Among dietary factors, a high intake of saturated fat (SF) and a low intake of dietary fibre have been most strongly associated with the incidence of type 2 diabetes mellitus (T2DM)( Reference Mann, De Leeuw and Hermansen 1 , Reference American Diabetes Association, Bantle and Wylie-Rosett 2 ), although there is some controversy whether high SF intake per se increases the risk of T2DM( Reference Hu, van Dam and Liu 3 , Reference Salmeron, Hu and Manson 4 ). Overweight and lack of physical activity are further known risk factors for T2DM( Reference InterAct Consortium, Ekelund and Palla 5 ). To investigate possible causative mechanisms, their associations with insulin secretion, peripheral and hepatic insulin sensitivity are of interest.
A high intake of SF has been associated with impaired insulin sensitivity( Reference Vessby, Uusitupa and Hermansen 6 ), predominantly hepatic( Reference Clore, Stillman and Li 7 , Reference Brons, Jensen and Storgaard 8 ) according to some studies. In addition, subjects with a low intake of SF and high fitness have a lower adiposity-related risk of elevated fasting plasma glucose concentrations( Reference Heikkila, Krachler and Savonen 9 ). In contrast, dietary fibre intake has been associated with both increased insulin sensitivity( Reference Weickert and Pfeiffer 10 ) and increased insulin secretion( Reference Juntunen, Laaksonen and Poutanen 11 , Reference Laaksonen, Toppinen and Juntunen 12 ). As assessment of insulin secretion per se without taking insulin sensitivity into account has been shown to provide only a limited insight; therefore, the use of the disposition index has been suggested for an assessment of insulin secretion( Reference Cobelli, Toffolo and Dalla Man 13 ). To our knowledge, only one study has reported an association between overall dietary fibre intake and the disposition index( Reference Liese, Schulz and Fang 14 ), and an association between habitual fat quality and the disposition index has not been reported in any study.
As various dietary factors and physical activity are known to accumulate( Reference van Dam, Willett and Rimm 15 ) and interact( Reference Coker, Williams and Yeo 16 ), simultaneous measurements of these lifestyle factors are essential. Yet, most studies of glucose metabolism have been carried out in selected groups of subjects, without objectively measured data on physical activity/cardiorespiratory fitness. The aim of the present study was to investigate the associations of dietary factors and glucose metabolism in a population-based sample with the objective measurement of cardiorespiratory fitness.
Subjects and methods
Study population
The present study was based on the baseline data from a population-based randomised controlled trial, the DR's EXTRA (Dose–Responses to Exercise Training) Study, described in detail previously( Reference Heikkila, Schwab and Krachler 17 ). The target population was a representative sample of 3000 men and women who lived in the city of Kuopio in Finland and who were 55–74 years of age in 2002. A total of 1479 individuals participated in the baseline examinations in 2005–6. The initial assessment consisted of four appointments at weekly intervals: (1) anthropometric data including waist circumference (WC); (2) dietary intake; (3) cardiorespiratory fitness; (4) oral glucose tolerance test (OGTT). After excluding the individuals with previously diagnosed T2DM, missing or insufficient data, the present study population consisted of 1114 individuals. The number of subjects with BMI < 25 kg/m2 was 334 (30·0 %), those with BMI 25–30 kg/m2 was 540 (48·5 %) and those with BMI >30 kg/m2 was 240 (21·5 %). The number of subjects with normal glucose metabolism (fasting plasma glucose concentration (fP-gluc) < 6·1 mmol/l and 2 h plasma glucose concentration (2hP-gluc) < 7·8 mmol/l) was 804 (72·2 % of the study population), with isolated impaired fasting plasma glucose concentration (fP-gluc 6·1–6·9 mmol/l and 2hP-gluc < 7·8 mmol/l) was 121 (10·9 %), isolated impaired glucose tolerance concentration (fP-gluc < 6·1 mmol/l and 2hP-gluc 7·8–11·1 mmol/l) was 94 (8·4 %), concurrent impaired fasting plasma glucose concentration and impaired glucose tolerance (fP-gluc 6·1–6·9 mmol/l and 2hP-gluc 7·8–11·1 mmol/l) was 47 (4·2 %) and newly diagnosed T2DM (fP-gluc ≥ 7 mmol/l and 2hP-gluc ≥ 11·2 mmol/l) was 48 (4·3 %). All subjects gave their written informed consent. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Joint Ethics Committee of the University of Kuopio and Kuopio University Hospital. Written informed consent was obtained from all subjects.
Assessment of dietary intake
Dietary intake was assessed by a 4 d food record, including three consecutive weekdays and one weekend day. A researcher defined the recording dates. The subjects received the food records with verbal and written instructions at the first study visit. The food records were returned at the second visit 1 week later. A nutritionist or a specially trained research nurse checked the food records and completed missing information. The amount of food consumed was estimated by a picture booklet of portion sizes( Reference Pietinen, Hartman and Haapa 18 ), household gauges or weighing. Data from the food records were analysed using the MicroNutrica® nutrient calculation software (version 2.5; Finnish Social Insurance Institution) based on Finnish analyses and international food composition tables( Reference Rastas 19 ).
