The small intestine (SI) is an important nutrient digestion and absorption site for mammals (including pigs and humans), and its health is closely related to the growth of young animals. In pig production, early-weaned piglets are susceptible to infection by enteropathogenic bacteria because the immune function of the gastrointestinal tract has not yet matured(Reference Campbell, Crenshaw and Polo1–Reference Den Hil, Litjens and Oudshoorn3). In particular, severe diarrhoea after weaning due to infection with enterotoxigenic Escherichia coli (ETEC) is a main cause of piglet growth performance decline and even death(Reference Pan, Zhao and Ma4,Reference Huang, Wang and He5) . ETEC and its derived enterotoxins can stimulate the secretion of pro-inflammatory factors and induce oxidative stress, which further cause epithelial cell apoptosis and villous atrophy in the SI(Reference Huang, Wang and He5–Reference Xu, Xu and Chen7). Atrophy of the villi in the SI (especially in the jejunum) directly disturbs the transport of nutrients and ultimately affects the growth performance and feed conversion efficiency of piglets(Reference Campbell, Crenshaw and Polo1,Reference Wijtten, Der Meulen and Verstegen2) . Dietary supplementation with pharmacological concentrations of inorganic Zn (1000–3000 mg/kg Zn2+) is an effective nutrition strategy and can improve intestinal health and alleviate weaning stress in weaned piglets(Reference Sun, Xia and Wu8,Reference Lei and Kim9) . However, high dietary Zn supplementation also increases the concentration of Zn in faeces, thereby polluting the environment(Reference Jensen, Kyvsgaard and Battisti10). As a result, many countries have issued policies to limit high-Zn diets in weaned piglets. At present, the Ministry of Agriculture and Rural Affairs of China has stipulated that the maximum Zn content in the feed of weaned piglets should be 1600 mg/kg(Reference Sun, Xia and Wu8). Therefore, it is necessary to find an effective and environmentally safe alternative.
Recently, the potential applications of nanotechnology in feed additives have been investigated(Reference Swain, Rao and Rajendran11). The use of nano-sized ZnO to alleviate weaning stress in piglets can reduce the final Zn concentration in the diet(Reference Sun, Xia and Wu8). However, the paradox is that nano-sized ZnO causes cytotoxicity in the SI and can also disrupt liver metabolism(Reference Moreno-Olivas, Tako and Mahler12,Reference Zhang, Zhao and Li13) . Chitosan (CS) is a natural product obtained from partial N-deacetylation of chitin, which is the second most abundant carbohydrate polymer in nature(Reference Hamed, Ozogul and Regenstein14). The structure of CS is well suited for Zn ion binding via ion exchange, complexation, physical sorption and inter- and intra-molecular entrapment(Reference Qi, Xu and Jiang15). Some studies have shown that CS–Zn chelate (in non-nanoparticle form) has a positive effect on the intestinal health of weaned piglets(Reference Han, Ma and Lv16,Reference Hu, Cheng and Li17) . However, the regulatory mechanism of the CS–Zn complex on piglet growth performance and intestinal antioxidant activity needs to be further clarified. In mammals, nanoparticles may have a higher bioavailability due to easier diffusion into the intestinal submucosa(Reference Desai, Labhasetwar and Amidon18,Reference Tian, Xu and Li19) . Thus far, some synthetic methods for loading Zn ions into CS-based nanoparticles have been widely studied in the field of agricultural science(Reference Rajasekaran and Santra20,Reference Deshpande, Dapkekar and Oak21) . However, little is known about the roles of CS–Zn nanoparticles in mammals. CS–Zn nanoparticles exhibit good antibacterial activity and have lower cytotoxicity than inorganic Zn in vitro (Reference AbdElhady22,Reference Kaur, Thakur and Barnela23) , suggesting that they may protect the intestine against ETEC infection in vivo. In this study, a nano chitosan–Zn complex (CP–Zn; particle diameter 50–150 nm) was synthesised by an ionotropic gelation method. Considering the similarities in anatomy and physiology of the gastrointestinal tract between pigs and humans(Reference Gonzalez, Moeser and Blikslager24), studying the intestinal health and growth mechanisms of weaned piglets could provide insights for future studies on infants and young children. Here, we hypothesised that dietary supplementation with a low concentration of Zn from CP–Zn could alleviate weaning stress in piglets. We combined an in vivo experiment on weaned piglets challenged with ETEC K88 and an in vitro experiment on IPEC-J2 cells to study the effects of CP–Zn on the growth performance and antioxidant capacity of the SI in weaned piglets and elucidate the underlying mechanisms.
Materials and methods
Ethics approval
All experimental protocols and procedures involving piglets were conducted in accordance with the guidelines established by the Institutional Animal Care and Use Committee of Nanjing Agricultural University. The study was approved by the Animal Care and Use Committee of Nanjing Agricultural University (approval no. SYXK 2019-0771).
Diets, experimental design and sample collection
Piglets received diets supplemented with different feed additives based on an antibiotic-free maize/soyabean meal that were formulated according to the recommendations of the National Research Council (NRC, 2012). The composition and nutrient content of the basal diet are listed in online Supplementary Table S1. CS with a deacetylation degree >95 % and an average molecular weight of 80 000–90 000 Da was purchased from Haidebei Marine Biological Engineering Co. Ltd. Zinc sulphate heptahydrate (ZnSO4) and ZnO, analytical reagents, were purchased from Sinopharm. CP–Zn (diameter 50–150 nm), a CS–Zn complex containing 5–10 % Zn and 50–60 % CS, was made in our laboratory at the College of Animal Science and Technology of Nanjing Agricultural University (Chinese patent: 201910039700.4).
A total of seventy-two piglets (Duroc × Landrace × Large White, male) weaned at day 21 with an average body weight of 6·21 (sd 0·49) kg were randomly divided into six treatment groups, twelve piglets in each treatment, and three piglets in one pen. The six dietary treatment groups are as follows: (1) basal diet (CON group); (2) the basal diet supplemented with 1600 mg/kg Zn from ZnO (H-ZnO group); (3) the basal diet supplemented with 100 mg/kg Zn from ZnSO4 (ZnSO4 group); (4) the basal diet supplemented with 100 mg/kg Zn from CP–Zn (CP–Zn group); (5) the basal diet supplemented with 100 mg/kg Zn from ZnSO4 and the same concentration of CS (766 mg/kg) as the CP–Zn group (CS + ZnSO4 group) and (6) the basal diet supplemented with the same concentration of CS as that in the CP–Zn group but not supplemented with Zn (CS group). All piglets had free access to feed and water. The pig pens were cleaned twice a day to maintain cleanliness, the ambient temperature of the pig house was maintained at 22–25°C, and the living environment of the piglets was in accordance with the animal welfare guidelines.
