INTRODUCTION
Ornamental aquatic organisms include over 1500 fish, 100 coral and 300 invertebrate species, usually kept in aquaria for display purposes. Freshwater fish species are farm-raised, and comprise 90–96% of the overall ornamental fish trade [Reference Livengood and Chapman1]. Globally, the industry is valued at over US$900 million and US$3 billion at the wholesale and retail levels, respectively, with the USA being the largest importer of ornamental fish [Reference Livengood and Chapman1, 2]. The movement pathways of ornamental fish (Fig. 1) are very complex and dynamic as ornamental fish shipments move thousands of miles from production sources and countries of origin via holding and transhipment facilities, wholesalers and retailers for eventual display in public aquariums or hobbyists' homes around the world [Reference Livengood and Chapman1]. It is estimated that one million Canadian and 12 million US households own aquariums [Reference Trust, Bartlett and Lior3]. Ornamental fish farms are traditionally small, family-run operations with often closely guarded husbandry practices developed through years of experimentation [Reference Chapman and Stickney4]. Numerous public health concerns have been linked with this industry, including zoonotic pathogens, antimicrobial use (AMU) practices and antimicrobial resistance (AMR). A lack of qualified production personnel and equipment, poor and unsustainable production and transport practices, routine or improper use of medications to suppress disease, and limited veterinary oversight of imports/exports is commonly noted in the industry [Reference Livengood and Chapman1, Reference Kleingold5, Reference Cole6]. Health and safety import regulations for ornamental fish might vary considerably among countries [Reference Chong and Whittington7–12].

Fig. 1. Pathways of ornamental fish production, shipment and acquisition (adapted from Livengood & Chapman [Reference Livengood and Chapman1]).
The data on the frequency of zoonotic bacterial infections in humans due to exposure to ornamental fish is limited and prone to under-reporting due to the difficulty or failure to associate exposure with disease occurrence. Similarly, the data on the frequency of these pathogens, AMU practices and levels of AMR in ornamental fish populations are lacking or are presented in a fragmented fashion [Reference Lowry and Smith13–Reference Cabello17]. Published reviews on these issues are relatively uncommon [Reference Lowry and Smith13]. Systematic reviews are frequently used to identify, critically appraise and summarize the existing knowledge and gaps on a given subject using transparent and replicable methodology [Reference Sargeant18]. Questionnaire surveys are also frequently used as a cost-effective way of eliciting opinions from targeted populations (e.g. field experts) or to identify and fill knowledge gaps, and inform future research and policy developments [Reference Windsor19]. Our objective was to apply these two methodologies in a complementary fashion and to integrate their findings through discussion and interpretation. A systematic review (SR) was conducted to evaluate and summarize the results of published research reporting the frequency of zoonotic bacterial pathogens, AMU and AMR in ornamental fish, or human illness due to (potential) exposure through ornamental fish. A questionnaire was administered in order to elicit opinions of aquaculture allied personnel on the frequency of AMU and AMR in ornamental fish. We discuss the results of the two independent yet complementary studies, which were part of a larger initiative investigating similar aspects in seafood-related aquaculture species, in terms of existing research knowledge and gaps, surveillance opportunities, and public health education needs.
METHODS
Literature search
Multiple population (e.g. ornamental fish) and outcome (e.g. bacterial prevalence) search terms were applied in six electronic databases in October 2008, and updated in October 2010, with the search restricted to research published during or after 1990. A complete list of search terms and combinations, and electronic databases is given in the Supplementary material (Appendix 1, available online). A simple Google internet search was conducted to identify additional potentially relevant references. Search verification included a manual search of reference lists of all electronically identified articles found to be relevant through the review process and reference list of our pilot study report (B. Mercier, unpublished results) developed previously to estimate the approximate amount of research in this area and guide our review. All electronic citations were downloaded and de-duplicated in a bibliographical management program Procite 5.0 (Thomson ResearchSoft, USA), followed by a manual de-duplication.
