Germ cell tumors (GCTs) comprise germinoma, teratoma, yolk sac tumor, embryonal carcinoma, choriocarcinoma, and mixed germ cell tumors. Central nervous system (CNS) GCTs account for 2–3% of all primary intracranial neoplasms. CNS GCTs are most prevalent in East Asian populations, with the highest percentage (15%) of GCTs among CNS neoplasms reported in Japanese pediatric patients.Reference Rosenblum, Nakazato and Matsutani1 The most common subtype of CNS GCT is germinoma, which makes up 55–65% of all CNS GCTs.Reference Thakkar, Chew and Villano2 CNS GCTs have subtype-specific treatment protocols and prognoses. Germinomas, in particular, have a reported long-term survival rate greater than 90% with craniospinal irradiation alone.Reference Millard and Dunkel3
CNS germinomas are usually located intracranially, in suprasellar, basal ganglia, and thalamic structures.Reference Hattab, Perry and Brat4 Primary spinal cord germinomas are very rare. Only 45 primary spinal cord germinomas have been reported, two-thirds (30/45) of which occurred in East Asian patients.Reference Nikitovic, Grujicic and Skender Gazibara5, Reference Hu, Yu and Du6 Eight of these 45 tumors were located in the cervical spinal cord, and only two were multifocal.Reference Nikitovic, Grujicic and Skender Gazibara5, Reference Hu, Yu and Du6 The average age at diagnosis was 25.5 (±9.4) years and the oldest previously reported patient at diagnosis was 39 years old.Reference Jennings, Gelman and Hochberg7
We report a 51-year-old East Asian man with primary spinal cord germinoma, the oldest reported patient with this diagnosis. He presented with a 4-week history of left lower limb and abdomen paresthesia and right lower limb weakness. His history was unremarkable apart from tuberculosis 20 years previously. Physical examination revealed a partial Brown-Sequard pattern of neurological deficit. Increased tone, decreased strength, and an extensor plantar response were present in the right lower limb. Decreased pinprick sensation to the T4 level was observed on the left. Posterior column function (vibration and position sense) was preserved bilaterally. CBC, biochemistry, immunology, and CSF were normal.
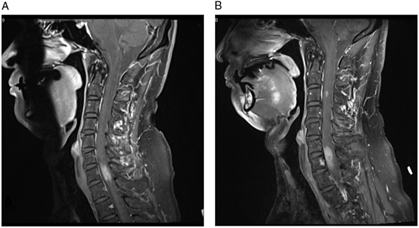
Magnetic resonance imaging (MRI) showed an expansile 2.5 cm enhancing lesion in the right cervical hemicord centered at C7 (Figure 1A). The radiographic features of germinoma are relatively nonspecific, usually hypointense on T1-weighted images and iso- or hyperintense on T2-weighted images.Reference Rosenblum, Nakazato and Matsutani1 They are often solid and more homogeneously contrast-enhancing on CT and MRI than other GCTs,Reference Rosenblum, Nakazato and Matsutani1 but the radiologic differential diagnosis remains broad. This includes neoplasm (glioma, hemangioblastoma, lymphoma, melanoma, GCT, metastatic carcinoma, and sarcoma) and non-neoplastic lesions comprising inflammatory (infectious causes such as tuberculosis and non-infectious causes such as sarcoidosis, demyelinating plaque, and progressive multifocal leukoencephalopathy) and other etiologies (e.g. vascular malformation).Reference Rosenblum, Nakazato and Matsutani1