Assessment of cardiorespiratory fitness
Cardiorespiratory fitness (VO2max) was assessed by defining maximal oxygen uptake from respiratory gas analysis during a maximal symptom-limited exercise stress test on a bicycle ergometer (Ergoline) to exhaustion as described previously( Reference Hassinen, Lakka and Savonen 20 ). The measured VO2max of each participant was transformed into the percentage of expected normal VO2max on the basis of sex, body weight, height and age × body weight using the residual method. In the residual method, VO2max in ml of oxygen per min was set as the dependent variable and sex, body weight, height and age × body weight as independent variables into linear regression analysis. The value of expected normal VO2max was calculated for each participant using the sex-specific formula derived from the linear regression analysis. The linear regression analysis and the calculation of expected normal VO2max for each participant were performed separately for men and women due to the known difference in VO2max between sexes. The percentage of expected normal VO2max (fitness) was then calculated by dividing the value of the participant's measured VO2max by the sex-specific expected normal VO2max of the participant. This measure limits the body-weight bias introduced by dividing VO2max by total body weight( Reference Savonen, Krachler and Hassinen 21 ).
Assessment of insulin sensitivity and insulin secretion
The selected indices for insulin sensitivity and insulin secretion were the best-performed indices in a validation study with a large selection of indices( Reference Stancakova, Javorsky and Kuulasmaa 22 ). The participants of that study were of same ethnicity (Finnish) as the subjects in the DR's EXTRA Study. HOMA-IR (homeostatic model assessment of insulin resistance) was the selected index for the assessment of whole-body insulin resistance with an emphasis on the assessment of hepatic insulin resistance( Reference Matsuda and DeFronzo 23 , Reference Abdul-Ghani, Jenkinson and Richardson 24 ) due to its comparable performance with other surrogate indices of hepatic insulin resistance( Reference Hattersley, Mohlig and Roden 25 ) and its predictive power on the incidence of T2DM( Reference Morimoto, Tatsumi and Deura 26 ). The following indices for insulin sensitivity and insulin secretion were used: Matsuda-IS (Matsuda insulin sensitivity index, i.e. whole-body insulin sensitivity with an emphasis on the assessment of peripheral insulin sensitivity; units used in the calculation were in mU/l for insulin and in mg/dl for glucose (a conversion factor of 18·014 was used to convert units of glucose from mmol/l into mg/dl))( Reference Matsuda and DeFronzo 23 ); HOMA-IR (fasting glucose (mmol/l) × fasting insulin (mU/l)/22·5); Secr120 (InsAUC120/GluAUC120, i.e. an index of total insulin secretion during the OGTT; calculated as a quotient of insulin AUC and glucose AUC between the time points 0 and 120 min of OGTT, where the trapezoidal method was used for the calculation of the entire AUC (not incremental AUC); units used in the calculation were as follows: glucose (mmol/l) and insulin (pmol/l) (a conversion factor of 6·945 was used to provide insulin into pmol/l))( Reference Stancakova, Javorsky and Kuulasmaa 22 ); Secr30 (InsAUC30/GluAUC30, i.e. an index of early-phase insulin secretion during the OGTT; calculated for the time interval between 0 and 30 min of OGTT, otherwise calculated in the same way as for Secr120)( Reference Stancakova, Javorsky and Kuulasmaa 22 ); total disposition index (DI120, i.e. an index of total insulin secretion during the OGTT that takes whole-body insulin sensitivity into account; calculated as a product of Secr120 and Matsuda-IS)( Reference Stancakova, Javorsky and Kuulasmaa 22 ); early-phase disposition index (DI30, i.e. an index of early-phase insulin secretion that takes whole-body insulin sensitivity into account; calculated as a product of Secr30 and Matsuda-IS)( Reference Stancakova, Javorsky and Kuulasmaa 22 ).
Statistical analyses
Statistical analyses were performed using the SPSS statistical software for Windows, version 19.0 (IBM Corporation Released 2010) and IBM SPSS Statistics for Windows, version 19.0 (IBM Corporation). A linear regression analysis was used to assess the associations between the selected dietary factors and the indices of glucose and insulin metabolism. The analysis was adjusted for age, sex, fitness and WC with sex-specific standardisation. Combined effects of WC, SF intake, dietary fibre intake and fitness were studied with a general linear model. The analyses with the general linear model were adjusted for age and sex, and Bonferroni correction was used for controlling multiple pairwise testing. For the combined analysis, fitness was dichotomised at above or below 100 % of expected normal VO2max. Cut-offs for WC (97·0 cm in men and 86·5 cm in women), reported SF intake (11·4 % of energy (E%) in men and 11·4 E% in women) and dietary fibre intake (3·0 g/MJ or 12·7 g/1000 kcal in men and 3·3 g/MJ or 13·9 g/1000 kcal in women) were derived from median values by sex, i.e. low and high. Combined effects were studied by comparing the means of each index of glucose and insulin metabolism among eight categories defined either by dichotomised SF intake, fitness and WC or by dichotomised dietary fibre intake, fitness and WC. The subjects with an optimal lifestyle profile, i.e. those with either low SF intake, high fitness and low WC or those with high dietary fibre intake, low fitness and low WC, were used as a reference group for seven pairwise comparisons. In addition, three pairwise comparisons of means were performed in a subpopulation of subjects with high WC where a reference category of subjects was defined by low SF intake and high fitness or by high dietary fibre intake and low fitness. Analyses with SF intake were performed also in a subpopulation with total fat intake below the median intake of 30·5 E% (n 557) based on the observation by Vessby et al. ( Reference Vessby, Uusitupa and Hermansen 6 ) on the effects of dietary fat quality on whole-body insulin resistance only in subjects with total fat below the median intake. Logarithmic transformations (log10) based on visually interpreted distribution histograms of normality were applied for all outcome variables to provide normally distributed variables. All independent variables followed a normal distribution except for sex and intakes of cheese and butter, of which the latter two were skewed to the right. However, residuals of the linear regression analysis with cheese or butter intakes were normally distributed. A P value of < 0·05 was considered as significant.
