Chemotherapy the most common therapy for cancerous tumours, and chemotherapy-induced diarrhoea, is one of the most prevalent side effects(Reference Richardson and Dobish1). Severe diarrhoea affects around 20–45 % of all patients receiving chemotherapy(Reference Arbuckle, Huber and Zacker2). Chemotherapy-induced diarrhoea can be induced by many chemotherapeutics, but mainly 5-fluorouracil and irinotecan (CPT-11), which account for up to 80 % of all cases(Reference Van Den Heuvel, Peeters and Hendlisz3). Moreover, with more abdominal and pelvic tumours being treated with radiation, radiotherapy-induced diarrhoea is becoming more prevalent(Reference Benson, Ajani and Catalano4). Radiotherapy-induced diarrhoea is common during the third week of therapy, with incidence rates ranging from 20 % to 70 %(Reference Classen, Belka and Paulsen5).
Diarrhoea caused by radiotherapy or chemotherapy may reduce the quality of life and sometimes cause treatment suspension or termination(Reference Dranitsaris, Maroun and Shah6). Radiation can change bacterial flora, intestinal motility and mucosal cell vascular permeability(Reference Salminen, Elomaa and Minkkinen7,Reference Bounous8) . Additionally, chemotherapy alters the composition of the intestinal microflora, which generates numerous enzymes and controls intestinal angiogenesis and immunological processes to preserve the integrity of the gut barrier(Reference Miller and Elamin9). The gut microbiota impacts human health by influencing the gut mucosal barrier, nutrient utilisation and immunological function, as well as by directly interacting with the gastrointestinal epithelium(Reference Guarner and Malagelada10,Reference Hooper and Gordon11) .
Probiotics are described as living microorganisms that, when administered in sufficient concentrations, provide a health benefit to the host(Reference Reid, Sanders and Gaskins12,Reference Guarner, Perdigon and Corthier13) . Lactobacilli and bifidobacteria are two genera that may be found in a variety of consumer products, including yogurt(Reference Holzapfel, Haberer and Geisen14). Lactobacillus rhamnosus is a bacterium that has the potential to boost the healing of mucosa damaged by radiation and/or chemotherapy exposure with the mechanisms of stimulating the immune response and increasing the production of enterocytes, according to researchers at the University of Bristol in the UK(Reference Banasaz, Norin and Holma15). Furthermore, these lactobacilli may help restore bacterial balance in the intestine, preventing bacterial translocation into tissues and boosting local and systemic immune responses to pathogens(Reference Mack, Ahrne and Hyde16–Reference Vaarala18).
So far, several systematic reviews and meta-analyses (SRMA) have evaluated the effect of probiotic supplements on diarrhoea caused by radiotherapy and/or chemotherapy. Nevertheless, the certainty of the evidence for the effect has not been assessed.
For this purpose, overviews of SRMA (also called ‘umbrella reviews’) are useful, as they summarise the evidence from published SRMA on a particular topic(Reference Aromataris, Fernandez and Godfrey19,Reference Pollock, Campbell and Brunton20) . We conducted an overview of SRMA to evaluate the evidence from published SRMAs of randomised clinical trials (RCT) that examine the effects of probiotics on diarrhoea caused by chemotherapy and radiotherapy.
Methods
For the present overview of SRMA, we used the Cochrane Handbook’s methodology for conducting systematic reviews of interventional trials(Reference Cumpston, Li and Page21), where applicable, and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework(Reference Guyatt, Oxman and Vist22). The study’s protocol was registered in PROSPERO (CRD42022312083), prior to initiating the study selection process. The study was reported according to ‘The Preferred Reporting Items for Overviews of Reviews’ statement(Reference Pollock, Fernandes and Pieper23,Reference Gates, Gates and Pieper24) .
Eligibility criteria
Studies that fulfilled the following criteria were included in this overview of SRMA: SRMA that (1) included RCT conducted on adults with cancer who were receiving chemotherapy and/or radiotherapy; (2) evaluated the efficacy of probiotic supplementation for the prevention or treatment of chemotherapy and/or radiotherapy-related diarrhoea compared with a control group; (3) considered the incidence of diarrhoea (in terms of any diarrhoea, as well as diarrhoea of certain severities) during the intervention; (4) reported relative risk or OR of diarrhoea incidence with 95 % CI. Narrative reviews and systematic reviews without meta-analyses were excluded. If multiple meta-analyses were available for each outcome, the meta-analysis with the largest number of RCT was selected for each outcome(Reference Neuenschwander, Ballon and Weber25).