A 14-d feeding trial was performed, the diarrhoea incidence, average daily gain (ADG), average daily feed intake and feed:gain ratio of weaned piglets were determined according to the methods described by Long et al. (Reference Long, Xu and Pan25) and the pen was used as the experimental unit. The piglet faeces were visually assessed every day by observers blinded to the treatments. Fresh excreta were graded according to the following scale: 1 = hard faeces, 2 = slightly soft faeces, 3 = soft faeces (partially formed), 4 = loose (semi-liquid) faeces and 5 = watery (mucous-like) faeces. Piglets with stool scores of 4 and 5 were identified as having diarrhoea. The diarrhoea incidence was calculated according to the following formula: diarrhoea rate (%) = 100 × (number of piglets with diarrhoea × number of diarrhoea days)/(total number of piglets × number of experiment days). After the feeding trial, at 08.00 hours on the 15th day, six piglets were randomly selected from each treatment group for oral ingestion of ETEC K88 at a concentration of approximately 1 × 109 colony-forming units/ml in 10 ml of PBS per piglet according to the methods of Huang et al. (Reference Huang, Wang and He5). After 48 h of ETEC K88 challenge, all challenged piglets were slaughtered and sampled. The piglets were anaesthetised with intravenous sodium pentobarbital (50 mg/kg body weight), and the piglets were euthanised by a competent person in a separate room out of sight of the other piglets. Blood was sampled from the anterior vena cava and centrifuged at 860 g for 15 min to obtain serum. Then, the serum samples were stored at −80°C for subsequent analysis. The contents of the gastrointestinal tract (stomach, duodenum and jejunum), the mucosa of the SI (duodenum and jejunum) and the longissimus dorsi muscle were collected and immediately stored in liquid N2 for subsequent analysis. In addition, fresh faeces at the end of the rectum of each piglet were collected and immediately stored at 4°C. The live E. coli in faeces were quantitated using MacConkey agar (Beijing AOBOX Biotechnology) within 24 h according to the methods of a previous study(Reference Long, Xu and Pan25).
Digestive organ index assessment and haematoxylin–eosin staining
After piglets were slaughtered, the stomach and SI of each piglet were removed. The wet weights of these organs were determined after gently removing the mesentery and fat on the organ surface. Then, the length of the SI was measured with a ruler. Next, samples of duodenal and jejunal tissue were lightly cut and fixed in a 4 % paraformaldehyde solution for 24 h. The fixed samples were dehydrated with alcohol, cleared with xylene and finally embedded in paraffin. The embedded samples were sectioned into 5 μm thick sections and stained with haematoxylin–eosin. The stained sections were observed under a light microscope (Nikon YS100; Nikon Corporation). The villus height (VH) and crypt depth were measured using ImageJ software.
ELISA assay
The levels of growth hormone, insulin, insulin-like growth factor 1 (IGF1) and epidermal growth factor in serum were measured with ELISA kits purchased from CUSABIO Biotechnology according to the manufacturer’s instructions. In addition, the levels of inflammatory cytokines, including TNF-α, IL-1β, interferon-γ and myeloperoxidase in serum were determined with ELISA kits purchased from Enzyme-linked Biotechnology according to the manufacturer’s instructions. For each type of assay, the intra-assay CV (CV%) was <5 % and the inter-assay CV% was <8 %.
Cell culture and treatment
Cells of the porcine jejunal epithelial cell line (IPEC-J2) were cultured in an antibiotic-free DMEM-F12 medium (Life Technologies) containing 10 % fetal bovine serum (Life Technologies). The IPEC-J2 cells were maintained at 37°C in a humidified atmosphere of 5 % CO2. To evaluate the oxidative status and the expression levels of nuclear factor erythroid 2-related factor 2 (Nrf2) pathway-related proteins, the IPEC-J2 cells were pretreated with ZnSO4, CP–Zn, CS + ZnSO4 or CS for 24 h and then co-treated with lipopolysaccharide (LPS) for another 6 h. The appropriate treatment concentrations of ZnSO4, CP–Zn, CS + ZnSO4 and LPS were chosen based on the results of a cell viability assay conducted using an MTT kit (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s instructions.
Analysis of digestive enzymes and antioxidant parameters
To determine the levels of digestive enzymes and antioxidant parameters, tissue and cell samples were homogenised and then centrifuged at 9500 g at 4°C for 15 min to obtain the supernatant. The protein concentrations of jejunal mucosal samples from weaned piglets and in vitro cell samples were determined with bicinchoninic acid kits (Solarbio Life Sciences). The activity levels of the digestive enzymes pepsin, trypsin, amylase, lipase, maltase, sucrase and lactase in the jejunums of weaned piglets were determined with commercial reagent kits (Nanjing Jiancheng Bioengineering Institute). Antioxidant parameters, namely, the levels of total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-px), catalase, malondialdehyde (MDA), GSSG and GSH in the jejunal mucosa and serum of weaned piglets and in IPEC-J2 cells, were assayed with commercial reagent kits (Nanjing Jiancheng Bioengineering Institute). In addition, the intracellular reactive oxygen species (ROS) levels in IPEC-J2 cells were determined by the DCFH-DA method (Sigma) according to the manufacturer’s instructions.
Quantitative real-time PCR analysis
Quantitative real-time PCR analysis was performed according to the methods in a previous study(Reference Tang, Huang and Liu26). Briefly, total RNA was extracted from the jejunal mucosae of piglets using an RNAiso Plus Kit (TaKaRa) according to the instructions. The concentration and purity of RNA were determined with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific), and the quality and integrity of the PCR products were evaluated by electrophoresis on 1 % agarose gels. Total RNA was reverse-transcribed into cDNA using a PrimeScript™ RT Reagent Kit (TaKaRa). The cDNA samples were twofold-diluted to determine the amplification efficiency. Quantitative real-time PCR was conducted with a QuantStudio® 5 Real-Time PCR Instrument (Applied Biosystems) using a TB Green® Premix Ex Taq™ II Kit (TaKaRa) according to the manufacturer’s instructions. The relative mRNA expression levels were calculated using the 2−ΔΔCT method with β-actin serving as the housekeeping gene(Reference Livak and Schmittgen27). The relative mRNA expression of each target gene was normalised to the expression in the control group. The primers are listed in online Supplementary Table S2.