Results
Intakes of dietary fibre and whole-grain bread were associated with Matsuda-IS, independent of cardiorespiratory fitness and WC (Table 1, n 1114). In women, dietary fibre was also associated with DI30 and inversely with HOMA-IR (Table 2). In men, no other associations independent of WC were found (Table 3).
Table 1 Associations between dietary factors and indices of glucose metabolism in DR's EXTRA (Dose–Responses to Exercise Training) baseline among the total study-specific population of 1114 subjects (β-Coefficients and P values)

HOMA-IR, index of whole-body insulin resistance with an emphasis on the assessment of hepatic insulin resistance; Matsuda-IS, index of whole-body insulin sensitivity with an emphasis on the assessment of peripheral insulin sensitivity; Secr30, index of early-phase insulin secretion; Secr120, index of later-phase insulin secretion; DI30, index of early-phase insulin secretion that takes insulin sensitivity into account; DI120, index of later-phase insulin secretion that takes insulin sensitivity into account; SF, saturated fat intake in percentage of energy intake.
* Log-transformed glucose metabolism indices were analysed as outcome variables using linear regression analysis. Dietary factors assessed by 4 d food records were analysed separately (no mutual adjustment) as explanatory variables, adjusted in model 1 for age and sex, in model 2 for age, sex and fitness (percentage of expected normal cardiorespiratory fitness assessed as maximal oxygen uptake) and in model 3 for age, sex, fitness and sex-specifically standardised waist circumference. The strength of an association is expressed per 1 sd (standardised coefficient) of the selected dietary factor and indicated by a β value. A P value of < 0·05 was considered as significant.
† Daily food intake (g) divided by daily energy intake (MJ). Due to the interpretation of the strength of associations with standardised coefficients, daily food intakes in g per 4184 kJ (1000 kcal) provided the same β values.
Table 2 Associations between dietary factors and indices of glucose metabolism in DR's EXTRA (Dose–Responses to Exercise Training) baseline among women (β-Coefficients and P values, n 576)

HOMA-IR, index of whole-body insulin resistance with an emphasis on the assessment of hepatic insulin resistance; Matsuda-IS, index of whole-body insulin sensitivity with an emphasis on the assessment of peripheral insulin sensitivity; Secr30, index of early-phase insulin secretion; Secr120, index of later-phase insulin secretion; DI30, index of early-phase insulin secretion that takes insulin sensitivity into account; DI120, index of later-phase insulin secretion that takes insulin sensitivity into account; SF, saturated fat intake in percentage of energy intake.
* Log-transformed glucose metabolism indices were analysed as outcome variables using linear regression analysis. Dietary factors assessed by 4 d food records were analysed separately (no mutual adjustment) as explanatory variables, adjusted in model 1 for age, in model 2 for age and fitness (percentage of expected normal cardiorespiratory fitness assessed as maximal oxygen uptake) and in model 3 for age, fitness and sex-specifically standardised waist circumference. The strength of an association is expressed per 1 sd (standardised coefficient) of the selected dietary factor and indicated by a β value. A P value of < 0·05 was considered as significant.
† Daily food intake (g) divided by daily energy intake (MJ). Due to the interpretation of the strength of associations with standardised coefficients, daily food intakes in g per 4184 kJ (1000 kcal) provided the same β values.
Table 3 Associations between dietary factors and indices of glucose metabolism in DR's EXTRA (Dose–Responses to Exercise Training) baseline among men (β-Coefficients and P values, n 538)

HOMA-IR, index of whole-body insulin resistance with an emphasis on the assessment of hepatic insulin resistance; Matsuda-IS, index of whole-body insulin sensitivity with an emphasis on the assessment of peripheral insulin sensitivity; Secr30, index of early-phase insulin secretion; Secr120, index of later-phase insulin secretion; DI30, index of early-phase insulin secretion that takes insulin sensitivity into account; DI120, index of later-phase insulin secretion that takes insulin sensitivity into account; SF, saturated fat intake in percentage of energy intake.