Relevance screening, methodological assessment, data extraction and summarization
A flow of the SR process used in the larger initiative and as it pertains to this study is shown in Figure 2. Initial, abstract-level relevance screening (RS) was conducted to identify primary research, published in English, French and Spanish (the first language of various project team members), investigating bacterial zoonoses, reported AMU and/or AMR in ornamental fish or human illness due to any of these exposures. All potentially relevant abstracts were procured as full articles, confirmed for relevance, type of study design and evaluated for methodological soundness and reporting (MS). An a priori decision was made to restrict MS assessment to very basic evaluation and to conduct it simultaneously with data extraction (DE). A traditional MS assessment approach was not feasible because our pilot study indicated that all relevant research was of descriptive nature, precluding such assessment. Instead, full articles were evaluated for two general (exclusion) criteria; reporting of minimum sufficient raw or adjusted data, and replicable method protocols (e.g. laboratory methods). The former did not apply to case or case-series articles. The studies that met these criteria proceeded to DE, along with any case or case-series studies, and were included in the review. Study-specific data, for example study location(s), population(s), outcome(s), sample type and point, laboratory testing (including AMR), and reported results were extracted. RS and simplified MS assessment were completed by two independent reviewers using previously designed and pre-tested forms. DE was conducted by two independent reviewers (M. W., N. C., L. D.) on the first 20 articles, using a priori developed DE forms; however, the remaining 68 articles were extracted by a single reviewer due to a good agreement observed for the initial 20 articles. All forms used in this SR are given in the Supplementary material (Appendix 2, online). RS and MS steps were conducted using a web-based SR format (Distiller®, Evidence Partners Inc., Canada), while DE was conducted in Excel (Microsoft, USA) spreadsheet format. The extracted data were cleaned, summarized and reported by study design and bacterial species (outcome). Reported resistance to antimicrobials was grouped by antibiotic class to enhance consistency and interpretation across studies.

Fig. 2. Flow of studies that entered the larger scoping study on the same topics in aquaculture species intended for human consumption as pertaining to selection of studies entering the ornamental zoonoses review. a Excluded due to: lack of raw/unadjusted data and/or measures of association/effect (n=5); irrelevant study to review question (n=9); Slavic language, unable to translate (n=1). b Initial electronic database searches did not include Mycobacterium marinum as a search term, resulting in an additional 44 M. marinum case reports/series; reference list searches and simple Google searches identified an additional 22 bacterial, pathogen and outbreak reports not captured otherwise. c In-depth quality assessment precluded due to study design (case report/series, outbreak reports).
Questionnaire
Database of aquaculture-allied professionals
Thirteen aquaculture-allied professionals, indicated by our team aquaculture experts, were contacted by email and asked to provide names and/or contacts of other colleagues and professionals with expertise in aquaculture, zoonotic bacteria and AMU/AMR. Additional participants were recruited via a blog on Aquavetmed E-news. All contact information received was entered into our ‘target respondents’ database (Microsoft Excel).
Questionnaire description, administration and analysis
The questionnaire included five sections, and was pre-tested by five professionals with expertise in veterinary medicine and/or aquaculture and/or microbiology and epidemiology. From 26 questions pertaining to various aquatic species, four closed questions (using a five-point scale, e.g. ‘never’ to ‘always’ and one multiple-choice question) related to ornamental fish. These included: (1) frequency of AMU in ornamental fish by antimicrobial drug classes, (2) frequency of AMU in ornamental fish by production phase, (3) purpose of AMU in ornamental fish and (4) frequency of resistance observed in ornamental fish to various antimicrobial drug classes. The respondents were given the option to skip ‘ornamental fish’ questions if they felt that they did not have sufficient expertise in this field. A Spanish version, translated from English into Spanish by a bilingual doctoral student, was developed for administration in Spanish-speaking regions (South and Central America and the Caribbean). The questionnaire was administered using Survey Monkey®, a web-based application (Survey Monkey, USA). Two weeks prior to initial administration a letter was sent by email to all individuals listed in the above-mentioned database inviting their participation. Each participant was provided with a unique link and had a choice to refuse and opt-out of further communication. In addition, a brief questionnaire was designed for non-responders to assess non-response bias. An email was sent to 75 (randomly selected) English and all 17 Spanish non-respondents. Spanish non-respondents were also contacted up to five times via telephone to elicit additional responses. A full copy of both questionnaires is available in the Supplementary material (Appendix 3, online). Ethical approval for the surveys was received from the University of Guelph Review Ethics Board (protocol no. 09MY010).