Western blot analysis
Western blot analysis was performed according to Tang’s work(Reference Tang, Huang and Liu26). Briefly, all samples (longissimus dorsi muscle, jejunal mucosa and IPEC-J2 cells) were lysed using radio-immunoprecipitation assay (RIPA) buffer with protease inhibitors (Solarbio Life Sciences). Then, the samples were homogenised and centrifuged at 9500 g for 15 min at 4°C, and the supernatant was collected. For standardisation, the total protein concentrations of all samples were determined with a bicinchoninic acid kit. The extracted protein samples were electrophoresed on an 8 % SDS-PAGE gel (GenScript) at 140 V for 50 min and then electrotransferred onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad) at 200 mA for 40–200 min. Primary antibodies against insulin receptor substrate 1 (IRS1), ribosomal S6 kinase (p70S6K), mammalian target of rapamycin (mTOR), Nrf2, NAD(P)H:quinone oxidoreductase 1 (NQO1), haeme oxygenase 1 (HO1) and β-actin (1:500–5000) were purchased from Proteintech. Primary antibodies against phospho-p70S6K and phospho-mTOR (1:1000) were purchased from Cell Signaling Technology. The PVDF membranes with migrated proteins were incubated with the primary antibodies overnight at 4°C. After washing with TRIS-buffered saline with Tween, the immunoblots were incubated with horseradish peroxidase-conjugated anti-rabbit (or anti-mouse) IgG (1:2000; Cell Signaling Technology) for 1 h at room temperature. Subsequently, the Western blot bands were visualised using a Tanon™ High-sig ECL Western Blotting Substrate (Tanon) with a Tanon 4200SF Gel Imaging System (Tanon), and the band densities were quantitated by densitometry using ImageJ software (NIH). The levels of all phosphorylated proteins were normalised to those of their corresponding non-phosphorylated proteins, and β-actin served as an internal reference.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 7.01 (GraphPad Software Inc.) and SPSS 22.0 software. Significant differences among treatment groups were evaluated by one-way ANOVA (with Tukey’s multiple comparisons test) if the data exhibited a Gaussian distribution and had equal variance or by the Kruskal–Wallis test (with Dunn’s multiple comparisons test) if the data were not normally distributed. The data are presented as mean values with their standard errors. P values <0·05 were considered to indicate significant differences.
Results
Growth performance and diarrhoea rate
There were no adverse events in piglets during the whole experimental period. As shown in Table 1, there were no significant differences in the body weight of piglets on day 15 among all treatment groups (P > 0·05). However, the ADG in the H-ZnO group was significantly higher than that in the CON group (H-ZnO, 293·33 g; CON, 236·76 g; P < 0·05), and the ADG in the CP–Zn group was significantly higher than that in the CON and ZnSO4 groups (ZnSO4, 247·86 g; CP–Zn, 312·02 g; P < 0·05). Compared with the values in the CON group, the average daily feed intake values in the H-ZnO and CP–Zn groups were significantly higher (H-ZnO, 465·36 g; CON, 384·82 g; CP–Zn, 471·49 g; P < 0·05), while the feed:gain ratio in the CP–Zn group was significantly lower (CON, 1·63; CP–Zn, 1·51; P < 0·05). In addition, compared with the values in the CON group, the diarrhoea rate (H-ZnO, 7·14 %; CON, 18·45 %; CP–Zn, 5·95 %; day 1–15) and the count of E. coli in faeces (log10 colony-forming units/g wet faeces) (H-ZnO, 7·37; CON, 8·06; CP–Zn, 7·34; day 17) in the H-ZnO and CP–Zn groups were significantly lower (P < 0·05).
Table 1. Effects of nano chitosan–zinc complex (CP–Zn) on growth performance and the diarrhoea rate in weaned piglets
(Mean values and standard errors)
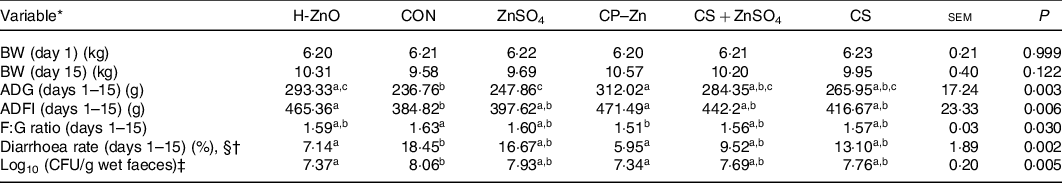
H-ZnO, basal diet supplemented with 1600 mg/kg Zn from ZnO; CON, basal diet; ZnSO4, basal diet supplemented with 100 mg/kg Zn from ZnSO4; CP–Zn, basal diet supplemented with 100 mg/kg Zn from CP–Zn; CS + ZnSO4, basal diet supplemented with 100 mg/kg Zn from ZnSO4 and 766 mg/kg of chitosan; CS, basal diet supplemented with 766 mg/kg of chitosan but not supplemented with Zn; BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; F:G ratio, feed:gain ratio; CFU, colony-forming units.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* BW (n 12); ADG (n 4); ADFI (n 4); F:G ratio (n 4); diarrhoea rate (n 4).
† With §: Kruskal–Wallis test; without §: ordinary one-way ANOVA.
‡ CFU = count of Escherichia coli (log10 CFU/g wet faeces) in faeces was determined on day 17 (n 6).
Digestive organ indexes and small intestine morphology
The digestive organ indexes of weaned piglets are presented in online Supplementary Table S3, and the results showed that the SI weight in the CP–Zn group was significantly higher than that in the CON group (CON, 827·93 g; CP–Zn, 991·77 g; P < 0·05). In addition, as shown in Fig. 1, in the duodenum, there were no significant differences in the VH, villus width, crypt depth or VH:crypt depth ratio among all treatment groups (P > 0·05). However, the VH in the jejunum was significantly higher in the CP–Zn group than in the CON and ZnSO4 groups (CON, 359·03 μm; ZnSO4, 383·17 μm; CP–Zn, 483·91 μm; P < 0·05) and was significantly higher in the CS + ZnSO4 group than in the CON group (CS + ZnSO4, 440·72 μm; P < 0·05). Compared with the CON group, the CP–Zn group had a significantly higher VH:crypt depth ratio (CON, 1·52; CP–Zn, 2·15; P < 0·05).

