and Trejo2008). The aims of the study reported here were to derive minimum population numbers for condors in north-western Patagonia, to estimate the size of the local population, and to describe the use of the roosts by season and age classes. The estimates are a reference for future studies requiring data on population size and trends of the Andean condor and other large scavengers.
Study area
I selected the 10 main Andean condor communal roosts within an area of c. 6,300 km2 in north-western Patagonia, Argentina (Fig. 1). The area encompasses a mosaic of woodlands and steppes, used mainly for extensive livestock ranching and where there are increasing numbers of introduced mammals (red deer Cervus elaphus, wild boar Sus scrofa and European hare Lepus europaeus). Carcasses consumed by condors are thus abundant throughout the study area (Lambertucci et al., Reference Lambertucci, Trejo, Di Martino, Sánchez-Zapata, Donázar and Hiraldo2009b). The climate of the region is cool temperate, with precipitation mainly concentrated in winter and autumn, and with summer being the driest season (Paruelo et al., Reference Paruelo, Beltrán, Jobbágy, Sala and Golluscio1998). The area’s geological history, along with more recent erosive processes, has created numerous cliffs that are used by the Andean condor for roosting and breeding.
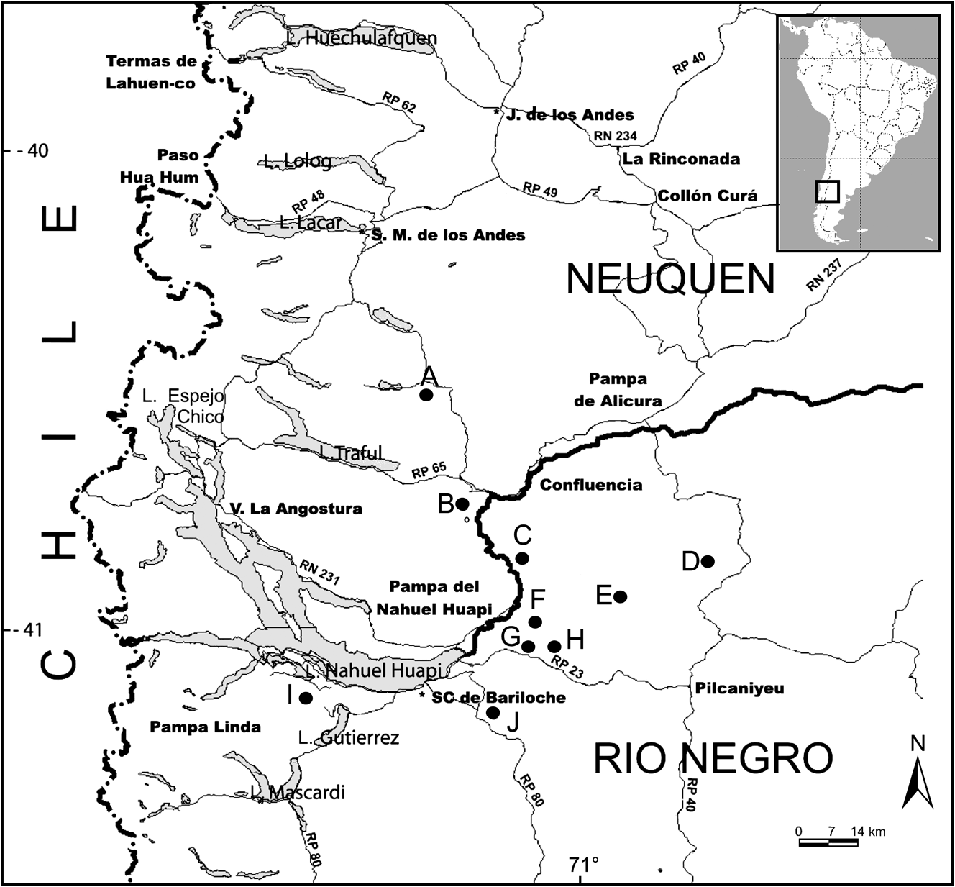
Fig. 1 Locations of the 10 communal roosts (A–J) of the Andean condor Vultur gryphus surveyed in north-west Patagonia, Argentina. Inset indicates the location of the main figure in South America.