The data from both questionnaires were exported separately to spreadsheets (Microsoft Excel), cleaned and imported into Stata 10 (Stata Corporation, USA) for frequency tabulations. A Fisher's exact test (P<0·05) was used to evaluate potential differences in proportions between respondents and non-respondents.
RESULTS
Systematic review
General characteristics of the studies included in the review
Eighty-eight articles were included in the review (Fig. 2). The ‘human illness’ articles included: 41 case studies reporting single occurrence of human illness, 16 case-series studies reporting two or more occurrences of human illness, and six outbreak studies reporting clusters of human illness due to various bacterial zoonotic infections and suspected or confirmed exposure to ornamental fish. In addition, 25 studies of small to modest size surveys (2–533 fish-level samples) of ornamental fish populations originating from various sources and measuring prevalence of various bacterial pathogens and/or AMR were also included in the review. No studies were identified reporting AMU in ornamental fish, although in one study levels of antibiotic residues in ornamental fish were reported [Reference Kleingold20]. AMR was mainly reported in studies surveying ornamental fish from imported and/or domestic sources (see Supplementary material, Appendix 4, online). The most frequent country importation sources of ornamental fish were from Asian and South American regions. In seven, 13 and two studies, only samples of healthy, sick, or both healthy and sick, ornamental fish were collected and tested, respectively, and in six studies fish transport or production water was also examined. Farm-level sample collection of ornamental fish was conducted in only six studies. Only one relevant article (published in Czech language) was excluded due to language. A complete list of articles included in the review is given in Appendix 4 (online).
Reported bacterial illness in humans due to (potential) exposure to ornamental fish
Case reports
Globally, Mycobacterium marinum (n=32 case reports, n=16 case series) was the most frequently reported zoonotic pathogen linking human cases to ornamental fish exposure (individual study data are not shown for reasons of brevity) [Reference Boubaker21–67]. Besides frequently reported cutaneous lesions, osteomyelitis, tendinitis, septic arthritis and synovitis were also reported. Antibiotic treatment was reported in all cases, and resistance profiles of isolates recovered from humans in 11 studies. Deaths were reported in three immunocompromised patients of various ages. Mycobacterium szulgai was reported in four cases and Aeromonas hydrophila, Comomonas spp., Edwardsiella tarda, Erysipelothrix rhusiopathiae, Vibrio cholera and Salmonella Paratyphi B var. Java (now renamed Salmonella Paratyphi B var. L-tartrate+) were each reported as a single case (Appendix 4, online).
Outbreak reports
All reported outbreaks linked to ornamental fish exposure involved Salmonella serovars [Reference Gaulin, Vincent and Ismail68–Reference Boxall73]. In Australia, clusters of cases associated with S. Paratyphi B var. Java [Reference Levings70, Reference Musto71] were linked to home aquaria as identical isolates were recovered from human case and tank samples. Similarly, officials in New Zealand reported a cluster of S. Paratyphi B var. Java cases (n=14 cases) [Reference Riley, Hanson and Ramsey72] and identical isolates were also confirmed in the fish-tank water and humans in 6/10 cases [Reference Boxall73]. S. Paratyphi B var. Java was also reported in two children in the UK, where this pathogen was isolated from the children's family home aquaria and a tank of the wholesaler that had supplied fish to the children's families retailers [Reference Riley, Hanson and Ramsey72]. Two outbreaks of S. Paratyphi B var. Java were reported in the province of Québec, Canada [Reference Gaulin, Vincent and Ismail68, Reference Gaulin, Vincent, Alain and Ismail69], initially indicating [Reference Gaulin, Vincent, Alain and Ismail69] that 3/6 aquaria owned by cases were positive for several Salmonella serovars, including Paratyphi B var. Java, Matopeni, and Typhimurium phage-type 104. Through epidemiological traceback, 3/7 retail fish tanks were found positive for S. Blockley, S. Matopeni, S. Agona, S. Stanley, S. Hadar and S. Kallo and 1/18 wholesaler tanks supplying retail shops was positive for S. Blockley and S. Wandsworth [Reference Gaulin, Vincent, Alain and Ismail69]. In an additional investigation (2000–2003), S. Paratyphi B var. Java was detected in 18/31 (58%) of home aquaria and 8/34 (23·5%) of retail fish tanks, and in a follow-up survey (2003–2004) of two fish importers from 19·7% of samples. The former were mostly collected from consignments imported from Malaysia and Thailand, and 28 different serovars were confirmed, with S. Schwarzendgrund being the most common (23%) (C. Vincent, Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec, unpublished results).