Fig. 1. Effects of nano chitosan–zinc complex (CP–Zn) on intestinal morphology in weaned piglets. (a) Villus height (VH), crypt depth (CD), VH:CD ratio and villus width (VW) in the duodenum; (b) VH, CD, VH:CD ratio and VW in the jejunum. The VH:CD ratio in the duodenum and the CD in the jejunum were analysed with the Kruskal–Wallis test, and the other variables were analysed by ordinary one-way ANOVA. The data are mean values with their standard errors, n 6. * P < 0·05, ** P < 0·01, *** P < 0·001. ![]() , Basal diet;
, Basal diet; ![]() , basal diet supplemented with 100 mg/kg Zn from ZnSO4;
, basal diet supplemented with 100 mg/kg Zn from ZnSO4; ![]() , basal diet supplemented with 100 mg/kg Zn from CP–Zn;
, basal diet supplemented with 100 mg/kg Zn from CP–Zn; ![]() , basal diet supplemented with 100 mg/kg Zn from ZnSO4 and 766 mg/kg of chitosan;
, basal diet supplemented with 100 mg/kg Zn from ZnSO4 and 766 mg/kg of chitosan; ![]() , basal diet supplemented with 766 mg/kg of chitosan but not supplemented with Zn.
, basal diet supplemented with 766 mg/kg of chitosan but not supplemented with Zn.
Digestive enzymes and nutrient transporters in the gastrointestinal tract
The digestive enzyme results are presented in Table 2. In the stomach, no significant differences were observed in the pepsin activity of the mucosa or the mucosal contents among all treatment groups (P > 0·05). In the SI, no significant differences were observed in the activity levels of trypsin and lipase in the duodenum and jejunum among all treatment groups (P > 0·05). Compared with the CON group, the CP–Zn, CS + ZnSO4 and CS groups exhibited significantly higher amylase activity in the duodenum (CON, 327·10 U/mg protein; CP–Zn, 466·57 U/mg protein; CS + ZnSO4, 460·89 U/mg protein; CS, 451·95 U/mg protein; P < 0·05) and jejunum (CON, 175·37 U/mg protein; ZnSO4, 185·42 U/mg protein; CP–Zn, 269·49 U/mg protein; CS + ZnSO4, 256·29 U/mg protein; CS, 251·17 U/mg protein; P < 0·05). The amylase activity of the jejunum was also significantly higher in the CP–Zn and CS + ZnSO4 groups than in the ZnSO4 group (P < 0·05). With regard to disaccharide-splitting enzymes in the jejunum, the activity levels of maltase (CON, 50·74 U/mg protein; ZnSO4, 58·94 U/mg protein; CP–Zn, 81·54 U/mg protein; CS + ZnSO4, 84·44 U/mg protein; CS, 80·81 U/mg protein) and sucrase (CON, 21·97 U/mg protein; ZnSO4, 24·63 U/mg protein; CP–Zn, 34·57 U/mg protein; CS + ZnSO4, 31·62 U/mg protein; CS, 32·16 U/mg protein) were significantly higher in the CP–Zn, CS + ZnSO4 and CS groups than in the CON group (P < 0·05). In addition, the activity of maltase in the CS + ZnSO4 group and sucrase in the CP–Zn group was significantly higher than those in the ZnSO4 group (P < 0·05). Compared with the CON group, the CP–Zn and CS + ZnSO4 groups exhibited significantly higher lactase activity (CON, 5·70 U/mg protein; CP–Zn, 9·26 U/mg protein; CS + ZnSO4, 9·09 U/mg protein; P < 0·05).
Table 2. Effects of nano chitosan–zinc complex (CP–Zn) on digestive enzymes of weaned piglets (n 6)
(Mean values and standard errors)

CON, basal diet; ZnSO4, basal diet supplemented with 100 mg/kg Zn from ZnSO4; CP–Zn, basal diet supplemented with 100 mg/kg Zn from CP–Zn; CS + ZnSO4, basal diet supplemented with 100 mg/kg Zn from ZnSO4 and 766 mg/kg of chitosan; CS, basal diet supplemented with 766 mg/kg of chitosan but not supplemented with Zn.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* With §: Kruskal–Wallis test; without §: ordinary one-way ANOVA.
The mRNA expression levels of nutrient transporters in the jejunum are shown in Fig. 2. The relative mRNA expression levels of Na+-dependent glucose transporter 1 (SGLT1), glucose transporter type 2 (GLUT2) and excitatory amino acid carrier 1 (EAAC1) in the CP–Zn group were significantly higher than those in the CON and ZnSO4 groups (P < 0·05), and the relative mRNA expression level of peptide transporter 1 (PEPT1) in the CP–Zn group was significantly higher than that in the CON group (P < 0·05). In addition, the relative mRNA expression level of GLUT2 in the CS + ZnSO4 group was significantly higher than that in the CON group (P < 0·05), and the relative mRNA expression level of EAAC1 in the CS + ZnSO4 group was significantly higher than those in the CON and ZnSO4 groups (P < 0·05).

Fig. 2. Effects of nano chitosan–zinc complex (CP–Zn) on the mRNA expression levels of nutrient transporters in the jejunums of weaned piglets. SGLT1, Na+-dependent glucose transporter 1; GLUT2, glucose transporter type 2; PEPT1, peptide transporter 1; EAAC1, excitatory amino acid carrier 1; SLC6A19, neutral amino acid transporter; FABP2, fatty acid binding protein 2. The data for PEPT1 and FABP2 were analysed with the Kruskal–Wallis test, and the other data were analysed by ordinary one-way ANOVA. The data are mean values with their standard errors, n 6. * P < 0·05, ** P < 0·01. ![]() , Basal diet;
, Basal diet; ![]() , basal diet supplemented with 100 mg/kg Zn from ZnSO4;
, basal diet supplemented with 100 mg/kg Zn from ZnSO4; ![]() , basal diet supplemented with 100 mg/kg Zn from CP–Zn;
, basal diet supplemented with 100 mg/kg Zn from CP–Zn; ![]() , basal diet supplemented with 100 mg/kg Zn from ZnSO4 and 766 mg/kg of chitosan;
, basal diet supplemented with 100 mg/kg Zn from ZnSO4 and 766 mg/kg of chitosan; ![]() , basal diet supplemented with 766 mg/kg of chitosan but not supplemented with Zn.