Information sources and search strategy
We searched three electronic databases, including MEDLINE (via PubMed), Scopus and ISI Web of Science (R.A and P.S) from inception up to 2 February 2022 without any language restriction. To ensure that no relevant publications were missed, the reference lists of all relevant SRMA were screened. Details on the search strategy and keywords are given in Supplementary Table 1.
Study selection
Two independent investigators (R.A and P.S) independently screened the references based on title and abstract and subsequently assessed the full texts for eligibility. To ensure that no publication was missed, the reference lists of any pertinent meta-analysis were also searched. Disagreements were resolved by discussion and consulting a third reviewer. For the quantitative synthesis, we included RCT from the eligible SRMA.
Data extraction
Two independent investigators (R.A and S.ZM) extracted the data from the eligible SRMA and their RCT. The data items extracted from the SRMA: Last name of the first author, year of publication, number of participants, number of primary RCT in the main (largest) meta-analysis, and number of primary RCT discovered from similar meta-analyses. The data items extracted from the RCT: Study design, number of participants and events within both intervention and control groups, sex, mean age, treatment modality (chemotherapy or radiotherapy), type of cancer, follow-up length, probiotic supplementation (type, duration, route of administration, daily dose, genus, strain and species), medication usage, criteria for diarrhoea and severity of diarrhoea (grade). The arm with the higher dose was chosen in RCT with two intervention arms and the same control group.
Assessment of methodological quality
We applied ‘A Measurement Tool to Assess Systematic Reviews’ (AMSTAR2) tool to assess the methodological quality of the included SRMA(Reference Shea, Reeves and Wells26). The Cochrane tool was used to evaluate the risk of bias (RoB) of the RCT included in each SRMA(Reference Higgins, Altman and Gøtzsche27). Moreover, the credibility of subgroup differences was examined according to eight criteria determined by the ‘Instrument to Assess the Credibility of Effect Modification Analyses’ (ICEMAN)(Reference Schandelmaier, Briel and Varadhan28). The assessments were conducted by two independent investigators (R.A and S.ZM) and disagreements were resolved by consensus.
Data synthesis and analysis
We briefly summarised the findings of the eligible SRMA Subsequently, we included RCT from the SRMA in meta-analyses. The outcomes included incidences of diarrhea (any grade), grade ≥ 2 diarrhea, grade ≥ 3 diarrhea, use of anti-diarrheal drug, soft stool consistency, and watery stool consistency. We selected OR and 95 % CI as the effect estimate for our analysis. OR and 95 % CI were calculated for each outcome using the quality effects model. Quality effects model was preferred to the random effects model because the random effects model suffers from serious overdispersion (especially when there is heterogeneity)(Reference Doi and Furuya-Kanamori29), and cannot be recommended anymore, even if the between-studies variance estimate is not the DerSimonian-Laird method.
In meta-analyses, the risk of bias in primary studies is evaluated, but usually, the results of this evaluation are not included in the analysis. Quality effects model is one method that considers the quality of studies, and it also reduces the estimator’s mean square error. Compared with the random effects model, this model maintained the correct coverage of the CI regardless of the heterogeneity level and showed lower variance(Reference Doi and Furuya-Kanamori29). Extracting quantitative data from the Cochrane RoB tool for input in the quality effects model was done using the method developed by Stone et al(Reference Stone, Glass and Munn30). A score of 1 was used for a domain with a low risk of bias, a score of 0 was used for high and unclear, and finally, the total score of the domains for each study was used in the analysis
The reason why we used OR rather than RR can be due to the fact that RR changes toward its null value as the prevalence of the outcome increases. This change happens regardless of the strength of the relationship between the intervention and the outcome; RR is the ratio of the probability of the outcome in the intervention group to the probability of the outcome in the control group, and both depend on the prevalence of the outcome. Moreover, OR only measures the effect and has nothing to do with the prevalence of outcome in the study and does not overestimate. In other words, OR indicates the equal chance of the outcome from an unexposed state to an exposed state(Reference Bakbergenuly, Hoaglin and Kulinskaya31,Reference Doi, Furuya-Kanamori and Xu32) .