* Log-transformed glucose metabolism indices were analysed as outcome variables using linear regression analysis. Dietary factors assessed by 4 d food records were analysed separately (no mutual adjustment) as explanatory variables, adjusted in model 1 for age, in model 2 for age and fitness (percentage of expected normal cardiorespiratory fitness assessed as maximal oxygen uptake) and in model 3 for age, fitness and sex-specifically standardised waist circumference. The strength of an association is expressed per 1 sd (standardised coefficient) of the selected dietary factor and indicated by a β value. A P value of < 0·05 was considered as significant.
† Daily food intake (g) divided by daily energy intake (MJ). Due to the interpretation of the strength of associations with standardised coefficients, daily food intakes in g per 4184 kJ (1000 kcal) provided the same β values.
Indices of insulin secretion that take whole-body insulin sensitivity into account, i.e. DI30 and DI120, were directly associated with dietary fibre intake. Indices for insulin secretion per se, i.e. Secr30 and Secr120, were negatively associated with dietary fibre intake. The directions of associations were similarly reversed for a vast majority of dietary factors studied.
In subjects with a total fat intake below 30·5 E%, intake of SF was positively associated with HOMA-IR and inversely with Matsuda-IS and DI30, independent of cardiorespiratory fitness (Table 4). The subjects with low SF intake together with high fitness were protected against the high WC-associated lower DI30 (Table 5). In women, the decrease in DI30 caused by high WC was nearly halved with the optimal lifestyle profile; i.e. a significant difference between 207 and 142 (Δ 65) and a non-significant difference between 207 and 173 (Δ 34) were observed (Table 6). The subjects in the other three categories with high WC and with non-optimal SF intake and fitness profile had a lower DI30 when compared with the reference group with low WC, low SF intake and high fitness. These findings were not present in men (Table 7), but were observed also in the subpopulation with total fat intake below the median intake of 30·5 E% (Table 8). Similarly, the optimal lifestyle profile also protected against the high WC-associated lower DI120 in the subpopulation with low total fat intake.
Table 4 Associations between saturated fat intake and indices of glucose metabolism in DR's EXTRA (Dose–Responses to Exercise Training) baseline among subjects with total fat intake below the median of 30·5 % of energy (E%) (β-Coefficients and P values, n 557)

HOMA-IR, index of whole-body insulin resistance with an emphasis on the assessment of hepatic insulin resistance; Matsuda-IS, index of whole-body insulin sensitivity with an emphasis on the assessment of peripheral insulin sensitivity; Secr30, index of early-phase insulin secretion; Secr120, index of later-phase insulin secretion; DI30, index of early-phase insulin secretion that takes insulin sensitivity into account; DI120, index of later-phase insulin secretion that takes insulin sensitivity into account; SF, saturated fat intake in percentage of energy intake.
* Log-transformed glucose metabolism indices were analysed as outcome variables using linear regression analysis. Dietary factors assessed by 4 d food records were analysed separately (no mutual adjustment) as explanatory variables, adjusted in model 1 for age and sex, in model 2 for age, sex and fitness (percentage of expected normal cardiorespiratory fitness assessed as maximal oxygen uptake) and in model 3 for age, sex, fitness and sex-specifically standardised waist circumference. The strength of an association is expressed per 1 sd (standardised coefficient) of the selected dietary factor and indicated by a β value. A P value of < 0·05 was considered as significant.
Table 5 Means of indices of glucose metabolism defined by dichotomised cardiorespiratory fitness, waist circumference (WC) and saturated fat (SF)‡ intake in DR's EXTRA (Dose–Responses to Exercise Training) baseline among the total study-specific population of 1114 subjects§
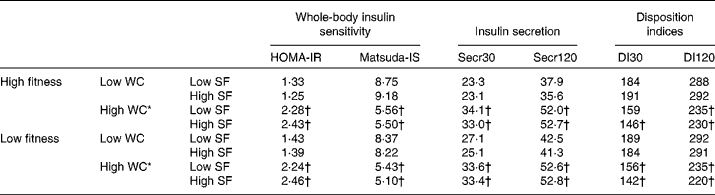
HOMA-IR, index of whole-body insulin resistance with an emphasis on the assessment of hepatic insulin resistance; Matsuda-IS, index of whole-body insulin sensitivity with an emphasis on the assessment of peripheral insulin sensitivity; Secr30, index of early-phase insulin secretion; Secr120, index of later-phase insulin secretion; DI30, index of early-phase insulin secretion that takes insulin sensitivity into account; DI120, index of later-phase insulin secretion that takes insulin sensitivity into account; fitness, percentage of expected normal cardiorespiratory fitness assessed as maximal oxygen uptake; low v. high, cut-off defined by the median value for WC (97·0 cm (n 538, 48 %) in men and 86·5 cm (n 576, 52 %) in women) and SF (11·4 % of energy (E%) in men and 11·4 E% in women) and for fitness dichotomised at above or below 100 % of expected normal maximal oxygen uptake.
* Among the four categories with high WC, significantly different from the subjects with high fitness, high WC and low SF intake. No significant differences were found.
† Significantly different from the subjects with high fitness, low WC and low SF intake.
‡ SF intake assessed by 4 d food records.