, basal diet supplemented with 766 mg/kg of chitosan but not supplemented with Zn.
Growth factors and inflammatory cytokines in serum
The concentrations of hormones in serum are shown in Fig. 3(a)–(d). The results showed that there were no significant differences in the levels of growth hormone and epidermal growth factor among all treatment groups (P > 0·05). The insulin content in the CP–Zn group was significantly higher than that in the CON group (CON, 10·09 μIU/ml; CP–Zn, 15·47 μIU/ml; P < 0·05), and the IGF1 content in the CP–Zn group was significantly higher than that in the CON and ZnSO4 groups (CON, 65·22 ng/ml; ZnSO4, 68·48 ng/ml; CP–Zn, 93·74 ng/ml; P < 0·05).

Fig. 3. Effects of nano chitosan–zinc complex (CP–Zn) on the concentrations of growth factors and inflammatory cytokines in the serum of weaned piglets. (a) Growth hormone (GH); (b) insulin; (c) insulin-like growth factor 1 (IGF1); (d) epidermal growth factor (EGF); (e) TNF-α; (f) IL-1β; (g) interferon-γ (IFN-γ); (h) myeloperoxidase (MPO). The EGF data were analysed with the Kruskal–Wallis test, and the other data were analysed by ordinary one-way ANOVA. The data are mean values with their standard errors, n 6. * P < 0·05, ** P < 0·01.
The inflammatory cytokine content of serum is shown in Fig. 3(e)–(h). The content of TNF-α in the CP–Zn group was significantly lower than that in the CON and ZnSO4 groups (CON, 171·19 pg/ml; ZnSO4, 160·55 pg/ml; CP–Zn, 115·29 pg/ml; P < 0·05), and the content of TNF-α in the CS + ZnSO4 group was also significantly lower than that in the CON group (P < 0·05). The levels of IL-1β (CON, 568·44 pg/ml; ZnSO4, 565·84 pg/ml; CP–Zn, 421·25 pg/ml; CS, 553·60 pg/ml) and myeloperoxidase (CON, 512·39 ng/ml; ZnSO4, 483·69 ng/ml; CP–Zn, 311·91 ng/ml; CS, 456·04 ng/ml) in the CP–Zn group were significantly lower than those in the CON, ZnSO4 and CS groups (P < 0·05).
Mammalian target of rapamycin pathway-related proteins in muscle
As shown in Fig. 4, Western blot analysis showed that the relative protein expression levels of IRS1 (IRS1/β-actin), phosphorylated mTOR/mTOR and phosphorylated p70S6K (p-p70S6K/p70S6K) in muscle were significantly higher in the CP–Zn group than in the CON and ZnSO4 groups (P < 0·05).

Fig. 4. Effects of nano chitosan–zinc complex (CP–Zn) on the mammalian target of rapamycin (mTOR) pathway in muscle in weaned piglets. (a, b) The protein expression levels of insulin receptor substrate 1 (IRS1), phosphorylated mammalian target of rapamycin (p-mTOR) and phosphorylated p70S6K (p-p70S6K) in muscle were detected by Western blot analysis. The data were analysed by ordinary one-way ANOVA. The data are mean values with their standard errors, n 6. * P < 0·05, ** P < 0·01. ![]() , Basal diet;
, Basal diet; ![]() , basal diet supplemented with 100 mg/kg Zn from ZnSO4;
, basal diet supplemented with 100 mg/kg Zn from ZnSO4; ![]() , basal diet supplemented with 100 mg/kg Zn from CP–Zn;
, basal diet supplemented with 100 mg/kg Zn from CP–Zn; ![]() , basal diet supplemented with 100 mg/kg Zn from ZnSO4 and 766 mg/kg of chitosan;
, basal diet supplemented with 100 mg/kg Zn from ZnSO4 and 766 mg/kg of chitosan; ![]() , basal diet supplemented with 766 mg/kg of chitosan but not supplemented with Zn.
, basal diet supplemented with 766 mg/kg of chitosan but not supplemented with Zn.
Antioxidant parameters and nuclear factor erythroid 2-related factor 2 pathway-related proteins in the jejunum
The antioxidant parameters in the serum and jejunums of weaned piglets are presented in Table 3. In serum, the MDA content in the CP–Zn group was significantly lower than that in the CON and ZnSO4 groups (CON, 10·34 nmol/ml; ZnSO4, 9·88 nmol/ml; CP–Zn, 6·42 nmol/ml; P < 0·05) and the GSH content in the CP–Zn group was significantly higher than that in the CON group (CON, 23·17 nmol/ml; CP–Zn, 36·74 nmol/ml; P < 0·05). In the jejunum, the activity of T-SOD (CON, 52·88 U/mg protein; ZnSO4, 60·62 U/mg protein; CP–Zn, 83·36 U/mg protein) and GSH-px (CON, 94·44 U/mg protein; ZnSO4, 100·86 U/mg protein; CP–Zn, 155·01 U/mg protein; CS + ZnSO4, 137·51 U/mg protein) and the content of GSH (CON, 16·93 nmol/mg protein; ZnSO4, 19·30 nmol/mg protein; CP–Zn, 31·49 nmol/mg protein; CS + ZnSO4, 27·70 nmol/mg protein) in the CP–Zn group were significantly higher than those in the CON and ZnSO4 groups (P < 0·05). The activity of GSH-px and the content of GSH in the CS + ZnSO4 group were also significantly higher than those in the CON group (P < 0·05). The MDA content in the jejunum was significantly lower in the CP–Zn group than in the CON, ZnSO4 and CS groups (CON, 2·07 nmol/mg protein; ZnSO4, 1·83 nmol/mg protein; CP–Zn, 1·10 nmol/mg protein; CS + ZnSO4, 1·46 nmol/mg protein; CS, 1·70 nmol/mg protein; P < 0·05) and was significantly lower in the CS + ZnSO4 group than in the CON group (P < 0·05).