To calculate the absolute effect, we estimated risk difference (RD) and its 95 % CI by using logittorisk module in Stata and an estimated baseline risk(Reference Furuya-Kanamori33). The estimated baseline risk required for computing RD was the event rate of the control group of all RCT for each outcome(Reference Elbarbary34). We examined and described heterogeneity quantitatively through the I2 statistic and conducted a chi-square test for homogeneity (Pfor heterogeneity > 0·10). For evaluating heterogeneity, we used Cochrane Handbook guidance, and I2 was considered as follows: may not be important (0–40 %), moderate heterogeneity (30–60 %), substantial heterogeneity (50–90 %), and considerable heterogeneity (75–100)(Reference Chandler, Cumpston and Li35). We conducted pre-defined subgroup analyses based on the type of anti-cancer therapy and outcome assessment method, as well as post hoc subgroup analyses based on the length of the intervention, probiotic genus, and singularity or combination of probiotics. To assess potential publication bias and small study effects, we used the Egger’s test(Reference Egger, Davey Smith and Schneider36). Stata version 16·0 was used for all analyses (StataCorp).
Grading of the evidence
The certainty of the evidence was rated with the GRADE approach(Reference Guyatt, Oxman and Vist22). High, moderate, low or very low are four possible categories for the overall quality of evidence when using the GRADE tool to judge the certainty of evidence.
Results
Study selection
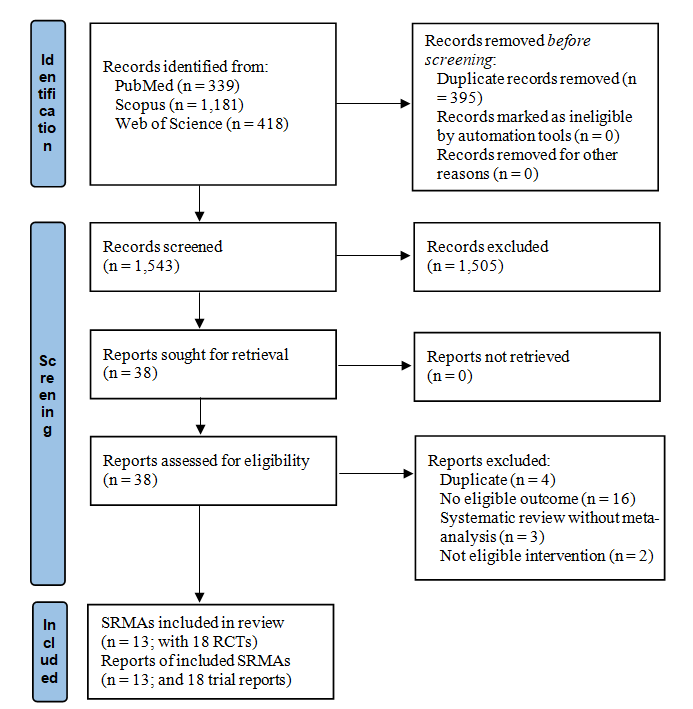
Figure 1 illustrates the study selection. Searching electronic databases resulted in 1938 records. After removing duplicates, there were 1543 studies left for screening based on titles and abstracts, which resulted in thirty-eight records for the full-text assessment. Finally, thirteen SRMA(Reference Neuenschwander, Ballon and Weber25,Reference Bartsch, Then and Harriss37–Reference Wei, Heus and van de Wetering48) were eligible for our qualitative and quantitative synthesis, contributing eighteen RCT(Reference Salminen, Elomaa and Minkkinen7,Reference Urbancsek, Kazar and Mezes49–Reference Chen, Xia and Shi65) . Supplementary Table 2 lists the excluded studies with reasons.

Fig. 1. Flow diagram showing the literature search and study selection process.
Study characteristics of the systematic reviews and meta-analyses
The thirteen SRMA included in this overview were published between 2009 and 2021. Six studies evaluated the anti-diarrhoeal effects of probiotics in patients receiving radiotherapy(Reference Bartsch, Then and Harriss37–Reference Hamad, Fragkos and Forbes40,Reference Liu, Yan and Ma43,Reference Qiu, Yu and Wang44) , three studies in chemotherapy(Reference Neuenschwander, Ballon and Weber25,Reference Lin and Shen42,Reference Wang, Yao and Wei46) and four studies in all cancer patients(Reference Hassan, Rompola and Glaser41,Reference Redman, Ward and Phillips45,Reference Wardill, Van Sebille and Ciorba47,Reference Wei, Heus and van de Wetering48) . One study included other nutritional supplements in addition to probiotics36, whereas the rest of the studies considered only probiotics.