Methods
Survey
Two or three trained observers visited each of the 10 communal roosts on 15 occasions from January 2006 to December 2008 to count the condors from blinds (> 300 m from the roost) with the aid of telescopes (20–60 × 60) and binoculars (10 × 50). Roosts were surveyed simultaneously (i.e. a team of 20–30 observers surveyed all 10 roosts on the same days) to avoid counting the same bird more than once and because there can be strong daily variations in the use of the communal roosts (Lambertucci et al., Reference Lambertucci, Jácome and Trejo2008). Condors at each roost were counted twice: a last-light census at dusk, at the time when the observers could still distinctly see individual condors, to coincide with the time when the majority of the condors were roosting and only a minority, or none, were flying, and a first-light census the following morning, before the condors left the roost, to verify the number of individuals counted the previous night. I used the larger of the two counts as the abundance of condors roosting. Condors were classified as immature (juveniles, brown without a collar, and subadults, brown-grey with a white collar) or adult (black with white coverts on the upper wing and a white collar; McGahan, Reference McGahan1972; del Hoyo et al., Reference del Hoyo, Elliott and Sargatal1994).
Data analysis
To analyse the number of condors in relation to the number of communal roosts, I constructed two accumulation curves from the complete set of censuses. These curves comprise a simple and reliable method used in biodiversity studies to estimate the number of species in a certain area (Soberón & Llorente, Reference Soberón and Llorente1993). As the sampling effort increases new species are added at a decreasing rate until an asymptote is reached. Application of accumulation curves facilitates estimation of how many communal roosts must be surveyed to census a high proportion (e.g. > 80%) of a population. They also allow comparison of abundances in different parts of a species' range. For the whole set of censuses I selected the communal roost with the highest number of individuals and then progressively added the combination of sites with the next highest number of individuals, and so on. To calculate the population of Andean condors in the area I fitted the data to the two asymptotic models, Clench’s, Sn = an/(1+bn), and the exponential model, Sn = a/b [1 – exp(-bn)]. In both models Sn is the expected size in the number of species (in this case number of individuals), a is the initial slope, b is a parameter related to the shape of the curve, and n is the sampling effort (in this case the number of communal roosts surveyed). The asymptote represents the total number of individuals predicted by the function, and is given by a/b (Soberón & Llorente, Reference Soberón and Llorente1993). Clench’s model is recommended for large sampling areas and has a good fit to real situations, whereas the exponential function is recommended when the area is smaller or all individuals have a high probability of being found (Soberón & Llorente, Reference Soberón and Llorente1993; Moreno & Halffter, Reference Moreno and Halffter2000). I considered that the exponential model would predict the lower limit whereas Clench’s model would provide an upper limit, with the true accumulation curve of individuals lying between them (Moreno & Halffter, Reference Moreno and Halffter2000). Therefore, I calculated the intermediate value between the asymptotes as an estimate of the number of individuals in the area.
On nine occasions I included other, smaller-sized, communal roosts (between one and four) to check that the use of the chosen 10 roosts accounted for a large proportion of the population. Using these further roosts I never obtained more individuals than in the maximum counts from the 10 selected roosts. I examined possible differences in condor abundances among seasons and age classes using an ANOVA, t-test, and χ2 tests and a significance level of P ≤ 0.05.
Results
The maximum number of condors counted simultaneously in the 10 communal roosts was 246. However, the variability of the data was large (range = 27–246; Fig. 2). There was a strong seasonal use of the roosts, mainly because condors do not aggregate strongly in this area during summer (Fig. 2). The maximum numbers of condors did not differ among years (![]() , P = 0.32) but there were clear seasonal differences in the use of the area (Fig. 2, ANOVA F3,11 = 7.23, P = 0.006). The main differences were between summer, and winter and spring (Tukey test, P summer-winter = 0.03; P summer-spring < 0.01). However, each roost varied in the month in which the number of individuals peaked (Fig. 3). Some seemed to be used more in autumn (Fig. 3A,C,J), others in spring (Fig. 3B,D,F,G) and/or in winter (Fig. 3E,H,I,J).
, P = 0.32) but there were clear seasonal differences in the use of the area (Fig. 2, ANOVA F3,11 = 7.23, P = 0.006). The main differences were between summer, and winter and spring (Tukey test, P summer-winter = 0.03; P summer-spring < 0.01). However, each roost varied in the month in which the number of individuals peaked (Fig. 3). Some seemed to be used more in autumn (Fig. 3A,C,J), others in spring (Fig. 3B,D,F,G) and/or in winter (Fig. 3E,H,I,J).