Table 3. Effects of nano chitosan–zinc complex (CP–Zn) on the antioxidant parameters of weaned piglets (n 6)
(Mean values and standard errors)

CON, basal diet; ZnSO4, basal diet supplemented with 100 mg/kg Zn from ZnSO4; CP–Zn, basal diet supplemented with 100 mg/kg Zn from CP–Zn; CS + ZnSO4, basal diet supplemented with 100 mg/kg Zn from ZnSO4 and 766 mg/kg of chitosan; CS, basal diet supplemented with 766 mg/kg of chitosan but not supplemented with Zn; T-SOD, total superoxide dismutase; GSH-px, glutathione peroxidase; CAT catalase; MDA, malondialdehyde.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* With §: Kruskal–Wallis test; without §: ordinary one-way ANOVA.
In addition, the results of Western blot analysis of Nrf2 pathway-related proteins in the jejunum are shown in Fig. 5. The results showed that the relative protein expression level of Nrf2 was significantly higher in the CP–Zn group than in the CON, ZnSO4 and CS + ZnSO4 groups (P < 0·05) and also significantly higher in the CS + ZnSO4 group than in the CON group (P < 0·05). The relative protein expression level of NQO1 in the CP–Zn group was significantly higher than those in the CON and ZnSO4 groups (P < 0·05), and the relative protein expression level of HO1 in the CP–Zn group was significantly higher than those in the CON, ZnSO4 and CS groups (P < 0·05).

Fig. 5. Effects of nano chitosan–zinc complex (CP–Zn) on the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway of the jejunum in weaned piglets. (a, b) The protein expression levels of Nrf2, NAD(P)H:quinone oxidoreductase 1 (NQO1) and haem oxygenase 1 (HO1) in the jejunum were detected by Western blot analysis. The data were analysed by ordinary one-way ANOVA. The data are mean values with their standard errors, n 6. * P < 0·05, ** P < 0·01. ![]() , Basal diet;
, Basal diet; ![]() , basal diet supplemented with 100 mg/kg Zn from ZnSO4;
, basal diet supplemented with 100 mg/kg Zn from ZnSO4; ![]() , basal diet supplemented with 100 mg/kg Zn from CP–Zn;
, basal diet supplemented with 100 mg/kg Zn from CP–Zn; ![]() , basal diet supplemented with 100 mg/kg Zn from ZnSO4 and 766 mg/kg of chitosan;
, basal diet supplemented with 100 mg/kg Zn from ZnSO4 and 766 mg/kg of chitosan; ![]() , basal diet supplemented with the 766 mg/kg of chitosan but not supplemented with Zn.
, basal diet supplemented with the 766 mg/kg of chitosan but not supplemented with Zn.
Antioxidant parameters and nuclear factor erythroid 2-related factor 2 pathway-related proteins in IPEC-J2 cells
The cell viability of IPEC-J2 cells is shown in online Supplementary Fig. S1. The results showed that treatment with 0·5–2 mm Zn from ZnSO4, 1–2 mm Zn from CP–Zn and 1–2 mm Zn from CS + ZnSO4 significantly decreased the viability of IPEC-J2 cells (P < 0·05) (online Supplementary Fig. S1A). Treatment with 5–20 μg/ml LPS also significantly decreased the viability of IPEC-J2 cells (P < 0·05) (online Supplementary Fig. S1B). In addition, the protective effect of CP–Zn on IPEC-J2 cells challenged with LPS is presented in online Supplementary Fig. S1C. Compared with the LPS-treated group, the groups treated with CP–Zn containing 0·05 mm, 0·1 mm and 0·2 mm Zn exhibited significantly higher cell viability (P < 0·05). Based on these results, we chose 0·05 mm Zn for the ZnSO4, CP–Zn and CS + ZnSO4 groups and 1 μg/ml LPS as the further treatment concentrations.
The antioxidant parameters of IPEC-J2 cells are shown in Fig. 6(a)–(e). Compared with the values in the LPS group, the MDA content (CON, 4·37 nmol/mg protein; LPS, 7·86 nmol/mg protein; CP–Zn, 5·59 nmol/mg protein; CS + ZnSO4, 5·66 nmol/mg protein) and ROS intensity (CON, 100 %; LPS, 208 %; CP–Zn, 144 %; CS + ZnSO4, 148 %) in the CON, CP–Zn and CS + ZnSO4 groups were significantly lower (P < 0·05). Compared with the LPS group, the ZnSO4, CP–Zn and CS + ZnSO4 groups exhibited significantly higher T-SOD activity (LPS, 12·29 U/mg protein; ZnSO4, 16·64 U/mg protein; CP–Zn, 18·65 U/mg protein; CS + ZnSO4, 19·48 U/mg protein; P < 0·05), and the CP–Zn and CS + ZnSO4 groups exhibited significantly higher GSH-px activity (LPS, 33·02 U/mg protein; CP–Zn, 54·90 U/mg protein; CS + ZnSO4, 55·72 U/mg protein; P < 0·05). In addition, as shown in Fig. 6(f) and (g), Western blot analysis showed that the relative protein expression levels of Nrf2, NQO1 and HO1 in the CS + ZnSO4 and CP–Zn groups were significantly higher than those in the ZnSO4 and CS groups (P < 0·05).

Fig. 6. Effects of nano chitosan–zinc complex (CP–Zn) on antioxidant parameters and the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway in IPEC-J2 cells. IPEC-J2 cells were pretreated with ZnSO4, CP–Zn, chitosan (CS) + ZnSO4 and CS for 24 h (the zinc concentration was 0·05 mm in the ZnSO4, CP–Zn, CS + ZnSO4 groups, and the content of CS (25 μg/ml) was the same in the CP–Zn, CS + ZnSO4 and CS groups) and then co-treated with lipopolysaccharide (LPS) (1 μg/ml) for another 6 h. (a) Malondialdehyde (MDA); (b) reactive oxygen species (ROS); (c) total superoxide dismutase (T-SOD); (d) catalase (CAT); (e) glutathione peroxidase (GSH-px); (f, g) protein expression levels of Nrf2, NAD(P)H:quinone oxidoreductase 1 (NQO1) and haem oxygenase 1 (HO1) in muscle as detected by Western blot analysis. (g) +LPS: ![]() , ZnSO4;
, ZnSO4; ![]() , CP–Zn;
, CP–Zn; ![]() , CS + ZnSO4;
, CS + ZnSO4; ![]() , CS. The data were analysed by ordinary one-way ANOVA. The data are mean values with their standard errors, n 6. (a–e) Compared with the LPS group. * P < 0·05, ** P < 0·01, *** P < 0·001.