Study characteristics of randomised controlled trial from the systematic reviews and meta-analyses
A total of eighteen RCT, with a total population of 2152 participants, could be included in our meta-analysis, and their characteristics are shown in Table 1. Three of the RCT featured a parallel design(Reference Okawa, Niibe and Arai50,Reference Mansouri-Tehrani, Khorasgani and Roayaei52,Reference Fang, Wu and Song61) and RCT(Reference Yi63–Reference Chen, Xia and Shi65), one was a parallel double-blind two-arm(Reference Demers, Dagnault and Desjardins58), one was a randomised phase II trial(Reference Lacouture, Keefe and Sonis54), one was a crossover(Reference Liu, Zhou and Zhang62) and the others were a parallel double-blind RCT(Reference Salminen, Elomaa and Minkkinen7,Reference Urbancsek, Kazar and Mezes49,Reference Osterlund, Ruotsalainen and Korpela51,Reference Linn, Thu and Win53,Reference Delia, Sansotta and Donato55–Reference Chitapanarux, Chitapanarux and Traisathit57,Reference Giralt, Regadera and Verges59,Reference Mego, Chovanec and Vochyanova-Andrezalova60) . The RCT were conducted between 1988 and 2018 in many different countries across the world. Four of the studies(Reference Salminen, Elomaa and Minkkinen7,Reference Okawa, Niibe and Arai50,Reference Linn, Thu and Win53,Reference Chitapanarux, Chitapanarux and Traisathit57) included exclusively gynecological malignancies, such as uterine and cervical cancers, while the others(Reference Urbancsek, Kazar and Mezes49,Reference Osterlund, Ruotsalainen and Korpela51,Reference Mansouri-Tehrani, Khorasgani and Roayaei52,Reference Lacouture, Keefe and Sonis54–Reference Delia, Sansotta and Donato56,Reference Demers, Dagnault and Desjardins58–Reference Chen, Xia and Shi65) included abdominal pelvic tumours, such as sigmoid, colon, prostate and bladder cancers, as well as gynecological cancers. The participants’ age ranged from 18 to 85 years. Radiotherapy with or without chemotherapy was used as an anti-cancer treatment in eleven trials(Reference Salminen, Elomaa and Minkkinen7,Reference Urbancsek, Kazar and Mezes49,Reference Okawa, Niibe and Arai50,Reference Mansouri-Tehrani, Khorasgani and Roayaei52–Reference Giralt, Regadera and Verges59) , chemotherapy was used in six studies(Reference Osterlund, Ruotsalainen and Korpela51,Reference Mego, Chovanec and Vochyanova-Andrezalova60–Reference Liu, Zhou and Zhang62,Reference Wei, He and Mai64,Reference Chen, Xia and Shi65) , and for one study, the type of anti-cancer treatment was not specified(Reference Yi63). In one study, the control group received no intervention(Reference Osterlund, Ruotsalainen and Korpela51), in another, the control group received diet counseling(Reference Salminen, Elomaa and Minkkinen7), in eleven studies, placebo(Reference Urbancsek, Kazar and Mezes49,Reference Okawa, Niibe and Arai50,Reference Mansouri-Tehrani, Khorasgani and Roayaei52–Reference Mego, Chovanec and Vochyanova-Andrezalova60) , and in the remaining five studies, the control treatment was unclear(Reference Fang, Wu and Song61–Reference Chen, Xia and Shi65). Follow-up range and probiotic dose varied between 6 and 208 weeks and 3 × 108 to 1·35 × 1012 CFU/g, respectively. The intervention dose was given to the participants from twice a day to four times a day. The major probiotics were Lactobacillus, Bifidobacterium, and Streptococcus. The outcomes, any grade(Reference Salminen, Elomaa and Minkkinen7,Reference Okawa, Niibe and Arai50–Reference Linn, Thu and Win53,Reference Delia, Sansotta and Donato55–Reference Chen, Xia and Shi65) , grade ≥ 2(Reference Mansouri-Tehrani, Khorasgani and Roayaei52,Reference Delia, Sansotta and Donato56–Reference Liu, Zhou and Zhang62) , grade ≥ 3 diarrhoea(Reference Osterlund, Ruotsalainen and Korpela51,Reference Linn, Thu and Win53,Reference Delia, Sansotta and Donato55–Reference Liu, Zhou and Zhang62,Reference Wei, He and Mai64) , use of anti-diarrhoeal drugs(Reference Salminen, Elomaa and Minkkinen7,Reference Urbancsek, Kazar and