1. Introduction
Fetal growth and development depend on the selective placental transport of long-chain polyunsaturated fatty acids (LCPUFAs). After premature birth, this transfer is disrupted and the infant becomes reliant on fatty acids (FAs) provided from intravenous lipid emulsions, human milk, and/or preterm formula. Accumulating evidence indicates that infants born at extremely low gestation obtain insufficient amounts of LCPUFAs to fulfill their nutritional needs in the perinatal period (Robinson & Martin, Reference Robinson and Martin2017), particularly the omega-3 FA docosahexaenoic acid (DHA, 22:6 n-3) and the omega-6 FA arachidonic acid (AA, 20:4 n-6; Hellström et al., Reference Hård, Nilsson, Lund, Hansen-Pupp, Smith and Hellström2021).
Human milk is considered the best nutrition for the preterm infant (Hård et al., Reference Hellström, Nilsson, Wackernagel, Pivodic, Vanpee, Sjöbom, Hellgren, Hallberg, Domellöf, Klevebro, Hellström, Andersson, Lund, Löfqvist, Elfvin, Sävman, Hansen-Pupp, Hård, Smith and Ley2019), providing FAs to support energy demands as well as omega-3 and omega-6 type LCPUFAs. However, mother’s own milk, donor human milk, or formula alone do not supply the necessary quantities of LCPUFA required for the rapidly growing infant (Klevebro et al., Reference Klevebro, Juul and Wood2019). Maternal milk LCPUFAs content must be considered when the best clinical supplementation strategies in this fragile group of infants are investigated.
2. Objective
We have previously reported on the FA profiles of milk from women delivering at extremely low gestation and how the FA composition changes during three stages of lactation: at 1 week after delivery (Day 7, transitional milk), and at postmenstrual weeks 32 and 40 (PMW 32 and PMW 40, mature milk; Nilsson et al., Reference Nilsson, Löfqvist, Najm, Hellgren, Sävman, Andersson, Smith and Hellström2018). This study aimed to expand on these data in a larger up-dated cohort of women recruited at three centers in Sweden.
3. Methods
This study is part of the Mega Donna Mega trial (ClinicalTrials.gov Identifier: NCT03201588; Hellström et al., Reference Hård, Nilsson, Lund, Hansen-Pupp, Smith and Hellström2021). The trial protocol was approved by the Regional ethics review board in Gothenburg. Informed consent was obtained from all mothers included in the study. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
The study was performed at three Swedish centers from December 15, 2016 to December 15, 2019. Expressed milk was collected from 151 women who had given birth to 159 infants. Mean (standard deviation [SD]) weeks of gestation at delivery was 25.6 (1.4). Milk (125 μl) FAs were converted to fatty acid methyl esters by direct acid transesterification (Cruz-Hernandez et al., Reference Cruz-Hernandez, Goeuriot, Giuffrida, Thakkar and Destaillats2013) and analyzed by gas chromatography–mass spectrometry (GC–MS; Nilsson et al., Reference Nilsson, Löfqvist, Najm, Hellgren, Sävman, Andersson, Smith and Hellström2018). GC oven parameters were as follow: 100°C for 2 min, 15°C/min to 150°C for 1 min, 2.5°C/min to 214°C for 1 min, then 70°C/min to 240°C for 2 min. One microliter of sample was injected in a 10:1 split ratio. The between analysis coefficient of variation for AA and DHA for the method was 9.6% and 11.3%, and within batch 4.0% and 6.7%, respectively. Statistical analyses were performed in SIMCA 17 (Umetrics AB, Umeå, Sweden) and IBM SPSS Statistics version 26 (IBM Corp, Armonk NY).