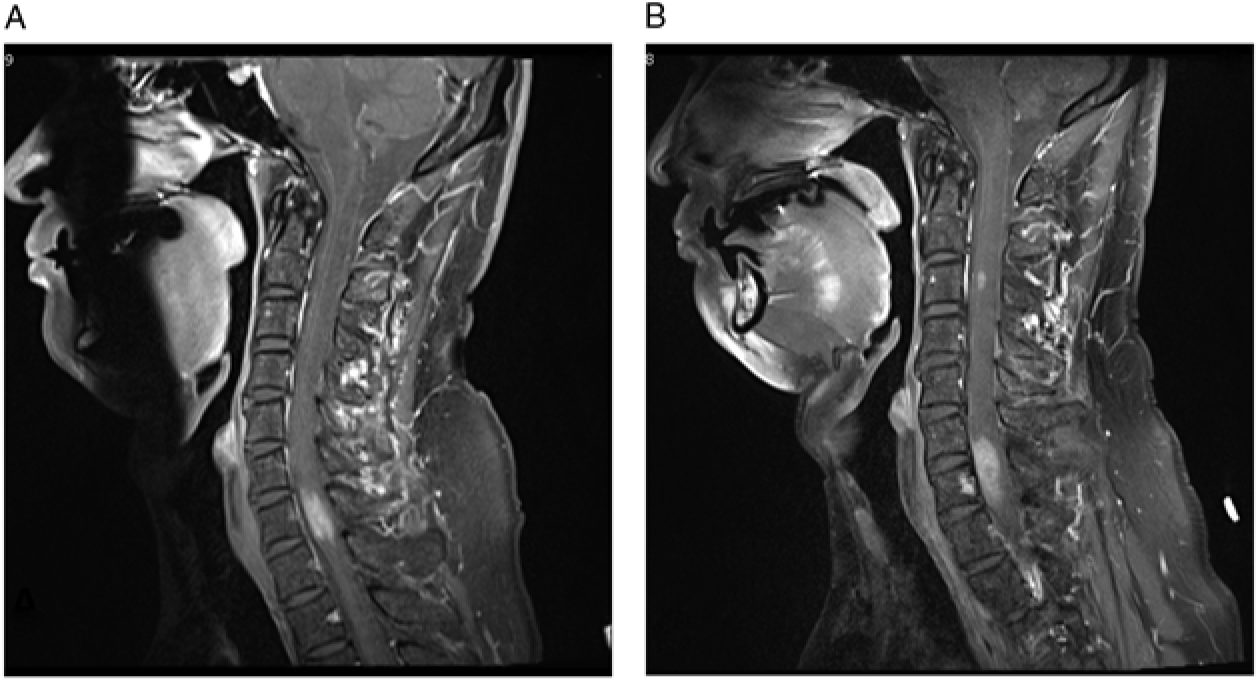
Figure 1: (A–B) T1-weighted post-contrast images demonstrate the progression of cervical spine lesions over time. (A) MRI scan at first admission, demonstrating hyperintense lesion centered at C7. (B) MRI scan 11 months after initial presentation, demonstrating enlargement of the original lesion and a new lesion at C3 level.
Our patient’s MRI of brain, CT of chest, abdomen, and pelvis, and scrotal sonogram were normal at the time of presentation and 18 months later. MR angiography of the neck and intracranial vessels was normal. He did not respond to a trial of steroids, but his symptoms remained fairly stable. Serial MRIs revealed no change until 11 months after presentation at which time a new enhancing nodule (5 mm) appeared at the C3 level, along with slight progression of the original C7 lesion (Figure 1B). Around this time, the patient noted numbness involving his right thorax, but he declined biopsy for a further number of months.
An open biopsy of the lesion was performed 18 months after presentation (fragments of the largest sample were in aggregate 0.5 cm in greatest dimension). Intraoperative assessment (smears and frozen section) showed prominent perivascular lymphoid infiltrate, but the presence of rare large atypical mitotic figures raised the question of a second cell population obscured by inflammation (Figure 2A). The formalin-fixed paraffin-embedded, hematoxylin and eosin (H&E)-stained sections revealed large, moderately pleomorphic cells scattered within densely inflamed areas. These cells had variably vacuolated cytoplasm (PAS-positive) and polygonal nuclei with prominent nucleoli (Figure 2B–F). In less inflamed areas, these cells formed nests well demarcated from CNS parenchyma. No syncytiotrophoblastic giant cells were seen. Mitotic figures were frequent. No necrosis was present.
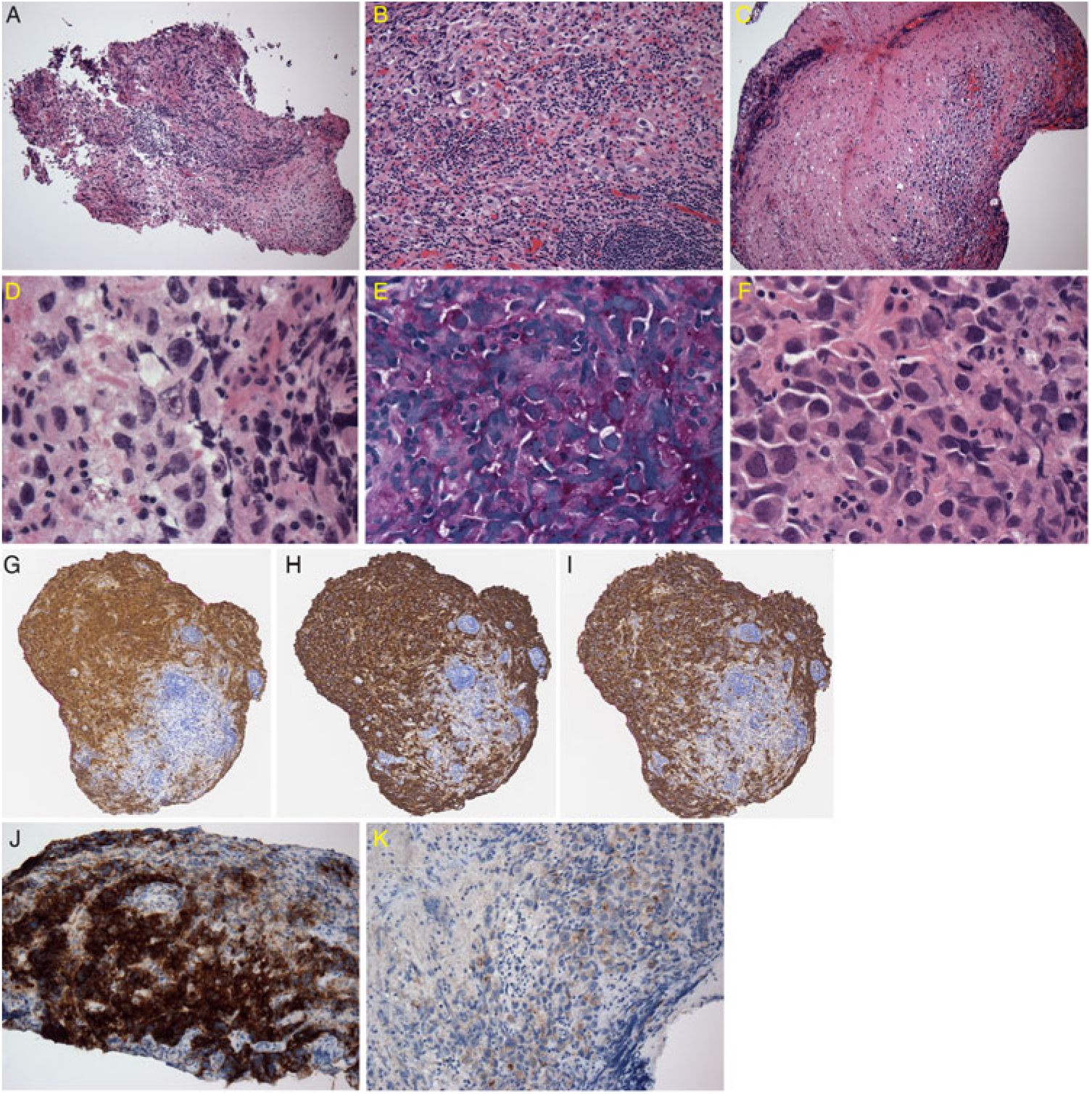
Figure 2: Neuropathological findings. (A) Intraoperative frozen section of lesion with dense lymphocytic infiltrate. (B) H&E; medium-power view of intense lymphocytic infiltrate masking scattered germinoma cells. (C) H&E; low-power view of cluster of tumor cells. (D) H&E; prominent nucleoli in tumor cells with prominent nucleoli and variably vacuolated cytoplasm. (E) Periodic acid-Schiff stain; tumor cells positive. (F) H&E; frequent mitotic figures in the tumor cells. (G) OCT 3/4; tumor cells positive. (H) PLAP; tumor cells positive. (I) CD117; tumor cells positive. (J) CK Cam5.2; tumor cells focally strongly positive. (K) CK Cam5.2; other areas of tumor weakly positive.