§ Log-transformed glucose metabolism indices were compared using the general linear model, adjusted for age and sex, and using Bonferroni correction for multiple pairwise testing. Means displayed are non-log-transformed and non-adjusted values. A P value of < 0·05 was considered as significant.
Table 6 Means of indices of glucose metabolism defined by dichotomised cardiorespiratory fitness, waist circumference (WC) and saturated fat (SF)‡ intake in 576 women of the DR's EXTRA (Dose–Responses to Exercise Training) baseline§

HOMA-IR, index of whole-body insulin resistance with an emphasis on the assessment of hepatic insulin resistance; Matsuda-IS, index of whole-body insulin sensitivity with an emphasis on the assessment of peripheral insulin sensitivity; Secr30, index of early-phase insulin secretion; Secr120, index of later-phase insulin secretion; DI30, index of early-phase insulin secretion that takes insulin sensitivity into account; DI120, index of later-phase insulin secretion that takes insulin sensitivity into account; fitness, percentage of expected normal cardiorespiratory fitness assessed as maximal oxygen uptake; low v. high, cut-off defined by the median value for WC (86·5 cm) and SF (11·4 % of energy) and for fitness dichotomised at above or below 100 % of expected normal maximal oxygen uptake.
* Among the four categories with high WC, significantly different from the subjects with high fitness, high WC and low SF intake. No significant differences were found.
† Significantly different from the subjects with high fitness, low WC and low SF intake.
‡ SF intake assessed by 4 d food records.
§ Log-transformed glucose metabolism indices were compared using the general linear model, adjusted for age, and using Bonferroni correction for multiple pairwise testing. Means displayed are non-log-transformed and non-adjusted values. A P value of < 0·05 was considered as significant.
Table 7 Means of indices of glucose metabolism defined by dichotomised cardiorespiratory fitness, waist circumference (WC) and saturated fat (SF)‡ intake in 538 men of the DR's EXTRA (Dose–Responses to Exercise Training) baseline§

HOMA-IR, index of whole-body insulin resistance with an emphasis on the assessment of hepatic insulin resistance; Matsuda-IS, index of whole-body insulin sensitivity with an emphasis on the assessment of peripheral insulin sensitivity; Secr30, index of early-phase insulin secretion; Secr120, index of later-phase insulin secretion; DI30, index of early-phase insulin secretion that takes insulin sensitivity into account; DI120, index of later-phase insulin secretion that takes insulin sensitivity into account; fitness, percentage of expected normal cardiorespiratory fitness assessed as maximal oxygen uptake; low v. high, cut-off defined by the median value for WC (97·0 cm) and SF (11·4 % of energy) and for fitness dichotomised at above or below 100 % of expected normal maximal oxygen uptake.
* Among the four categories with high WC, significantly different from the subjects with high fitness, high WC and low SF intake. No significant differences were found.
† Significantly different from the subjects with high fitness, low WC and low SF intake.
‡ SF intake assessed by 4 d food records.
§ Log-transformed glucose metabolism indices were compared using the general linear model, adjusted for age, and using Bonferroni correction for multiple pairwise testing. Means displayed are non-log-transformed and non-adjusted values. A P value of < 0·05 was considered as significant.
Table 8 Means of indices of glucose metabolism defined by dichotomised cardiorespiratory fitness, waist circumference (WC) and saturated fat (SF)‡ intake in DR's EXTRA (Dose–Responses to Exercise Training) baseline among the subjects with total fat intake below the median of 30·5 % of energy (E%) (n 557)§

HOMA-IR, index of whole-body insulin resistance with an emphasis on the assessment of hepatic insulin resistance; Matsuda-IS, index of whole-body insulin sensitivity with an emphasis on the assessment of peripheral insulin sensitivity; Secr30, index of early-phase insulin secretion; Secr120, index of later-phase insulin secretion; DI30, index of early-phase insulin secretion that takes insulin sensitivity into account; DI120, index of later-phase insulin secretion that takes insulin sensitivity into account; fitness, percentage of expected normal cardiorespiratory fitness assessed as maximal oxygen uptake; low v. high, cut-off defined by the median value for WC (96·9 cm (n 252, 45 %) in men and 86·5 cm (n 305, 55 %) in women) and SF (9·4 E% in men and 9·6 E% in women) and for fitness dichotomised at above or below 100 % of expected normal maximal oxygen uptake.
* Among the four categories with high WC, significantly different from the subjects with high fitness, high WC and low SF intake. No significant differences were found.
† Significantly different from the subjects with high fitness, low WC and low SF intake.
‡ SF intake assessed by 4 d food records.
§ Log-transformed glucose metabolism indices were compared using the general linear model, adjusted for age and sex, and using Bonferroni correction for multiple pairwise testing. Means displayed are non-log-transformed and non-adjusted values. A P value of < 0·05 was considered as significant.
∥ Age- and sex-adjusted log-transformed mean for the non-adjusted DI30 value of 158 was 2·146 (95 % CI 2·092, 2·200) and, respectively, the adjusted value for the non-adjusted DI30 value of 164 was 2·131 (95 % CI 2·081, 2·181). This explains why the estimated log-transformed mean for 164 is indicated (P= 0·026) and for 158 (P= 0·166) is not indicated to differ from the estimated log-transformed mean for the reference value of 192.