Zoonotic bacterial pathogens and AMR in various ornamental fish populations
Mycobacterium, Aeromonas and Salmonella genera were the most frequently reported pathogens (Appendix 4). AMR was tested and reported mainly for Aeromonas spp. with resistance to the tetracycline, sulfonamide and quinolone classes frequently reported with wide ranges of resistance of 24–96%, 2·9–88%, and 6–91%, respectively (Table 1). Generally, isolates recovered from healthy warm-water and cold-water ornamental fish had considerable differences in frequency of AMR [Reference Kleingold5, Reference Kleingold20, Reference Verner-Jeffreys74]. Five studies also reported multi-drug resistance and the presence of resistance genes/plasmids [Reference Verner-Jeffreys74–Reference Čižek78]. For brevity, data are shown only for studies reporting AMR on ⩾20 bacterial isolates (Table 1).
Table 1. Summary of studies (n⩾20 isolates) reporting antimicrobial resistance (AMR) in ornamental fish

n.r., Not reported.
* Isolated through the use of selective bacteriological culture media.
† Isolates selected for chloramphenicol-resistance.
‡ Serovars not fully described.
§ Motile Aeromonas.
Questionnaire
Demographic characteristics of survey participants self-rating their level of experience with ornamental fish as ‘medium to high’ or ‘high’ (n=113, 56·8% of respondents) is shown in Table 2. Almost 70% of these performed primarily clinical or field work and 78·6% said they had experience with AMU; however, not all these respondents provided responses to all questions (Tables 3 and 4).
Table 2. Demographic characteristics of 113 survey respondents self-rating their professional experience with ornamental fish at medium-high to high

Table 3. Reported frequency of usage of selected antimicrobial drugs in ornamental fish
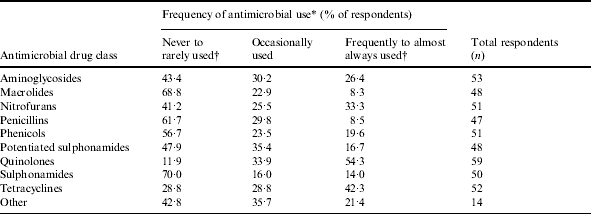
* Categories for frequency of antimicrobial use were based on a five-point ordinal scale: 1, never used; 2, rarely used, 3, occasionally used; 4, frequently used; 5, always used.
† Categories ‘never used’ (1) and ‘rarely used’ (2) and categories ‘frequently used’ (4) and ‘always used’ (5) were collapsed.
Table 4. Reported usage of antimicrobial drugs in ornamental fish by production phase and purpose for using antimicrobial drugs
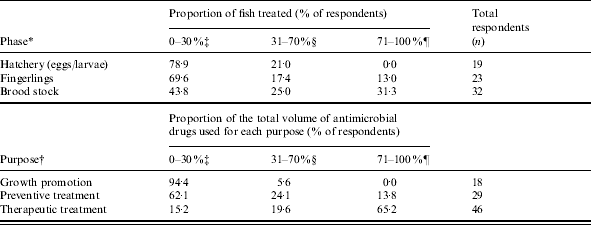
* Estimate of what proportion of ornamental fish production was treated with antimicrobial drugs in each production phase.
† Estimate of the proportion of the total volume of antimicrobial drugs used in ornamental fish culture for the purposes listed.
‡ Collapsed to include 0–10 %, 11–20 % and 21–30 %.
§ Collapsed to include 31–40 %, 41–50 %, 51–60 % and 61–70 %.
¶ Collapsed to include 71–80 %, 81–90 % and 91–100 %.