A full panel of immunostains for carcinoma/GCT narrowed the diagnosis to germinoma with the positive triad of octamer-binding transcription factor (OCT) 3/4, placental alkaline phosphatase (PLAP), and c-Kit (CD117) (Figure 2G–I), despite regionally strong cytokeratin staining (Figure 2J–K). Immunostaining was negative for antibodies to human chorionic gonadotropin (HCG), alpha-fetoprotein (AFP), human placental lactogen (HPL), CK7, CK20, CD30, melanoma-associated antigen recognized by T cells (MART-1/melanA), and Human Melanoma Black45 (HMB45). The lymphocytic infiltrate was a mixture of T-cells and B-cells. The morphology and immunophenotype were diagnostic of germinoma. Normal AFP and beta-HCG serological values also supported the diagnosis of pure germinoma rather than mixed germ cell tumor.
Once the diagnosis of germinoma was established, further clinical and radiologic studies were needed to rule out a metastasis to the spinal cord.Reference Hattab, Perry and Brat4 As CNS GCTs are morphological and immunophenotypic homologues of germ cell neoplasms outside the nervous system,Reference Rosenblum, Nakazato and Matsutani1 germinoma is morphologically identical to seminoma in the testis (and dysgerminoma in the ovary). In our patient, normal ultrasound of the scrotum and the absence of any extracranial lesion on radiologic imaging helped to rule out metastatic seminoma.
The patient was treated with four cycles of chemotherapy with cisplatin and etoposide. Three weeks after completing his fourth cycle of chemotherapy, he felt improved with respect to both right lower limb strength and left lower limb/truncal sensation. Repeat MRI at that time showed no residual contrast-enhancing disease at either C7/T1 or C3. Six months post-biopsy, he remains clinically stable.
In conclusion, this 51-year-old East Asian man is the oldest reported patient with the rare diagnosis of primary spinal cord germinoma. Initial microscopic views of the small biopsy were not entirely clear due to heavy inflammation. Rigorous attention to morphology and extensive immunostaining are needed to rule out lymphoma, melanoma, high-grade glioma, and components of other germ cell tumors. Clinical correlation confirmed the primary site of this germinoma in the spinal cord.
acknowledgements
We are grateful to the patient for allowing us to publish this case report.
disclosures
None of the authors has any conflicts of interest to disclose.
statement of authorship
AM did most of the manuscript preparation and background research, with help from SKr. BS, PB, PD, JS, MP, and SKa contributed to manuscript preparation and reviewing.