The combined high dietary fibre intake and high fitness did not protect against high WC-associated lowering of DI30 (Tables 9–11), in contrast to the observation with SF intake together with fitness on DI30. The combined high dietary fibre intake and high fitness also did not differentiate the DI30 among the subjects with low WC. The combined low dietary fibre intake and low fitness was associated with lower Matsuda-IS among the subjects with low WC; however, this lifestyle combination did not modify the Matsuda-IS among the subjects with high WC. Further analyses found that these results were evident only in women.
Table 9 Means of indices of glucose metabolism defined by dichotomised cardiorespiratory fitness, waist circumference (WC) and dietary fibre intake in DR's EXTRA (Dose–Responses to Exercise Training) baseline among the total study-specific population of 1114 subjects‡

HOMA-IR, index of whole-body insulin resistance with an emphasis on the assessment of hepatic insulin resistance; Matsuda-IS, index of whole-body insulin sensitivity with an emphasis on the assessment of peripheral insulin sensitivity; Secr30, index of early-phase insulin secretion; Secr120, index of later-phase insulin secretion; DI30, index of early-phase insulin secretion that takes insulin sensitivity into account; DI120, index of later-phase insulin secretion that takes insulin sensitivity into account; fitness, percentage of expected normal cardiorespiratory fitness assessed as maximal oxygen uptake; fibre, dietary fibre intake assessed by 4 d food records; low v. high, cut-off defined by the median value for WC (97·0 cm in men and 86·5 cm in women) and fibre (3·0 g/MJ or 12·7 g/1000 kcal in men and 3·3 g/MJ or 13·9 g/1000 kcal in women) and for fitness dichotomised at above or below 100 % of expected normal maximal oxygen uptake.
† Significantly different from the subjects with high fitness, low WC and high fibre intake.
* Among the four categories with high WC, significantly different from the subjects with high fitness, high WC and high fibre intake. No significant differences were found.
‡ Log-transformed glucose metabolism indices were compared using the general linear model, adjusted for age and sex, and using Bonferroni correction for multiple pairwise testing. Means displayed are non-log-transformed and non-adjusted values. A P value of < 0·05 was considered as significant.
Table 10 Means of indices of glucose metabolism defined by dichotomised cardiorespiratory fitness, waist circumference (WC) and dietary fibre intake in 576 women of the DR's EXTRA (Dose–Responses to Exercise Training) baseline‡

HOMA-IR, index of whole-body insulin resistance with an emphasis on the assessment of hepatic insulin resistance; Matsuda-IS, index of whole-body insulin sensitivity with an emphasis on the assessment of peripheral insulin sensitivity; Secr30, index of early-phase insulin secretion; Secr120, index of later-phase insulin secretion; DI30, index of early-phase insulin secretion that takes insulin sensitivity into account; DI120, index of later-phase insulin secretion that takes insulin sensitivity into account; fitness, percentage of expected normal cardiorespiratory fitness assessed as maximal oxygen uptake; fibre, dietary fibre intake assessed by 4 d food records; low v. high, cut-off defined by the median value for WC (86·5 cm) and fibre (3·3 g/MJ or 13·9 g/1000 kcal) and for fitness dichotomised at above or below 100 % of expected normal maximal oxygen uptake.
* Among the four categories with high WC, significantly different from the subjects with high fitness, high WC and high fibre intake. No significant differences were found.
† Significantly different from the subjects with high fitness, low WC and high fibre intake.
‡ Log-transformed glucose metabolism indices were compared using the general linear model, adjusted for age, and using Bonferroni correction for multiple pairwise testing. Means displayed are non-log-transformed and non-adjusted values. A P value of < 0·05 was considered as significant.
Table 11 Means of indices of glucose metabolism defined by dichotomised cardiorespiratory fitness, waist circumference (WC) and dietary fibre intake in 538 men of the DR's EXTRA (Dose–Responses to Exercise Training) baseline‡

HOMA-IR, index of whole-body insulin resistance with an emphasis on the assessment of hepatic insulin resistance; Matsuda-IS, index of whole-body insulin sensitivity with an emphasis on the assessment of peripheral insulin sensitivity; Secr30, index of early-phase insulin secretion; Secr120, index of later-phase insulin secretion; DI30, index of early-phase insulin secretion that takes insulin sensitivity into account; DI120, index of later-phase insulin secretion that takes insulin sensitivity into account; fitness, percentage of expected normal cardiorespiratory fitness assessed as maximal oxygen uptake; fibre, dietary fibre intake assessed by 4 d food records; low v. high, cut-off defined by the median value for WC (97·0 cm) and fibre (3·0 g/MJ or 12·7 g/1000 kcal) and for fitness dichotomised at above or below 100 % of expected normal maximal oxygen uptake.
* Among the four categories with high WC, significantly different from the subjects with high fitness, high WC and high fibre intake. No significant differences were found.
† Significantly different from the subjects with high fitness, low WC and high fibre intake.