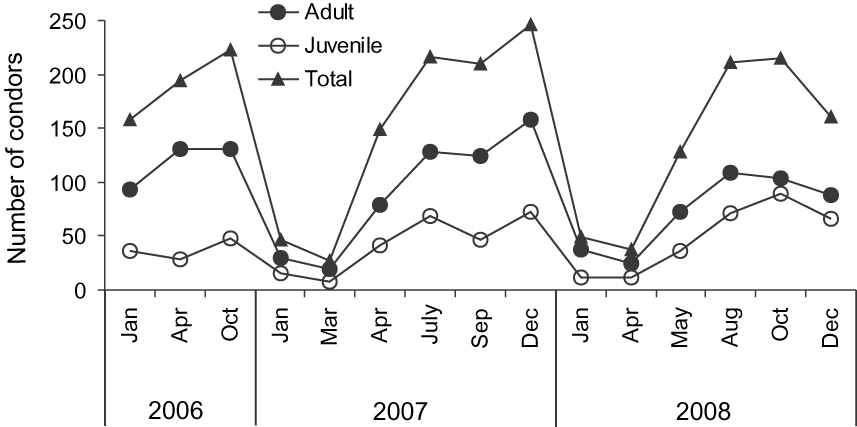
Fig. 2 Temporal trends in the numbers of Andean condors observed during 2006–2008 at 10 communal roosts (Fig. 1) surveyed simultaneously in north-west Patagonia, Argentina.

Fig. 3 Temporal trends in the numbers of Andean condors observed at each of the 10 communal roosts (A–J, Fig. 1) surveyed simultaneously during 2006–2008 in north-west Patagonia, Argentina. Note the differing y-axis scales.
I observed more adults than immatures in the area year-round (t = 3.39, df = 28, P < 0.01; Fig. 2), with a maximum of 159 adults (68.5%) and 73 immatures (31.5%) for the same year (2007). The maximum number of immatures was 89 during December 2008. The ratio of adults to immatures, based on the maximum number of each age class counted simultaneously, was strongly biased toward adults (1 : 0.46). Although both age classes used the same area synchronously (Fig. 2) there were differences in monthly use, which varied between roosts (Fig. 3).
Both of the applied accumulation models fitted the observed data well (r2 > 0.97; Table 1, Fig. 4). As expected, I obtained a larger population estimate with Clench’s function than with the exponential model (Fig. 4). The intermediate value between the asymptotes of the two models was 296.

Fig. 4 Observations (circles) and fitted accumulation curves (lines) for the maximum number of condors in 1–10 communal roosts surveyed simultaneously. Two asymptotic models (Table 1), Clench’s (dashed line) and the exponential (solid line), are fitted to estimate the increment in the number of individuals in relation to the increment in the area surveyed.
Table 1 Estimates of the Andean condor Vultur gryphus population in north-west Patagonia obtained by fitting two asymptotic accumulation models. In both models a is the slope at the beginning of the sampling, b is a parameter related to the shape of the curve, n is the sampling effort, a/b is the asymptote of the curve and represents the total population, R 2 is the proportion of variance accounted for each model, and CI is the confidence interval.

Discussion
The Andean condor population of north-west Patagonia is the most important known for the species. Minimum and estimated population sizes are 246 and 296 (range 260–332), respectively, in an area of c. 6,300 km2. Of the estimated total population of 6,200 (Díaz et al., Reference Díaz, Cuesta, Abreu and Mujica2000) this population comprises c. 4.8%. In the same region 136 condors have previously aggregated in just one communal roost, and up to 196 in three roosts during 2001 (Lambertucci et al., Reference Lambertucci, Jácome and Trejo2008). Explanations for communal roosting in such large groups may include, for example, social interaction, a climate effect, and food and space availability (Beauchamp, Reference Beauchamp1999; Lambertucci, Reference Lambertucci2007).
My estimate of the population may be conservative as it does not account for condors breeding away from the communal roosts or for fledglings that remain in the nest for several months (Lambertucci & Mastrantuoni, Reference Lambertucci and Mastrantuoni2008). In addition, this population could be an open one that uses an area larger than that surveyed. The Andean condor’s home range has not been fully studied but five satellite tagged juvenile condors from the same area flew almost 600 km north-south and 100 km west-east, and almost 200 km in one day (Astore, Reference Astore2001; L. Jácome pers. comm.). However, counting condors in different communal roosts simultaneously, as in the present work, avoids counting an individual more than once per census and provides estimates of the minimum and total population of an area. Application of this type of census across the species' range will allow comparisons between populations. Satellite tagging or newer techniques (e.g. Wilson et al., Reference Wilson, Shepard and Liebsch2008) could provide information on the species' home range and on the time condors dedicate to communal roosting.
During the 3-year study the abundance of condors was stable, although birds used the region with a clear seasonality, increasing from autumn to spring and decreasing in summer. The Egyptian vulture Neophron percnopterus has a similar pattern in Europe coinciding with its breeding season (Donázar et al., Reference Donázar, Palacios, Gangoso, Ceballos, González and Hiraldo2002). Breeding is unlikely to be the main reason for the seasonal movements of condors, as the breeding season extends throughout the year (Lambertucci & Mastrantuoni, Reference Lambertucci and Mastrantuoni2008). In this study I included almost all of the communal roosts in the study area and therefore it appears that condors move away from the study area, mainly during the summer. Because the main movements are latitudinal (Astore, Reference Astore2001) condors could, for example, be following livestock movements initiated by farmers during summer, be moving to areas with better weather and flying conditions, or be dispersing to areas less accessible to researchers.