Mezes49,Reference Mansouri-Tehrani, Khorasgani and Roayaei52–Reference Lacouture, Keefe and Sonis54,Reference Chitapanarux, Chitapanarux and Traisathit57–Reference Giralt, Regadera and Verges59) and stool consistency(Reference Chitapanarux, Chitapanarux and Traisathit57–Reference Giralt, Regadera and Verges59) were evaluated in 16, 8, 11, 8 and 3 studies, respectively. Bristol scale was used to measure the consistency of stool(Reference Chitapanarux, Chitapanarux and Traisathit57–Reference Giralt, Regadera and Verges59). The severity of diarrhoea was evaluated with the National Cancer Institute Common Toxicity Criteria in five studies(Reference Mansouri-Tehrani, Khorasgani and Roayaei52,Reference Linn, Thu and Win53,Reference Chitapanarux, Chitapanarux and Traisathit57,Reference Giralt, Regadera and Verges59,Reference Mego, Chovanec and Vochyanova-Andrezalova60) and WHO criteria were used in four studies(Reference Osterlund, Ruotsalainen and Korpela51,Reference Delia, Sansotta and Donato55,Reference Delia, Sansotta and Donato56,Reference Demers, Dagnault and Desjardins58) . In the other studies, the diarrhoea measuring instrument was not reported(Reference Salminen, Elomaa and Minkkinen7,Reference Okawa, Niibe and Arai50,Reference Fang, Wu and Song61–Reference Chen, Xia and Shi65) . Among the eighteen RCT, diarrhoea was evaluated as any grade diarrhoea(Reference Salminen, Elomaa and Minkkinen7,Reference Okawa, Niibe and Arai50–Reference Linn, Thu and Win53,Reference Delia, Sansotta and Donato55–Reference Chen, Xia and Shi65) , grade ≥ 2 diarrhoea(Reference Mansouri-Tehrani, Khorasgani and Roayaei52,Reference Delia, Sansotta and Donato56–Reference Liu, Zhou and Zhang62) and grade ≥ 3 diarrhoea(Reference Osterlund, Ruotsalainen and Korpela51,Reference Linn, Thu and Win53,Reference Delia, Sansotta and Donato55–Reference Liu, Zhou and Zhang62,Reference Wei, He and Mai64) .
Table 1. Study characteristics of RCT from the eligible systematic reviews and meta-analyses

CT, chemotherapy; NCI-CTC, National Cancer Institute Common Toxicity Criteria; RCT, randomised control trial; RT, radiotherapy; ref, reference; NR, not reported.
* Women (uterus or the ovaries), men (prostatic cancers), rectum cancers and miscellaneous malignancies of the lower abdomen.
Methodological quality of the systematic reviews and meta-analyses
The results of the AMSTAR2 assessment for each SRMA are given in Supplementary Table 3. The main issue of the included SRMA was that the majority did not account for risk of bias when interpreting their results(Reference Bartsch, Then and Harriss37–Reference Hamad, Fragkos and Forbes40,Reference Lin and Shen42–Reference Qiu, Yu and Wang44,Reference Wardill, Van Sebille and Ciorba47) . Using the AMSTAR2 framework, we rated the overall quality as high, low and critically low for two (15·4 %)(Reference Neuenschwander, Ballon and Weber25,Reference Wei, Heus and van de Wetering48) , nine (69·2 %)(Reference Devaraj, Suppiah and Veettil38–Reference Wang, Yao and Wei46) and two (15·4 %)(Reference Bartsch, Then and Harriss37,Reference Wardill, Van Sebille and Ciorba47) SRMA, respectively.
Methodological quality of the RCTs from the systematic reviews and meta-analyses
The methodological quality of the RCT included from the SRMA was determined based on the Cochrane RoB tool. According to the results, one study was of good quality(Reference Mego, Chovanec and Vochyanova-Andrezalova60), four were fair(Reference Linn, Thu and Win53,Reference Lacouture, Keefe and Sonis54,Reference Chitapanarux, Chitapanarux and Traisathit57,Reference Demers, Dagnault and Desjardins58) and the rest were of poor quality(Reference Salminen, Elomaa and Minkkinen7,Reference Okawa, Niibe and Arai50–Reference Mansouri-Tehrani, Khorasgani and Roayaei52,Reference Delia, Sansotta and Donato55,Reference Delia, Sansotta and Donato56,Reference Giralt, Regadera and Verges59,Reference Fang, Wu and Song61–Reference Chen, Xia and Shi65) . The results are provided in detail in Supplementary Table 4.