4. Results
Milk samples collected at Day 7 versus at PMW 32 and 40 were separated in the first component in a principal component analysis (PCA, Figure 1a). This separation was largely driven by composition differences in LCPUFAs, long-chain saturated FAs, and long-chain monounsaturated FAs, being generally higher in milk from Day 7 (Figure 1b and Table 1). DHA and AA decreased from median (Q1–Q3) 0.35 (0.28–0.46) and 0.49 (0.41–0.61) to 0.22 (0.17–0.31) and 0.34 (0.28–0.40) mol% between Day 7 and PMW 32. This change corresponded to a 30% reduction in AA and 37% reduction in DHA from Day 7 to PMW 32 (Supplementary Table S1). Levels of DHA and AA were similar at PMW 32 and 40. The fraction of the n-3 LCPUFA eicosapentaenoic acid (EPA, 20:5 n-3) remained stable across all time points. FAs increasing in proportion during the progress of milk maturation included linoleic acid (18:2 n-6), γ-linolenic acid (18:3 n-6), and α-linolenic acid (18:3 n-3). Although samples separated according to milk maturation stage in the PCA, they were not well grouped, displaying a large variation in FA milk composition between the lactating women.
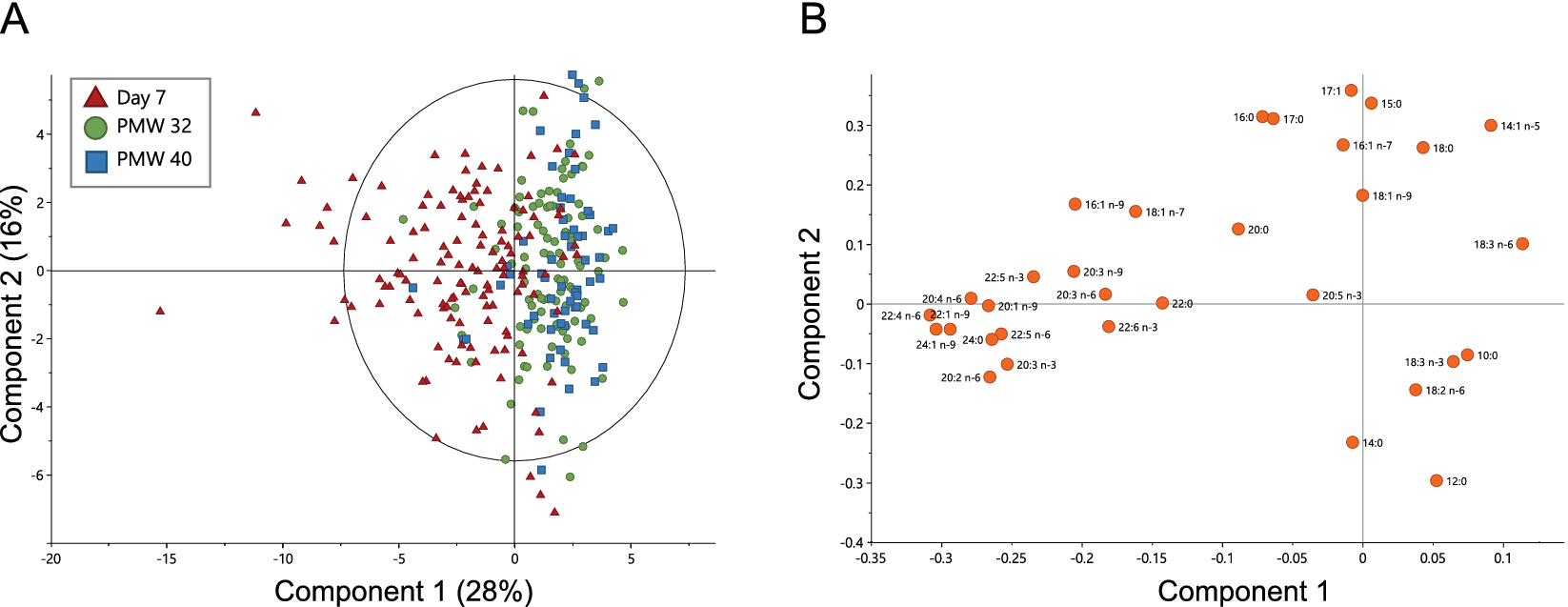
Figure 1. Principal component analysis (PCA) based on the fatty acid profiles of milk samples collected at Day 7, at postmenstrual week 32 (PMW 32), and PMW 40 (total n = 288). (a) PCA scatter plot colored according to sampling occasion and (b) the corresponding loading plot. The ellipse in (a) shows Hotelling’s T 2 (95% confidence limit). PCA model: six components, R 2X = 0.76 and Q 2 = 0.49.