‡ Log-transformed glucose metabolism indices were compared using the general linear model, adjusted for age, and using Bonferroni correction for multiple pairwise testing. Means displayed are non-log-transformed and non-adjusted values. A P value of < 0·05 was considered as significant.
Discussion
In this population-based study of 1114 middle-aged and elderly men and women, low SF intake together with high cardiorespiratory fitness attenuated the negative association between WC and the DI30. Intake of dietary fibre was positively associated with insulin sensitivity, independent of both physical fitness and WC. In women, intake of dietary fibre was also positively associated with DI30. Overall, the observations were stronger in women.
The observed associations between dietary fibre/whole-grain bread and insulin sensitivity are in line with an earlier finding of increased insulin sensitivity when on a diet rich in cereal fibre( Reference Weickert, Roden and Isken 27 ). The association between dietary fibre intake and the DI30 in women supports the earlier finding on the association between dietary fibre intake assessed with FFQ and pancreatic functionality assessed with the frequently sampled intravenous glucose tolerance test( Reference Liese, Schulz and Fang 14 ). In addition, the present finding suggests that this association is independent of cardiorespiratory fitness and stronger in women than in men. Because increase in physical activity is known to improve insulin sensitivity( Reference Shojaee-Moradie, Baynes and Pentecost 28 ), the present finding emphasises the independent contribution of dietary fibre and whole-grain products on insulin sensitivity. However, dietary fibre intake was inversely associated with the indices of insulin secretion per se, i.e. Secr30 and Secr120. The directions of associations for indices of insulin secretion per se and for the corresponding disposition indices were similarly reversed for a majority of dietary factors. This reversal of associations can be explained by the hyperbolic law: the product of β-cell function and insulin sensitivity remains unchanged in an individual whose β-cells respond to a decrease in insulin sensitivity by adequately increasing insulin secretion( Reference Cobelli, Toffolo and Dalla Man 13 ). Therefore, when a dietary factor is associated with insulin sensitivity and maximum β-cell secretion capacity has not been reached, the same dietary factor is consequently associated inversely with insulin secretion. Thus, in the present population-based data, dietary fibre (independent of cardiorespiratory fitness) is associated not only with the maintenance of insulin sensitivity, but also with early-phase insulin secretion.
Women with high WC, but with a suboptimal lifestyle profile, i.e. either high SF intake or low fitness, had a significantly lower DI30 when compared with the reference category. Similarly, the beneficial effects of the lowering of SF intake on the disposition index were observed in women, but not in men( Reference Kien, Bunn and Poynter 29 ). However, that study did not include obese subjects and baseline fitness correlated with the insulin-sensitising effect of a low-SF diet but not with diet-induced changes in disposition index. Obesity-induced inflammation is a possible mediator and may explain the observed sex differences: an increase in body weight has been shown to elevate inflammatory markers( Reference Ahonen, Vanhala and Kautiainen 30 ). This pro-inflammatory effect of adiposity is more pronounced in women( Reference Ahonen, Vanhala and Kautiainen 30 ). Inflammatory activity in obese subjects may cause insulin resistance( Reference Calder, Ahluwalia and Brouns 31 ), which in turn may cause decreased insulin secretion( Reference Weyer, Bogardus and Mott 32 ). Therefore, the pro-inflammatory effects of SF( Reference Calder, Ahluwalia and Brouns 31 )- and physical activity-induced reduction in inflammation( Reference Teixeira-Lemos, Nunes and Teixeira 33 ) may contribute to the more pronounced differences found in DI30 among women. The present study extends our knowledge that high fitness may have an important role together with low habitual SF intake to counteract even the adiposity-associated decrease in DI30 among women. High fitness alone could not counteract the negative association of high SF intake with DI30.