Findings of the systematic reviews and meta-analyses
The results of all SRMA generally stated that probiotics can reduce the incidence of diarrhoea in patients receiving anti-cancer treatments; However, in two studies, the result was not statistically significant(Reference Fuccio, Guido and Eusebi39,Reference Wardill, Van Sebille and Ciorba47) . The main outcome in these studies was the occurrence of diarrhoea of any degree, but in the case of other outcomes, such as varying degrees of diarrhoea or the use of anti-diarrhoeal drugs, there was a discrepancy between the results of the studies(Reference Bartsch, Then and Harriss37,Reference Hamad, Fragkos and Forbes40,Reference Lin and Shen42) . Hence, the SRMA did not clearly indicate whether probiotics would be effective. However, SRMA of high methodological quality (i.e., a AMSTAR2 rating of high) showed beneficial effects on some outcomes.
Effect of probiotics on the incidence of diarrhoea (any grade)
Of the eighteen RCT, sixteen studies(Reference Salminen, Elomaa and Minkkinen7,Reference Okawa, Niibe and Arai50–Reference Linn, Thu and Win53,Reference Delia, Sansotta and Donato55–Reference Chen, Xia and Shi65) from four SRMA reported incidence of diarrhea (any grade) at follow-up. The groups receiving probiotics significantly reduced the incidence of diarrhoea compared with the control group (OR of 0·35; 95 % CI: 0·22, 0·54; P < 0·001; RD: −25 %, 95 % CI: −34 %, −15 %; online Supplementary Fig. 1), but with a low certainty of evidence (Table 2 and online Supplementary Table 5). There was substantial heterogeneity between primary studies (I2 = 67·7 %) (online Supplementary Fig. 1). When exploring potential sources of heterogeneity (type of anti-cancer treatment, duration of the intervention, outcome measurement tool, the genus of the probiotics, and the singularity or combination of the probiotics), we found no statistically significant subgroup effects (Table 3). The credibility of the subgroup analyses was all rated as low with the ICEMAN tool for this outcome, as well as for the other outcomes (online Supplementary Table 6). There was no statistically significant publication bias according to Egger’s test (P = 0·172).
Table 2. The effect of probiotic supplementation on diarrhea related to chemotherapy and radiotherapy

GRADE, Grading of Recommendations Assessment, Development, and Evaluation;wk, week.
* Calculated by quality effects model.
† Calculated by Cochran’s Q test.
‡ 0·1 mg twice a week during radiotherapy, afterward 0·1 mg per two weeks.
Table 3. Subgroup analyses of the effect of probiotics in diarrhoea-related chemotherapy and radiotherapy*

NCI-CTC, National cancer institute Common Toxicity Criteria.
* Calculated by quality-effects model.
† P heterogeneity within subgroup.
Effect of probiotics on the incidence of grade ≥ 2 diarrhoea
Eight RCT(Reference Mansouri-Tehrani, Khorasgani and Roayaei52,Reference Delia, Sansotta and Donato56–Reference Liu, Zhou and Zhang62) reported the effect of probiotics on the incidence of diarrhoea of grade 2 or more. The use of probiotics significantly reduced the incidence (OR of 0·43; 95 % CI: 0·25, 0·74, P = 0·004; RD: −20 %, 95 % CI: −29 %, −7 %; online Supplementary Fig. 2), but with low certainty of evidence (Table 2 and online Supplementary Table 5). Egger’s test revealed no statistically significant publication bias (P = 0·999).
Effect of probiotics on the incidence of grade ≥ 3 diarrhoea
Eleven RCT reported the effect of probiotics on the incidence of grade ≥ 3 diarrhoea. Also, for this outcome, probiotics resulted in a lower incidence of grade ≥ 3 diarrhoea compared with control (OR of 0·30; 95 % CI: 0·15, 0·29; P = 0·004; RD: −18 %, 95 % CI: −23 %, −9 %; online Supplementary Fig. 3). However, the certainty of the evidence was rated very low (Table 2 and online Supplementary Table 5). There was substantial heterogeneity between the studies (I2 = 67·6 %). Subgroup analysis could not find the source of heterogeneity (Table 3). There was no indication of publication bias based on Egger’s test (P = 0·566).