Knowledge of age structure and sex ratio is important for demographic studies (Ezard et al., Reference Ezard, Becker and Coulson2006). In north-west Patagonia 68.5% of the individuals were adults and therefore c. 200 adult condors may potentially breed. However, given that, even during favourable conditions, condors can only nest up to once every other year (del Hoyo et al., Reference del Hoyo, Elliott and Sargatal1994; Lambertucci & Mastrantuoni, Reference Lambertucci and Mastrantuoni2008), a maximum of 100 may breed each year if the male to female ratio is 1 : 1. If this is the case (although it is probably not; M. Alcaide, L. Cadahía, S.A. Lambertucci & J.J Negro, unpubl. data), this population could produce c. 50 chicks per year. The ratio of adults to immatures of 1 : 0.46 in this population is similar to that in southern Chile (1 : 0.52; Sarno et al., Reference Sarno, Franklin and Prexl2000), and Peru (1 : 0.35; Wallace & Temple, Reference Wallace and Temple1988) but different from that in Bolivia (1 : 1.5; Ríos-Uzeda & Wallace, Reference Ríos-Uzeda and Wallace2007). It has been proposed that the adult : immature proportion may indicate the reproductive rate of the Andean condor (Wallace & Temple, Reference Wallace and Temple1988). However, a skewed adult : immature ratio could be because of the condors’ long life-span, low reproductive rate and low natural mortality rate. The ratio could also be influenced by differences in age-class habitat use (Donázar et al., Reference Donázar, Travaini, Ceballos, Rodríguez, Delibes and Hiraldo1999; Sarno et al., Reference Sarno, Franklin and Prexl2000).
The Andean condor was formerly more widely distributed, including in eastern South America (Lambertucci, Reference Lambertucci2007). However, no historic estimations for abundance exist for the entire range. The number of individuals found in this study, although the highest concentration known, is low compared to related species (Ferguson-Lees & Christie, Reference Ferguson-Lees and Christie2001). The griffon vulture Gyps fulvus in southern Spain, where it feeds on extensively raised livestock, can reach c. 1,500 pairs in c. 8,000 km2 (Del Moral & Martí, Reference Del Moral and Martí2001; J. Donázar, pers. comm.). My maximum estimate was c. 300 individuals (200 adults) in c. 6,200 km2. Given that food is abundant and human density is low in Patagonia, Andean condors may maintain low densities in the area because of threats such as persecution, poisoning and electrocution (Lambertucci, Reference Lambertucci2007). The low number of individuals could otherwise indicate that Andean condors are habitually rare. Notwithstanding, both possibilities make this a species of conservation concern.
The design of any protected areas for the Andean condor should include not one but a set of communal roosts used by different age classes and at different times of the year. However, considering the wide range of this species, it is also necessary to consider problems such as poisoning and hunting pressure. For instance, condors can aggregate around a carcass in groups of c. 30 in this area (Speziale et al., Reference Speziale, Lambertucci and Olsson2008; Lambertucci et al., Reference Lambertucci, Speziale, Rogers and Morales2009a; S.A. Lambertucci, pers. obs.). Thus, just 10 poisoned carcasses could extinguish the population. It is important to identify any new communal roosts and to obtain population estimations of the Andean condor for the whole of South America. Long-term monitoring is essential for the detection of potential declines, which could then be addressed before they become critical.
Acknowledgements
I am especially grateful to volunteers and coordinators from Global Vision International, and also to F. Barbar, O. Mastrantuoni, H. Pastore, V. Werenkraut, J. Benclowicz, C. Lambertucci and K.L. Speziale for help in the field. J.A. Donázar, A. Ruggiero, C. Wells, T. Rogers, J. Jones, K.L. Speziale, R. Wilson and two anonymous reviewers provided valuable suggestions that improved this article. Managers and owners of La Buitrera del Ñirihuau, San Ramón, Siete Cóndores, Pilcañeu, Chacabuco, Cerro López, La Lonja and Cerro Negro farms as well as the Argentinean National Park Administration gave permission to study the roosts. SAL was funded from the CONICET, ANPCYT(PICT 38148) and Universidad Nacional del Comahue during this work.
Biographical sketch
Sergio A. Lambertucci’s main interests are animal ecology and conservation, with a particular focus on the ecology, biodiversity and behaviour of raptors. He has worked on the ecology and conservation of the Andean condor in Patagonia for more than a decade.