Quinolones were the most frequently reported antimicrobial drug class used, followed by tetracyclines, nitrofurans, and aminoglycosides (Table 3). The highest proportion of fish treated was reported for the brood-stock production phase (31·3% of respondents stating ‘71–100% of fish were treated with antimicrobial drugs’, Table 4). Sixty-five percent of respondents indicated between 71% and 100% of antimicrobials were used for therapeutic use, while use as preventive treatment and growth promotion was less frequent (Table 4). Three factors, ‘inappropriate duration of treatment’ (74·5%), ‘absence of accurate diagnosis’ (73·5%) and ‘use of antimicrobials in place of improving husbandry’ (73·5%) were selected as the main contributors to the development of AMR. Resistance to tetracyclines was most frequently reported, followed by penicillins and potentiated sulphonamides (Table 5).
Table 5. Frequency of observation of antimicrobial resistance (AMR) by drug class in ornamental fish
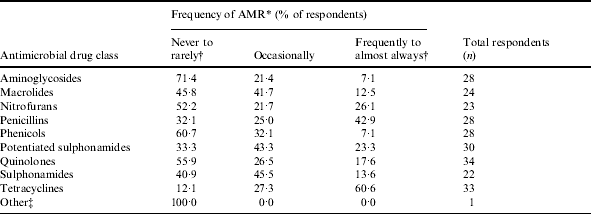
* Categories for frequency of observation of AMR by drug class were based on a five-point ordinal scale: 1, never; 2, rarely; 3, occasionally; 4, frequently, 5, almost always.
† Categories ‘never’ (1) and ‘rarely’ (2) and categories ‘frequently’ (4) and ‘almost always used’ (5) were collapsed.
‡ Responses under other are incomplete as no antimicrobials were described.
Twenty-three (32·9% of forms sent) English and no Spanish non-respondents answered the non-response form. From Fisher's exact test, no differences (P>0·05) were observed between responders and non-responders. Most non-responders selected ‘I don't believe I can contribute as it is not relevant to my professional experience’ as a reason for refusal in participation (60·9%, 14/23) followed by ‘I don't have time’ (21·7%, 5/23).
DISCUSSION
Systematic review
While the percentage of the population having contact with aquaria through hobby or work is largely unknown, reports indicate that individuals exposed to ornamental fish might be at greater risk for zoonotic infections, particularly for M. marinum and Salmonella serovars. This has prompted clinicians to advise caution around handling ornamental fish and aquaria for vulnerable populations [Reference Devchaud and Gullick58, Reference Musto71, Reference Cremonesini and Thomson86]. It is important to note that in the majority of the case reports analysed, clinicians hypothesized causation due to direct topical contact of cases with fish or aquarium water (other than Salmonella cases, which are caused by ingestion), but this was rarely confirmed by concurrent isolation of the suspected pathogen from the fish exposure source. In only 10 studies was the linkage confirmed through detection of a phenotypic match from the patient and their fish and/or aquarium water [Reference Parent35, Reference Gaulin, Vincent and Ismail68–Reference Boxall73, Reference Abalain-Colloc87–Reference Booth, Lang and Athersuch89], while a genotypic match was seldom confirmed [Reference Levings70, Reference Boxall73]. Lack of research reporting contributions of other zoonotic pathogens to human illness due to exposure to ornamental fish may be due to under-reporting. Infection may cause only relatively mild gastrointestinal disease, chiefly diarrhoea [Reference Lowry and Smith13, Reference Novotny15], and physicians might therefore only seldom see such cases [Reference Mead90, Reference Majowicz91]. Many pathogens of aquatic origin have fastidious culture requirements, making diagnosis difficult if not suspected and specifically requested for isolation by clinicians. The human case reports incriminating M. marinum in ‘fish handler's disease’ or ‘fish tank granuloma’ [Reference Lowry and Smith13] are probably over-represented in the literature due to external, easily visible (and photographable) lesions suitable for publication.