, CS. The data were analysed by ordinary one-way ANOVA. The data are mean values with their standard errors, n 6. (a–e) Compared with the LPS group. * P < 0·05, ** P < 0·01, *** P < 0·001.
Discussion
Body weight, ADG and average daily feed intake are considered to be key indicators of the growth performance of weaned piglets(Reference Huang, Wang and He5,Reference Zong, Huang and Zhang28) . Previous studies have shown that dietary supplementation with pharmacological doses of ZnO can improve the growth performance of weaned piglets(Reference Sun, Xia and Wu8,Reference Sales29,Reference Park, Jung and Kang30) . In the present study, we found that both H-ZnO treatment (1600 mg/kg Zn) and CP–Zn treatment (100 mg/kg Zn) significantly increased ADG and average daily feed intake, indicating that dietary CP–Zn supplementation can improve the growth performance of weaned piglets. Diarrhoea is usually an important cause of growth retardation in young piglets. Generally, fluids containing digestive factors are secreted in the SI to promote the digestion of nutrients, and the fluids are later reabsorbed in the large bowel (through a process that uses SCFA for energy)(Reference Topping and Clifton31,Reference Ramakrishna, Venkataraman and Srinivasan32) . However, ETEC adhesion can lead to fluid hypersecretion in the SI such that the fluid cannot be fully recovered by the large bowel, resulting in diarrhoea(Reference Pan, Zhao and Ma4,Reference Huang, Wang and He5,Reference Jin and Zhao33) . In the present study, CP–Zn treatment reduced the diarrhoea rate and the faecal E. coli count, suggesting that CP–Zn treatment inhibited adhesion of E. coli to the intestine. Similar findings were also observed in the H-ZnO treatment group. Improved growth performance and reduced diarrhoea rates are considered important signs of weaning stress alleviation in piglets. Therefore, the above results indicate that CP–Zn supplementation could be used as a substitute for high-Zn supplementation to relieve piglet weaning stress.
We further studied the mechanism by which dietary supplementation with CP–Zn improved the growth performance of weaned piglets. Growth performance is affected by digestive and absorptive functions. The endogenous enzymes of the gastrointestinal tract, including proteases, amylase and lipase, are critical for the digestion of nutrients(Reference Fan, Han and Xu34,Reference Patra, Amasheh and Aschenbach35) . In this study, dietary supplementation with CP–Zn did not change trypsin or lipase activity but significantly increased amylase activity in the duodenum and jejunum. Furthermore, dietary CP–Zn supplementation significantly increased maltase, sucrase and lactase activity in the jejunum. These results suggest that CP–Zn improves the digestibility of dietary carbohydrates in the SI of weaned piglets. Interestingly, our results also showed that amylase, sucrase and lactase activity was significantly increased by CS treatment and CS + ZnSO4 treatment. Therefore, we speculate that dietary supplementation with CP–Zn improves the digestibility of carbohydrates in the SI related to CS. Consistent with this hypothesis, previous reports have shown that CS can increase the activity of enzymes related to carbohydrate digestion in the SI of weaned piglets(Reference Guo36,Reference Zhang, Wan and Wu37) . One possible explanation for this effect is that CS, as a natural polysaccharide, can act as a substrate similar to disaccharidases and thus promote the activity of disaccharidases(Reference Collins, James and Smith38,Reference Toshinao and Sachiko39) .
Generally, after the diet is hydrolysed by digestive enzymes, nutrients are absorbed mainly in the jejunum(Reference Fan, Han and Xu34). Some key nutrient transporters of intestinal epithelial cells are responsible for transporting nutrients into the bloodstream(Reference Patra, Amasheh and Aschenbach35). Glucose uptake in epithelial cells depends primarily on two transporters, SGLT1 and GLUT2 (Reference Zong, Huang and Zhang28). PEPT1, which is highly expressed in the jejunal parietal membrane, plays a key role in absorbing dietary di- and tripeptides(Reference Zong, Huang and Zhang28). EAAC1 and neutral amino acid transporter (SLC6A19) are the major transporters of glutamate and neutral amino acids in the intestine, respectively(Reference Yang, Xiong and Wang40). In the present study, dietary CP–Zn supplementation increased the mRNA expression levels of SGLT1, GLUT2, PEPT1 and EAAC1 in the jejunum, suggesting that CP–Zn improved the nutrient absorption capacity of the SI. Notably, absorption of nutrients depends on the healthy development of the SI, which can be directly reflected by the VH and SI weight(Reference Zong, Huang and Zhang28). ETEC K88 gavage can disrupt the intestinal morphological integrity of piglets by reducing their VH(Reference Pan, Zhao and Ma4,Reference Huang, Wang and He5) . In this study, CP–Zn supplementation enhanced the jejunal VH. In addition, CP–Zn supplementation increased SI weight, indicating that CP–Zn promoted the development of the SI; however, the mechanism needs to be further studied. We also found that dietary supplementation with CP–Zn reduced the feed:gain ratio, which might have been attributable to the improvements in digestion and absorption in the SI.
Muscle growth is an important part of body development in young animals. E. coli LPS challenge impairs muscle growth in weaned piglets(Reference Liu, Li and Gong41). During muscle growth, IGF1, insulin, growth hormone and epidermal growth factor have pleiotropic effects that regulate cell proliferation, differentiation, metabolism, and myofibrillar growth and regeneration(Reference Wang, Yi and Hou42). The results of the present study showed that dietary supplementation with CP–Zn enhanced the levels of insulin and IGF1 in serum. The binding of insulin and IGF1 to their receptors on the cell surface in muscle tissue can activate the downstream mTOR signalling pathway through upstream IRS1 to mediate the PI3K-AKT pathway(Reference Glass43,Reference Hsieh, Chuang and Yang44) . In the mTOR signalling pathway, phosphorylated mTOR protein further phosphorylates p70S6K through a protein signalling cascade to promote cellular protein synthesis(Reference Hsieh, Chuang and Yang44). In this study, the protein expression levels of IRS1, phosphorylated mTOR and phosphorylated p70S6K in the CP–Zn treatment were significantly higher than those in the CON and ZnSO4 treatments, suggesting that CP–Zn supplementation promoted muscle development by activating the mTOR signalling pathway. On the other hand, previous studies have shown that weaned piglets subjected to immunological challenge (with ETEC or LPS) have increased expression levels of pro-inflammatory factors, which inhibit activation of the mTOR signalling pathway in muscle(Reference Huang, Wang and He5,Reference Wang, Yi and Hou42,Reference Kang, Wang and Wu45) . Notably, our results showed that dietary CP–Zn supplementation reduced the levels of the pro-inflammatory factors TNF-α, IL-1β and myeloperoxidase in the serum, suggesting that CP–Zn attenuated the inhibitory effects of overexpressed inflammatory factors on the mTOR signalling pathway in muscle.