Table 1. Proportions of fatty acids (FAs) in milk samples collected on three occasions during lactation
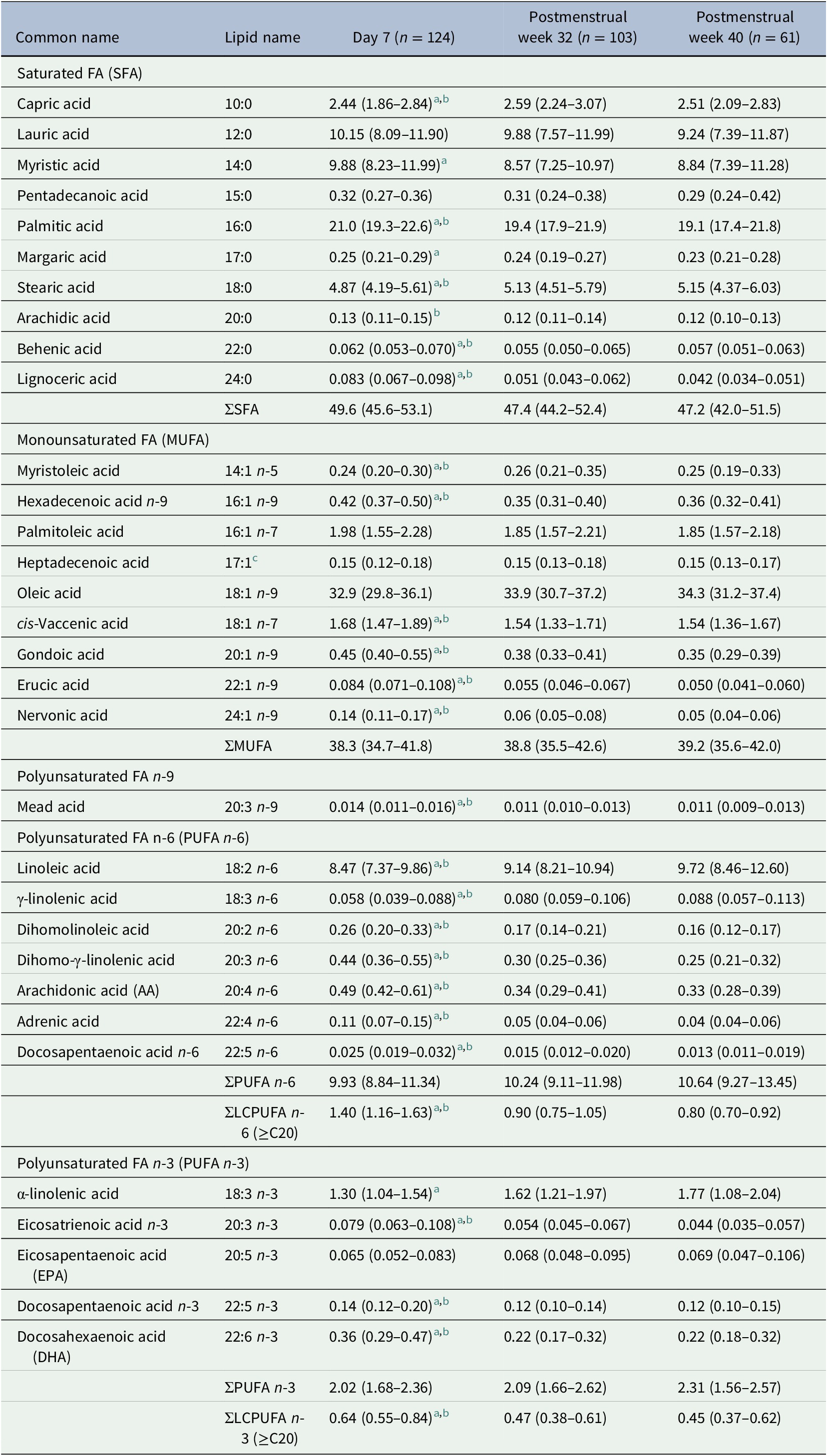
Data are expressed as the molar percent (mol%) of all analyzed fatty acids and presented as median (Q1–Q3). Minimum/maximum days for sample collection were 6/12 for Day 7 samples, 29/68 for postmenstrual week 32 samples, and 69/128 for postmenstrual week 40 samples.