Effect of probiotics on the use of anti-diarrhoeal drug
The effect of probiotic usage on anti-diarrhoeal drug use was assessed in eight RCT(Reference Salminen, Elomaa and Minkkinen7,Reference Urbancsek, Kazar and Mezes49,Reference Mansouri-Tehrani, Khorasgani and Roayaei52–Reference Lacouture, Keefe and Sonis54,Reference Chitapanarux, Chitapanarux and Traisathit57–Reference Giralt, Regadera and Verges59) . Participants who received probiotics used on average less frequently anti-diarrhoea medication than those in the control group (OR of 0·49; 95 % CI: 0·27, 0·88; P = 0·047; RD: –17 %, 95 % CI: –28 %, –3 %; online Supplementary Fig. 4), but with a low certainty of evidence (Table 2 and online Supplementary Table 5). The heterogeneity between studies was substantial (I2 = 63·4 %), and the source of the heterogeneity could not be identified from our subgroup analyses (Table 3). Based on Egger’s test, there was no evidence of publication bias (P = 0·084).
Effect of probiotics on the incidence of soft and watery stool consistency
Three RCT evaluated the effect of probiotics on stool consistency. For the incidence of soft stools, there was no difference between the groups (OR of 1·10; 95 % CI: 0·44, 2·76, P = 0·951; RD: 2 %, 95 % CI: –20 %, 21 %; moderate certainty of evidence; online Supplementary Fig. 5; Table 2 and online Supplementary Table 5). The incidence of watery stool was lower in the intervention group compared with the comparator group (OR of 0·52; 95 % CI: 0·29, 1·29; P = 0·173; RD: –16 %, 95 % CI: –28 %, 6 %; very low certainty of evidence online Supplementary Fig. 6; Table 2 and online Supplementary Table 5), but it was not statistically significant. Based on Egger’s test, there was no evidence of publication bias for any of the two outcomes (P for soft stool = 0·927; P for watery stool = 0·854).
Adverse events
Adverse events were reported for eight RCT. In four of these, no adverse effects were observed from probiotic use(Reference Delia, Sansotta and Donato55,Reference Chitapanarux, Chitapanarux and Traisathit57,Reference Giralt, Regadera and Verges59,Reference Mego, Chovanec and Vochyanova-Andrezalova60) . In one trial(Reference Demers, Dagnault and Desjardins58), a few cases of neutropenia were seen during treatment. In another study(Reference Mansouri-Tehrani, Khorasgani and Roayaei52), three patients complained of upper abdominal pain, and 45 patients reported bloating during treatment, of which 35 patients belonged to the intervention group and 10 to the control group. As a result of intradermally injected LC9018 (a biologic response modifier prepared from heat-killed Lactobacillus casei YIT9018), nine cases of fever were reported; in addition, complications such as pain, tenderness, induration, swelling, necrosis, and abscess formation at the injection site were reported(Reference Okawa, Niibe and Arai50). In the study by Urnancseka et al. (Reference Urbancsek, Kazar and Mezes49), following intake of Lactobacillus rhamnos, three participants showed side effects. In the intervention group, one participant reported mild to moderate gastrointestinal problems, and in the control group, two patients reported moderate to severe gastrointestinal problems and one labial edema.
Discussion
In this overview of SRMA, we included thirteen SRMA contributing eighteen RCT, assessing the efficacy of probiotics on radiation and chemotherapy-related diarrhoea. The SRMA did not clearly indicate whether probiotics would be effective. However, well-performed SRMA showed a beneficial impact on some outcomes. According to our meta-analyses, probiotics markedly reduced the incidence of diarrhoea, regardless of severity, and the use of anti-diarrhoeal medications compared with the control group; however, the evidence certainty ranged from very low to low. Additionally, we did not observe any significant effects on the incidence of watery and soft stools.
Our meta-analysis findings of the RCT were generally consistent with conclusions of the included SRMA that indicated a decrease in chemotherapy or radiotherapy-related diarrhoea from intake of probiotics(Reference Bartsch, Then and Harriss37,Reference Lin and Shen42) . The majority of SRMA(Reference Bartsch, Then and Harriss37,Reference Devaraj, Suppiah and Veettil38,Reference Hamad, Fragkos and Forbes40,Reference Liu, Yan and Ma43,Reference Qiu, Yu and Wang44) were conducted on patients who received radiotherapy; however, in our analysis, individuals who had only undergone chemotherapy were also included. The SRMA conducted by Fuccio et al. (Reference Fuccio, Guido and Eusebi39) concluded that the use of probiotics did not provide beneficial effects for individuals getting chemotherapy and/or radiotherapy. This discrepancy with our results might be due to their low number of included studies (four trials in their analysis v. eighteen trials in our analysis) and the low sample size (793 v. 2152 patients), which may decrease the statistical power to detect any effects.