Oxidative stress is an important factor that aggravates the intestinal inflammatory response, induces intestinal epithelial cell apoptosis and even destroys intestinal morphology(Reference Xu, Xu and Chen7,Reference Wu, Wang and Liu46) . MDA is produced by lipid peroxidation and is often regarded as a key indicator of oxidative injury(Reference Goethals, Vossen and Michiels47). The results of the present study showed that dietary CP–Zn supplementation reduced the MDA content of the jejunum, indicating that CP–Zn alleviated oxidative stress injury in the jejunum of piglets. In animals, nonenzymatic antioxidants (e.g. GSH) and some key antioxidative enzymes (e.g. SOD, GSH-px and catalase) protect cells from oxidative stress injury(Reference Goethals, Vossen and Michiels47,Reference Young, Fan and Mine48) . In the present study, the GSH content of the jejunum was significantly increased in the CP–Zn and CS + ZnSO4 groups, consistent with the mRNA expression levels of EAAC1. EAAC1 is abundantly expressed on the apical and brush border membranes of the SI in pigs and is responsible for glutamate transport(Reference Wang, Cui and Zhang49). Glutamate is an important precursor substance for the synthesis of GSH(Reference Wang, Cui and Zhang49). On the other hand, glutamine production via biotransformation of glutamate, the main energy substrate of enterocytes, can reduce oxidative stress and alleviate apoptosis of the intestinal epithelium, thereby preventing intestinal dysfunction(Reference Wang, Chen and Li50). In addition, studies have shown that immunological challenge (with LPS and ETEC K88) can impair the expression of antioxidant enzymes in weaned piglets(Reference Jiang, Li and Awati6,Reference Xu, Xu and Chen7,Reference Wu, Wang and Liu46) . In this study, our results showed that dietary CP–Zn supplementation enhanced the activity of T-SOD and GSH-px in the jejunum, indicating that dietary CP–Zn supplementation relieved oxidative stress in the jejunums of weaned piglets by promoting the expression of antioxidant enzymes.
The expression of antioxidant enzymes in cells is closely related to the Nrf2 signalling pathway(Reference Kobayashi and Yamamoto51). Under normal physiological conditions, Nrf2, a basic leucine zipper transcription factor, binds to its inhibitor protein Keap1 to form an inactive complex in the cytoplasm. Upon stimulation, Nrf2 dissociates from the Nrf2/Keap-1 complex and moves to the nucleus, where it binds to the antioxidant response element(Reference Kobayashi and Yamamoto51). As a result, HO1 and NQO1 are abundantly expressed and further promote the production of antioxidant enzymes(Reference Wu, Wang and Liu46,Reference Kobayashi and Yamamoto51) . In the present study, Western blot analysis revealed that dietary CP–Zn supplementation significantly increased the protein expression levels of Nrf2, HO1 and NQO1 in the jejunum, suggesting that CP–Zn regulated oxidative stress by activating the Nrf2 signalling pathway. Interestingly, we also found that CS + ZnSO4 increased the protein expression level of Nrf2 and the activity of GSH-px and reduced the content of MDA in the jejunum. To further clarify the mechanism by which CP–Zn improved antioxidant capacity, an in vitro experiment was performed on the porcine jejunal epithelial cell line IPEC-J2.
ROS are other key indicators of oxidative stress at the cellular level(Reference Wu, Wang and Liu46). In the present study, the levels of ROS and MDA were significantly higher in the LPS group than in the CON group, indicating that LPS induced oxidative stress in IPEC-J2 cells, consistent with the findings of a previous study(Reference Wu, Wang and Liu46). Interestingly, we found that CS + ZnSO4 and CP–Zn significantly decreased the levels of ROS and MDA in IPEC-J2 cells. In addition, in terms of antioxidant capacity, both CS + ZnSO4 and CP–Zn significantly increased T-SOD and GSH-px activity and activated the Nrf2 signalling pathway by increasing the protein expression levels of Nrf2, HO1 and NQO1 in IPEC-J2 cells. These results demonstrated that CS + ZnSO4 and CP–Zn alleviated oxidative stress by activating the Nrf2 signalling pathway in vitro. The results also suggested that CS and Zn had a synergistic beneficial effect on the antioxidant capacity of intestinal epithelial cells. However, CS + ZnSO4 did not significantly affect the protein expression levels of HO1 and NQO1 or the activity of T-SOD in the jejunums of weaned piglets, indicating that CS + ZnSO4 was less effective than CP–Zn in improving antioxidant capacity in vivo. We speculate that the effectiveness of CP–Zn may be attributable to its small particle size. Nano-sized CP–Zn may diffuse into the submucosal layers of the intestine easily and thus be able to interact efficiently with intestinal epithelial cells(Reference Desai, Labhasetwar and Amidon18,Reference Tian, Xu and Li19) .
In conclusion, the present study demonstrates that dietary supplementation with nano CP–Zn (100 mg/kg Zn) can improve growth performance and the antioxidant capacity of the SI, thus alleviating weaning stress in piglets. CP–Zn supplementation increases the activity of carbohydrate digestion-related enzymes and the mRNA expression levels of nutrient transporters in the jejunum and up-regulates the mTOR signalling pathway in muscle. In addition, CP–Zn improves the antioxidant capacity of the jejunum by activating the Nrf2 signalling pathway. These findings provide a valuable reference for research on the mechanisms by which nano Zn improves the intestinal health and growth of piglets and infants.
Acknowledgements
This study was supported by National Key R&D Program of China (2017YFD0500505) and the Graduate Research and Practice Innovation Program of Jiangsu Province (no. KYCX19_0573).
The authors’ contributions were as follows: M. Y. Z. and J. W. designed the experiment, analysed the experimental data and wrote this paper. M. Y. Z., G. J. H., P. H. and D. F. conducted feeding experiments and sample collection. J. W. and W. Y. Z. participated in editing the paper. All authors read and approved the final manuscript.
The authors declare that they have no competing interests.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520004766