Low SF intake and high fitness did not differentiate adiposity-associated insulin sensitivity/resistance significantly as was found with adiposity-associated DI30. This suggests that the long-term effects of SF intake and fitness on insulin sensitivity/resistance may be reflected better in DI30 than in insulin sensitivity/resistance per se. Also in a previous study, SF intake appeared to be more important for maintaining normal glucose metabolism in overweight and obese subjects( Reference Lovejoy, Smith and Champagne 34 ). The observed associations between HOMA-IR/Matsuda-IS and SF intake only in subjects with limited total fat intake are supported by earlier findings in an intervention setting( Reference Vessby, Uusitupa and Hermansen 6 ). SF intake-dependent changes in HOMA-IR and Matsuda-IS (β values) were similar, whereas fibre intake correlated with smaller changes in HOMA-IR compared with Matsuda-IS. This observation suggests that SF intake may have a greater relative impact on hepatic insulin resistance than dietary fibre intake. Similarly, a short-term intervention with a hyperenergetic diet providing 20 E% from SF induced hepatic, but not peripheral, insulin resistance( Reference Brons, Jensen and Storgaard 8 ). Possible pathophysiological mechanisms have been described as follows: insulin resistance pronounced in obese( Reference Weyer, Hanson and Bogardus 35 ), together with decreased insulin secretion, leads to hyperglycaemia( Reference Cobelli, Toffolo and Dalla Man 13 ), which, in turn, may decrease insulin secretion via hyperglycaemia-induced incretin resistance( Reference Herzberg-Schafer, Heni and Stefan 36 ) and via an overall glucotoxic effect on β-cells( Reference Stumvoll, Goldstein and van Haeften 37 , Reference Nolan and Prentki 38 ). Furthermore, elevated levels of SFA in the blood may via the induction of hexokinase impair endocrine pancreas function( Reference Hosokawa, Corkey and Leahy 39 ). Physical activity may augment physiological glucose metabolism via improved insulin sensitivity( Reference Shojaee-Moradie, Baynes and Pentecost 28 ). The lipotoxic effect of high levels of NEFA on β-cells( Reference Nolan and Prentki 38 ) is also a potential pathophysiological mechanism: high concentration of NEFA is common in overweight adults( Reference Frohnert, Jacobs and Steinberger 40 ), and a diet high in SF elevates( Reference Stefanovski, Richey and Woolcott 41 ), whereas physical activity attenuates plasma NEFA concentration( Reference Shojaee-Moradie, Baynes and Pentecost 28 ). Our data suggest that the DI30 is the most sensitive of the studied markers regarding lifestyle-mediated homeostasis of glucose metabolism. Our findings expand on our previous short report that SF intake and fitness may modulate the adiposity-related risk of elevated fasting plasma glucose( Reference Heikkila, Krachler and Savonen 9 ). Plasma glucose concentrations remain normal as long as the maximum capacity to secrete insulin is not reached (hyperbolic law)( Reference Cobelli, Toffolo and Dalla Man 13 ). Hence, the combination of both insulin sensitivity/resistance and insulin secretion is a more sensitive marker of the association between nutrient intake and glucose metabolism. Measures of insulin sensitivity and early-phase insulin secretion derived from the OGTT are less precise than intravenous methods. Conclusive specificity of OGTT-derived indices for either early- or second-phase insulin secretion cannot be postulated( Reference Stumvoll, Mitrakou and Pimenta 42 ), nor are HOMA-IR- and Matsuda-IS-specific methods that differentiate hepatic and peripheral insulin sensitivity/resistance, respectively( Reference Abdul-Ghani, Jenkinson and Richardson 24 ). Yet, the selected indices are less resource-intensive and allow an assessment of insulin sensitivity and insulin secretion also in larger samples, such as those in the present study. Moreover, early-phase insulin secretion calculated from an OGTT may be more physiologic than intravenous methods because the gastrointestinal contribution to insulin secretion is not bypassed( Reference Holst and Gromada 43 ). Furthermore, the OGTT-derived DI30 used in the present study is an independent predictor of T2DM incidence( Reference de Mello, Lindstrom and Eriksson 44 ).
The cross-sectional setting of the study cannot delineate causality. The possibility of the effect of unknown or residual confounding cannot be ruled out, either. Moreover, as illustrated by the Look AHEAD study, favourable changes in risk factors may not always translate into a lower incidence of endpoints( Reference Wing and Bolin 45 ). The major strengths of the present study are the use of a 4 d food record to assess dietary intake and maximal exercise tests with a direct measurement of oxygen consumption for cardiorespiratory fitness and the oral glucose tolerance test to assess insulin secretion and insulin sensitivity. The use of a population-based sample of middle-aged and elderly subjects increases the external validity of the present study. Seasonal variation in diet may not have been captured at an individual level; however, as the examination of the present study population was spread out over 1·5 years, introduction of a general bias is unlikely.
In conclusion, intake of dietary fibre and whole-grain bread were positively associated with insulin sensitivity, independent of physical fitness and WC. In women, dietary fibre intake was also positively associated with the markers of early-phase insulin secretion. Moreover, the deleterious effect of abdominal obesity may be attenuated by a combination of low SF intake together with high fitness.
Acknowledgements
The DR's EXTRA Study was supported by grants from the Ministry of Education and Culture of Finland (627; 2004–2011), the Academy of Finland (104943, 123885), the European Commission FP6 Integrated Project (LSHM-CT-2004-005272; EXGENESIS), the Kuopio University Hospital, the Finnish Diabetes Association, the Finnish Foundation for Cardiovascular Research, the Päivikki and Sakari Sohlberg Foundation, the City of Kuopio and the Social Insurance Institution of Finland. The present study, including the analysis of DR's EXTRA baseline data and reporting of the results, was supported by the Juho Vainio Foundation (to H. M. H.) and the Diabetes Research Foundation (to H. M. H.). None of the funders had any role in the design and analysis of the study or in the writing of this article.
The authors’ contributions are as follows: R. R. contributed to the study conception and design; H. M. H. contributed to the data collection; H. M. H., B. K., R. R. and U. S. S. contributed to the study-specific research questions; H. M. H. and B. K. analysed the data; H. M. H., B. K., R. R. and U. S. S. contributed to the data interpretation and discussion; H. M. H. drafted the manuscript; H. M. H., B. K., R. R. and U. S. S. revised the manuscript; R. R. was a guarantor of the study.
The authors declare that there are no conflicts of interest.