Epidemiological and bacterial links between cases and posited source-exposures were more frequently investigated in outbreaks of salmonellosis, probably as result of coordinated (and perhaps regulatory) response within respective jurisdictions [Reference Gaulin, Vincent and Ismail68–Reference Boxall73, Reference Senanayake88]. The detection of the ‘fish tank strain’ S. Paratyphi B var. Java in cattle, genetically different only in the presence of plasmids coding for sulphonamide and trimethoprim resistance [Reference Evans92], and S. Javiana, the predominant strain in ornamental fish in Trinidad also found in cases of human gastroenteritis, pet animals, cattle, and wildlife [Reference Newaj-Fyzul, Adesiyun and Mutani93], indicate possible ‘cross-over’ into terrestrial agriculture. Surveillance programmes for Salmonella in food and other animals should also include strains commonly found in ornamental fish populations as this source for the next epidemic strain of this particular genus must not be discounted. Future efforts to ascertain potential associations between exposure to ornamental fish and illness in humans should include well-designed and executed case-control studies along with genotyping of strains found in humans, and fish or the fish environment. Currently, the extent of population exposure to ornamental fish through hobby or work is largely unknown. Better understanding of this aspect is necessary before any larger surveillance initiative is considered. Recommendations for public promotion of good hygiene practices around ornamental fish, the institution of importation practices designed to curtail the entry of ornamental fish with antimicrobial resistant bacteria into the country [Reference Musto71, Reference Boxall73] and screening programmes aimed at ornamental fish imported facilities [Reference Gaulin, Vincent, Alain and Ismail69] were all called for by public health offices.
Aeromonas spp. were the most-frequently reported zoonotic pathogen in bacterial surveys of ornamental fish populations [Reference Kleingold5, Reference Verner-Jeffreys74–Reference Redondo, Jarero and Figueroa76, Reference Dixon and Issvoran79, Reference Del Rio-Rodriguez and Turnbull81–Reference Dixon and Issvoran84, Reference Taylor94], with purposive selection of this pathogen noted [Reference Verner-Jeffreys74, Reference Ansary75, Reference Dixon and Issvoran79, Reference Del Rio-Rodriguez and Turnbull81, Reference Dixon and Issvoran84]. This finding did not correlate with the number of case reports (n=1) [Reference Cremonesini and Thomson86] linking disease in humans to this pathogen, probably because this is primarily a fish health pathogen [Reference Lowry and Smith13]. The second most commonly investigated pathogen was Mycobacterium spp. [Reference Mouton, Basson and Impson95, Reference Prearo96–Reference Macri98], particularly M. fortuitum [Reference Mouton, Basson and Impson95, Reference Prearo96–Reference Macri98] and M. marinum [Reference Pate97]. Prearo et al. [Reference Prearo96] advocated that Mycobacterium spp. be listed in European Union (EU) ornamental fish importation guidelines as one of the pathogens required for ‘disease free’ certification, both from animal health and public health perspectives. The Centers for Disease Control (CDC) has already listed M. marinum as an emerging zoonotic pathogen [Reference Evans99].
When examined by antimicrobial pharmaceutical class a wide range of resistance was observed in most classes, particularly for warm-water fish species; however, the former also represented a greater number of fish species examined. AMR levels reported in research publications were largely in agreement with the survey respondents' perception of the level of AMR in ornamental fish populations, including high levels of tetracycline resistance (Tables 1 and 5). Several researchers in our review posited the potential for transfer of AMR via ornamental fish to human populations [Reference Verner-Jeffreys74, Reference Huys80]. In this context, resistance in ornamental fish zosonotic pathogens is somewhat concerning, particularly in the case of resistance to fluoroquinolones or other antimicrobial drugs of critical importance in human medicine [Reference MacDougall100, 101].
We were unable to identify, through SR, any study reporting AMU by ornamental fish producers. Due to lack of any published information on AMU in ornamental fish, data generated through our questionnaire fill an important gap in knowledge and provide initial baseline semi-quantitative information on AMU in ornamental fish. Comparisons between respondents and non-respondents did not indicate strong biases in our respondent population. Nevertheless, additional quantitative data measured through more robust and precise instruments are needed.