The SRMA by Wardill et al., which analysed data from seven trials (1091 patients), found a reached a similar conclusion of no beneficial effects from probiotics(Reference Wardill, Van Sebille and Ciorba47); however, positive results were obtained in the radiotherapy group, which might explain why the ‘Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology’ (MASCC/ISO) recommends Lactobacillus for preventing diarrhoea in pelvic cancers(Reference Lalla, Bowen and Barasch66).
Systemic chemotherapy causes changes in the gut microorganisms(Reference Manichanh, Varela and Martinez67). The phenotype of the gut bacteria shifts from Lactobacillus to Escherichia coli as a result of high-dose chemotherapy(Reference Van Sebille, Gibson and Wardill68). As a result, probiotics containing Lactobacillus have shown to be effective in chemotherapy-induced diarrhoea(Reference Montassier, Batard and Massart69,Reference Montassier, Gastinne and Vangay70) . Several mechanisms have been proposed for the effective action of probiotics in diarrhoea caused by cancer treatments. Probiotics exert beneficial effects by modifying dysbiosis, lowering intestinal pH, stimulating and regulating immune cell function, downmodulation apoptosis, lactase production, and aiding lactose digestion(Reference Demers, Dagnault and Desjardins58,Reference Paris, Fuks and Kang71–Reference Liu, Li and Shu73) . Spencer et al. found that mice who received probiotic supplements had more diverse microbiota than the control group in a study on mice with melanoma cancer(Reference Spencer, McQuade and Gopalakrishnan74). Additionally, interferon γ, CD+8, and the frequency of toxic T cells were significantly lower in the probiotic supplementation group(Reference Spencer, McQuade and Gopalakrishnan74). However, a previous narrative review indicated that the effects of probiotics are strain specific(Reference Rijkers, de Vos and Brummer75). Thus, these beneficial effects could not be attributed to all strains of probiotics. Although probiotics reduced any grade, grade ≥ 2, and grade ≥ 3 diarrhoea, the effects of probiotics on any grade and grade ≥ 3 are uncertain due to their very low and low certainty of evidence, respectively, as found in this overview of SRMA. Our subgroup analyses did not indicate any clear reason for the observed heterogeneity between studies. However, for the incidence of diarrhoea (any grade), the subgroup with the genus without Bifidobacterium did not reach significance, and for grade 2 and grade 3 diarrhoea, a single probiotic supplement did not reach significance. However, these subgroups include only few RCT, reducing the precision of the estimates.
This overview of SRMA has several strengths, but also some limitations. To our knowledge, this overview is presenting the most complete and comprehensive summary of the effects of probiotics on diarrhoea caused by radiation and chemotherapy. Our search strategy was comprehensive by involving many keywords applied to three electronic databases as well as screening references lists. Our study selection and data extraction were conducted by two independent investigators, increasing the methodological quality. We evaluated the methodological quality of the included SRMA and their RCT, and we evaluated the certainty of the evidence using the GRADE method. However, our study also had some limitations. Since we included RCT from SRMA, there is a risk that some newly published RCT were not identified for this study. The included RCT were heterogeneous in terms of criteria for evaluating diarrhoea, sex distributions (several RCT included women only) and chemotherapy regimens and types of radiotherapy. For some outcomes, only few RCT reported data, especially for stool consistency. Also, many SRMA and RCT were of low methodological quality, contributing to the low quality of evidence of our findings.
Conclusion
In conclusion, this overview of SRMA found evidence that probiotics can reduce the incidence of diarrhoea in cancer patients, as well as the need for anti-diarrhoeal medication. However, the certainty of evidence ranged from very low to low. There is an urgent need for high-quality RCT with a large sample size, better study design and more complete outcome assessment to provide high-quality evidence for the effects of probiotics on chemotherapy and radiotherapy-related diarrhoea as well as explore potential subgroup effects.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0007114523000910
Acknowledgements
The project was funded by the Students’ Scientific Research Center (SSRC) of Tehran University of Medical Sciences (code: IR.TUMS.MEDICINE.REC.1401.163). Section for Biostatistics and Evidence-Based Research, The Parker Institute (SMN) is supported by a core grant from The Oak Foundation (OCAY-18-774-OFIL), a group of philanthropic organisations giving grants to not-for-profit organisations around the world.
The details of the authors’ responsibility in this project were as follows. R. A., S. Z. M. and P. S. collected and analysed the data and wrote the initial version of the work. H. M. and S. M. N. carefully read the text and tables and revised them. The final version was reviewed and approved by all authors.
There was no conflict of interest in this project.