a Significantly different between postnatal day 7 and PMA 32 weeks (n = 78) according to the Wilcoxon signed-rank test (p < .05).
b Significantly different between postnatal day 7 and PMA 40 weeks (n = 44) according to the Wilcoxon signed-rank test (p < .05).
c Likely representing the cis-Δ9 17:1 isomer (17:1 n-8) (Precht & Molkentin, Reference Precht and Molkentin2000).
5. Discussion
This study confirms our previous results that the content of LCPUFAs is low in milk from Swedish mothers of extremely low gestational age infants and that levels decline significantly during milk maturation (Nilsson et al., Reference Nilsson, Löfqvist, Najm, Hellgren, Sävman, Andersson, Smith and Hellström2018). The proportions of DHA and AA in transitional and mature milk were similar in this cohort as reported for preterm milk in a recent meta-analysis (Floris et al., Reference Floris, Stahl, Abrahamse-Berkeveld and Teller2020). There was a high heterogeneity in milk FA composition between the lactating mothers, likely reflecting differences in diet, genetic background, and lifestyle choices, among other factors (Koletzko, Reference Koletzko2016).
6. Conclusions
Extremely preterm infants miss the maternal transfer of LCPUFAs during the third trimester and are thus born with low endogenous deposits. The low content of LCPUFAs in mother’s own milk may aggravate the deficit with potential consequences for the preterm infant’s growth and development. Supplementation strategies for infants born extremely preterm based on the known intake of LCPUFA in milk and other sources and the calculated needs of the infant are warranted. The data presented here may be used in decision-making when such supplementation strategies are designed.
Acknowledgments
The authors thank all participating mothers and their families who made this study possible. The authors also thank the nursing, medical, and research staff at the neonatal units in Gothenburg, Lund and Stockholm for their excellent work.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/exp.2022.4.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Funding statement
This work was primarily supported by the Swedish Medical Research Council (2015-00810). We also acknowledge the support from the Swedish Medical Research Council (2016-01131), the Gothenburg Medical Society, De Blindas Vänner, Government grants under the ALF agreements (ALFGBG-717971, ALFGBG-812951), and the Wallenberg Clinical Scholars.
Conflict of interest
The authors declare that they have no conflict of interest.
Authorship contributions
A.K.N. and A.H. conceived and designed the study. I.H.P., D.W., and K.S. were involved in sample collection. U.S. and O.C. prepared samples for analysis and A.K.N. conducted mass spectrometry data gathering. A.K.N. performed statistical analyses. A.K.N. drafted the manuscript. All authors critically revised and approved the final manuscript.





Comments
Comments to the Author: The authors report the extension of data from a previous study on the importance of the presence of polyunsaturated fatty acids in breast milk for infant development.
Although the study is interesting, it does not contribute anything new or innovative to the previously published study. This study only provides more data than the previously published study.
Furthermore, the authors base their results on exploratory data analysis (PCA), but the graph shown in figure 2 does not show clear trends in the grouping of the three types of samples analysed according to fatty acid composition. In fact, there is only a slight tendency to group the samples classified as “day 7” with negative scores for component 1 and the remaining two with positive scores, as the authors state in the results section.
For this reason, I recommend not to publish this study, as a more complete study is needed to correlate the authors’ hypothesis.