Over 65% of respondents linked development of AMR due to an emphasis on AMU in place of improvements in husbandry practices. While the country-level regulations regarding labelled AMU (along with withdrawal times) are generally in place for food-intended aquaculture production, such regulations are generally non-existent, or not enforced, for ornamental fish. Given the stressful shipping conditions facing ornamental fish (e.g. periods of hypoxia, long air flights), antibiotics are often routinely added to shipping water [Reference Cole6, Reference Kleingold20] to prevent disease. The end result of this practice may be the development of AMR. The Australian government has recommended that the use of medications on fish in quarantine (at importers' facilities) be limited [Reference Chong and Whittington7]. Efforts to ensure the health of ornamental fish in exporting countries will require a great deal of trust. For example, Singapore exporters ship an average of 50 consignments of tropical fish daily and inspection of every shipment by veterinary officials it is likely to be impractical [Reference Jing and Chuan8]. When Kleingold et al. [Reference Kleingold20] measured antibiotic residues in ornamental fish, only 1/8 species examined was free of antimicrobials. In a separate study, Kleingold et al. reported a marked increase in resistance index to enrofloxacin in all fish (food and ornamental species) attributing this phenomenon to the use of this antimicrobial by cold-water ornamental fish hobbyists [Reference Kleingold5].
The findings of our review and survey indicate that government authorities should seriously consider public health risks associated with zoonotic pathogens and AMR in ornamental fish. In Canada, others [Reference Trust, Bartlett and Lior3] have reported salmonellae in aquariums in 1981 recommending surveillance consideration at that time [Reference Trust, Bartlett and Lior3]. Risk-based surveillance is defined as ‘a surveillance programme in the design of which exposure and risk assessment methods have been applied together with traditional design approaches in order to ensure appropriate and cost-effective data collection’ [Reference Stark102]. This framework provides a sound approach for the development of effective and feasible surveillance that should be considered within the context of each country. It requires prior epidemiological knowledge in order to determine occurrence of disease in differing population strata or the influence of risk factors [Reference Stark102]. Our review provides some relevant and important baseline information for consideration of such potential surveillance or research programmes, globally and within the Canadian context. In 2008, Canada imported ornamental fish from over 110 different countries worth over Can$9·7 million [2], primarily from the USA, Singapore and Thailand [103]. Given the almost non-existent state of domestic ornamental fish production in Canada, targeting ornamental fish imports with risk-based sampling and testing for Salmonella and Mycobacterium spp. and AMR in zoonotic or indicator bacteria might be a reasonable focus of such potential surveillance efforts. Within the Canadian context, the baseline prevalence studies of healthy ornamental fish should be undertaken in a systematic manner to determine the bacterial flora, AMR patterns, and resistance genes present in fish and their transport/holding water, as well as countries of importation. The financial sustainability of such initiatives is very challenging, and requires considerable efforts from various government and industry stakeholders.
User-friendly educational material needs to target ornamental fish hobbyists and employees in the ornamental fish industry in order to increase awareness of the zoonotic potential of ornamental fish species. A study in 2003 by Schmoor et al. [Reference Schmoor104] in France surveyed tropical fish salespeople on their knowledge of M. marinum. Only 15% of respondents had in-depth knowledge of the pathogen and 75% ignored the problem. Among 22·5% of the respondents who were fish salespersons with some formal training, only one-third had been taught about the pathogen. Most of the workers immersed their hands in the tanks without wearing gloves, and only a few reported destroying all the fish from an infected tank. An analogous questionnaire to fish hobbyists in the UK revealed similar lack of knowledge both in terms of fish and zoonotic diseases [Reference Gray55]. A public education campaign aimed at Canadian hobbyists and aquarium workers should be considered to make them aware of the potential for illness and the steps they can take to prevent disease. A letter by Hay & Seal [Reference Hay and Seal105] responding to a study purporting the beneficial physiological and psychological effects of watching ornamental fish, sums up the dangers of aquarium fish, advising ‘ornamental fish – look but do not touch!’
NOTE
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/hyg).
ACKNOWLEDGEMENTS
The authors thank Drs Carol McClure, Sofie St-Hilaire, Victoria Alday and Roy Yanong for their assistance with the development and pre-testing of the questionnaire; and Dr America Mederos for translating the questionnaire from English to Spanish and for her generous help with administration of the non-respondent questionnaire in Spanish. We also thank Dr Chantal Vincent of the Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec. This study was funded by the Public Health Agency of Canada-Laboratory for Foodborne Zoonoses in Guelph, Canada.
The authors are saddened to report the sudden passing away of co-author Lucie Dutil, on 15 August 2011.
DECLARATION OF INTEREST
None.









